

植物学报 ›› 2024, Vol. 59 ›› Issue (3): 355-372.DOI: 10.11983/CBB24006 cstr: 32102.14.CBB24006
朱晓博1,2, 董张1,2, 祝梦瑾1,2, 胡晋1,2, 林程3, 陈敏2, 关亚静1,2,*( )
)
收稿日期:2024-01-10
接受日期:2024-03-30
出版日期:2024-05-10
发布日期:2024-05-10
通讯作者:
关亚静, 博士, 教授、博导, 浙江大学“求是”青年学者, 农业与生物技术学院现代种业研究所种子科学与工程中心主任, 浙江大学海南研究院种子工程与检验检疫团队方向负责人, 中国作物学会种子专业委员会副会长, 中国植物学会种子与技术专业委员会副主任。主要从事种子发育成熟和活力分子机理、多功能种子丸化及种子增值技术研究。E-mail: 基金资助:
Xiaobo Zhu1,2, Zhang Dong1,2, Mengjin Zhu1,2, Jin Hu1,2, Cheng Lin3, Min Chen2, Yajing Guan1,2,*( )
)
Received:2024-01-10
Accepted:2024-03-30
Online:2024-05-10
Published:2024-05-10
Contact:
E-mail: 摘要: 高等植物通常从种子萌发开始, 经过营养生长和生殖发育后重新形成种子, 由此完成世代更迭。种子中积累的碳水化合物、脂质、蛋白质及mRNA等大分子物质对于维持其发芽潜力至关重要, 其中部分mRNA可长期保存而不被降解, 被称为长寿命mRNA (即long-lived mRNA)。在水稻(Oryza sativa)中, 与萌发相关的long-lived mRNA在花后10-20天开始转录积累, 花后20天至种子完全成熟期间, 一些与休眠和胁迫响应相关的long-lived mRNA转录并保存在细胞中。Long-lived mRNA种类繁多, 主要包括蛋白质合成类mRNA、能量代谢类mRNA、细胞骨架类mRNA及逆境响应相关的mRNA, 如小热激蛋白和LEA家族蛋白。Long-lived mRNA的转录组分析表明, 很多基因的启动子区域都包含脱落酸(ABA)或赤霉素(GA)相关的顺式作用元件, 拟南芥(Arabidopsis thaliana) atabi5突变体种子中约有500个不同于野生型的差异表达long-lived mRNA, 暗示ABA和GA是影响long-lived mRNA种类的关键激素。Long-lived mRNA通常与单核糖体和RBP蛋白交联在一起, 以PBs (P-bodies)形式存在于细胞中, 保护mRNA不被降解。与种子休眠相关的long-lived mRNA在种子后熟过程中逐渐被降解, 而且一些特定long-lived mRNA的氧化修饰是种子打破休眠的一种生物现象。在种子长期贮藏过程中, long-lived mRNA的随机降解直接关系到种子的寿命和活力, 保留下来的mRNA在种子吸胀初期被翻译成蛋白质, 促进种子在吸胀早期快速萌发。该文综述了种子重要储存物质long-lived mRNA的特征和功能, 并提出了本领域需要进一步研究的科学问题, 以期为深入理解种子休眠、萌发与寿命的分子机制提供参考。
朱晓博, 董张, 祝梦瑾, 胡晋, 林程, 陈敏, 关亚静. 重要的种子储存物质长寿命mRNA. 植物学报, 2024, 59(3): 355-372.
Xiaobo Zhu, Zhang Dong, Mengjin Zhu, Jin Hu, Cheng Lin, Min Chen, Yajing Guan. Indispensable Material for Germination: Long-lived mRNAs of Plant Seed. Chinese Bulletin of Botany, 2024, 59(3): 355-372.
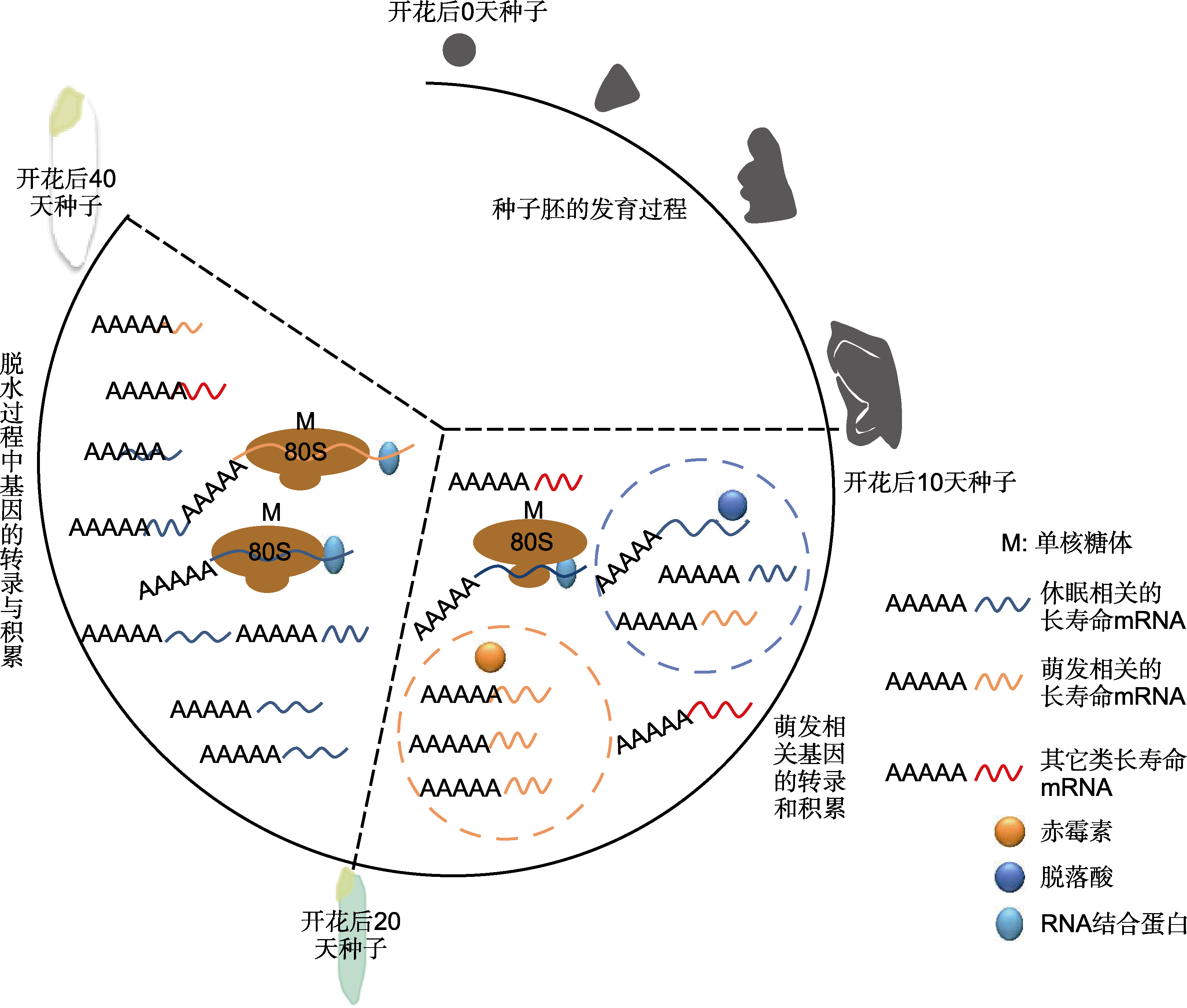
图1 长寿命mRNA在水稻种子发育成熟期的变化(种子胚发育过程参考Sano et al., 2015)
Figure 1 The changes of long-lived mRNAs during rice seed development (the development of seed embryo process refer to Sano et al., 2015)
| 基因名称 | ID | 表达特点 | 功能 | 分子机制 | 物种 | 参考文献 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| SDR3.1 | LOC_Os03g-11550 | 开花后5天开始表达, 15天达到峰值 | 反向调控 种子休眠 | 与ABI5互作, 并抑制ABI5的表达 | 水稻 | Guo et al., | ||||
| SRO1 | TraesCS4A-01G321300 | 在叶片、根和种子中 表达 | 反向调控 种子休眠 | 与TaVP1互作, 抑制穗上萌发基因TaPHS1和TaSdr的表达, 且影响TaVP1与TaABI5的互作 | 小麦 | Liu et al., | ||||
| OsWSD1 | LOC_Os03g-24460 | 在种子发育过程中, 其表达量逐渐降低; 在种子吸胀前36小时 稳定表达 | 正向调控 种子休眠 | 抑制GA的合成和α-淀粉酶的活性; 突变后对ABA不敏感 | 水稻 | Huang et al., | ||||
| AtAIL6 | AT5G10510 | 在整个种子发育过 程中维持高表达 | 正向调控 种子休眠 | FUS3直接调控AtAIL6的表达 | 拟南芥 | Liu et al., | ||||
| WRKY36 | AT1G69810 | 在种子中高表达 | 反向调控 种子休眠 | WRKY36与AFP2互作, 复合体直接抑制DOG1的表达 | 拟南芥 | Deng et al., | ||||
| AFP2 | AT1G13740 | 在种子中高表达 | 反向调控 种子休眠 | |||||||
| OsNAC2 | LOC_Os04g-38720 | 在种子发育和萌发过程中高表达 | 正向调控 种子休眠 | 直接抑制OsABAox1和OsABAox2的表达 | 水稻 | Zhao et al., | ||||
| BG14 | AT2G27500 | 在整个种子发育过程中维持高表达 | 正向调控 种子休眠 | 降解胚细胞间的胼胝质, 调控发育种子中ABA的积累 | 拟南芥 | Wang et al., | ||||
| SPT | AT4G36930 | - | 存在正向 和反向两 种调控种 子休眠机制 | 在Landsberg erecta与Columbia中种子休眠的遗传表现相反。直接抑制RGA和MFT的表达, 直接调控ABI5的表达 | 拟南芥 | Vaistij et al., | ||||
| OsDOR1 | LOC_Os03g-20770 | 在种子胚中特异表达 | 正向调控 种子休眠 | OsDOR1与OsGID1互作, 破坏OsGID1-OsSLR1复合体的形成, 影响GA信号转导 | 水稻 | Kim et al., | ||||
| FIP1 | AT5G58040 | 在干种子中表达 | 正向调控 种子休眠 | FIP1是一个加工pre-mRNA 3'端的蛋白, Abi5、DOG1和PYL12在突变体fip1中表达量下降 | 拟南芥 | Li et al., | ||||
| OsNCED3 | LOC_Os03g-44380 | 在种子胚发育过程中高表达 | 反向调控 种子休眠 | 突变后, 种子胚的ABA含量降低, GA含量升高; 而超表达植株中, 种子胚的ABA/GA比例关系到种子的休眠性 | 水稻 | Chen et al., | ||||
| SFL1 | AT1G27461 | 在种子发育过程中表达量逐渐升高 | 反向调控 种子休眠 | OsSdr4的同源基因, 且功能相同 | 拟南芥 | Zheng et al., | ||||
| AtMLP329 | AT2G01530 | 在种子胚的胚根中表达 | 正向调控 种子的初 级休眠 | DOF6直接调控MLP329的表达; 突变后, GA合成酶基因GA1表达量升高, ABA合成酶基因ZEP表达量下降 | 拟南芥 | Chong et al., | ||||
| TaETR1 | TraesCS4A-02G274300 | 在所有组织中都有表达, 在根中表达量最高 | 正向调控 种子休眠 | 超表达植株的种子对乙烯不敏感 | 小麦 | Wei et al., | ||||
| AtAAH | AT4G20070 | 在发育中的角果、根和干种子及萌发期的种子中高表达 | 反向调控 种子休眠 | 外施硝酸钾可部分恢复ataah种子的高休眠性表型 | 拟南芥 | Yazdanpanah et al., | ||||
| OsABA8ox1 | LOC_Os02g-47470 | 在种子中表达 | 反向调控 种子休眠 | 降低ABA含量 | 水稻 | Fu et al., | ||||
| OsABA8ox2 | LOC_Os08g-36860 | |||||||||
| OsABA8ox3 | LOC_Os09g-28390 | |||||||||
| KCS12 | Mtr.49305.1.- S1_at | 在种皮中表达 | 正向调控 种子休眠 | 控制种皮细胞中长链脂肪酸的合成 | 蒺藜苜蓿 | Chai et al., | ||||
| FHY3 | AT3G22170 | 随着角果的发育, 表达量逐渐升高, 在干种子中表达量达到峰值 | 反向调控 种子休眠 | 白光促进FHY3蛋白积累; FHY3与phyB互作, 直接调控RVE2和RVE7的表达, 直接抑制SPT的表达 | 拟南芥 | Liu et al., | ||||
| FT | AT1G65480 | 正向调控 种子休眠 | 在种子中特异表达FT或者TFL1, GA含量降低 | 拟南芥 | Chen et al., | |||||
| TFL1 | AT5G03840 | |||||||||
| OsbZIP09 | LOC_Os01g-59760 | ABA处理15分钟可诱 导OsbZIP09的表达 | 反向调控 种子休眠 | 利用RNA-seq和DAP-seq分析了52个OsbZIP09直接调控的基因, 包括休眠相关基因OsLOX2和LEA家族 | 水稻 | Zhu et al., | ||||
| TaAMY2 | TraesCS7D-02G380400 | 开花后5天高表达, 之后表达量逐渐降低 | 反向调控 种子休眠 | 在超表达TaAmy2植株中, α-淀粉酶的活性升高, 导致可溶性糖含量升高, 新鲜种子没有休眠性, 对ABA不敏感 | 小麦 | Zhang et al., | ||||
| OsGLP2-1 | LOC_Os02g-29000 | 在种子盾片中特异表达 | 正向调控 种子休眠 | 受ABA诱导表达, GA抑制其表达; ABI5和GAMYB拮抗地调控GLP2-1的表达 | 水稻 | Wang et al., | ||||
| REF6 | AT3G48430 | 在发育中的角果表达 | 反向调控 种子休眠 | 在角果发育过程中, REF6结合CYP707A1和CYP707A3, 并负责这2个基因的H3K27me3修饰 | 拟南芥 | Chen et al., | ||||
| HSFA9 | Medtr4g126-070 | 在种子中特异表达 | 反向调控 种子休眠 | 调控ABA代谢和信号通路 | 蒺藜苜蓿 | Zinsmeis- ter et al., | ||||
| SD6 | LOC_Os06g-06900 | 在种子发育过程中表 达量逐渐降低 | 反向调控 种子休眠 | 与OsICE2互作, 直接促进ABAOX3的表达 | 水稻 | Xu et al., | ||||
| OsICE2 | LOC_Os01g-70310 | 在种子发育过程中表 达量逐渐升高 | 正向调控 种子休眠 | 与SD6互作, 直接抑制ABAOX3的表达 | 水稻 | |||||
| OsDOG1L-3 | LOC_Os01g-20030 | 在开花后15天的种子 中表达量达到最大值, 后逐渐下降 | 正向调控 种子休眠 | 正反馈影响ABA合成基因的表达, 增加种子中ABA含量 | 水稻 | Wang et al., | ||||
| OsbZIP75 | LOC_Os09g-34060 | 在种子中高表达 | 正向调控 种子休眠 | OsbZIP75直接调控OsDOG1L-3 | 水稻 | |||||
| OsbZIP78 | LOC_Os10g-38820 | 在种子中高表达 | 正向调控 种子休眠 | 同OsbZIP75 | 水稻 | |||||
| OsBT1 | LOC_Os02g-10800 | 在种子中特异表达, 开花后21天达到峰值; 在茎、叶、叶鞘和穗中几乎不表达 | 正向调控 种子休眠 | - | 水稻 | Song et al., | ||||
| AtPER1 | AT1G48130 | 在种子中特异表达 | 正向调控 种子的初 级休眠 | 清除种子中的活性氧, 抑制ABA降解和促进GA合成 | 拟南芥 | Chen et al., | ||||
| ETR1/RDO3 | AT1G66340 | - | 正向调控 种子休眠 | ERF12与TPL相互作用, 直接抑制DOG1的表达, 而ETR1是ERF12的上游基因, 参与调控ERF12的表达 | 拟南芥 | Li et al., | ||||
| DOGL4 | At4g18650 | 在新鲜种子和干种子中都有高表达; 在开花后6天胚乳中、开花后8天胚中开始表达 | 反向调控 种子休眠 | 在F1代种子的胚乳细胞中, 来自父本的基因甲基化DOG4的启动子, 降低其表达; 纯合突变体种子的休眠能力增强 | 拟南芥 | Zhu et al., | ||||
| ROS1 | AT2G36490 | 在干种子中表达 | 反向调控 种子休眠 | 促进DOG4的表达 | ||||||
| GHNAC83 | GlaUn057212 | 在叶片、花和根中高表 达; 在球茎中低表达 | 正向调控 种子休眠 | GHNAC83直接抑制GHPP2C1的表达, 影响ABA信号; 直接结合GHIPT的启动子, 负调控CK的合成 | 唐菖蒲 | Wu et al., | ||||
| GHPP2C | GlaUn078852 | 在根、叶、球茎、雄蕊、雌蕊和花瓣中都有表达 | 反向调控 种子休眠 | 识别ABA分子 | ||||||
| ASPG1 | AT3G18490 | 在圆锥花序及萌发的种子中表达 | 反向调控 种子休眠 | 可能通过影响GA信号通路, 影响种子休眠及萌发 | 拟南芥 | Shen et al., | ||||
| EBS | AT4G22140 | 在种子发育早期及吸胀前24小时的种子中表达 | 正向调控 种子休眠 | 植物特有的一类转录调节蛋白, 与SHL在调控种子休眠上功能冗余, 并且与AGL67互作 | 拟南芥 | Narro-Die- go et al., | ||||
| GATA12 | AT5G25830 | 在新鲜种子中表达量最高, 在干种子中也有表达 | 正向调控 种子休眠 | RGL12与DOF6互作, 直接调控GATA12的表达 | 拟南芥 | Ravindran et al., | ||||
| KNOX4 | Medtr5g011-070 | 在种皮细胞中显著表达 | 反向调控 种子休眠 | 突变体种子的种皮角质层发生变化, 直接调控角质层合成基因的表达 | 蒺藜苜蓿 | Chai et al., | ||||
| OsGA20ox2 | LOC_Os01g- 66100 | 在发育中的种子和胚细胞中表达 | 反向调控 种子休眠 | 影响发育中种子的GA合成基因表达 | 水稻 | Ye et al., | ||||
| RDO5 | AT4G11040 | 在种子中特异表达, 在干种子中表达量最高 | 正向调控 种子休眠 | RDO5编码一个PP2C磷酸酶, 通过抑制RNA结合蛋白APUM9的表达调控种子休眠 | 拟南芥 | Xiang et al., | ||||
| APUM9 | AT1G35730 | 在开花后16天的种子中高表达, 之后表达量逐渐降低 | 反向调控 种子休眠 | |||||||
| WRKY41 | AT4g11070 | 在成熟种子、种子胚、 叶脉和下胚轴中表达 | 正向调控 种子休眠 | WRKY14直接调控ABI3的表达, 但与ABA通路无关联 | 拟南芥 | Ding et al., | ||||
| HON | AT1g07430 | 在开花后9天的种子中 表达, 开花后12天表达量达到峰值 | 反向调控 种子休眠 | 是PP2C家族蛋白, 在ABA存在时, 与PYR1/RCAR11互作; 抑制ABA信号而激活GA信号 | 拟南芥 | Kim et al., | ||||
| ABI4 | AT2G40220 | 在种子中高表达 | 反向调控 种子休眠 | ABI4直接抑制CYP707A1和CYO- 707A2的表达; 突变体中ABA含量降低, GA含量升高, abi4突变体可以恢复ga1-t不发芽的表型 | 拟南芥 | Shu et al., | ||||
| SNL1 | AT3G01320 | 随着种子成熟, 表达量逐渐升高 | 正向调控 种子休眠 | SNL1与SNL2形成组蛋白去乙酰化复合体, SNL1与组蛋白去乙酰化酶HDA19互作, 调控乙烯合成基因及ABA合成、降解基因的乙酰化水平 | 拟南芥 | Wang et al., | ||||
| SNL2 | AT5G15020 | |||||||||
| AtDOF6 | AT3G45610 | 在新鲜种子中表达, 在后熟过程和吸胀时期逐渐降低 | 正向调控 种子休眠 | 与TCP14互作, TCP14反向调控种子休眠 | 拟南芥 | Rueda-Ro mero et al., | ||||
| AtET2 | AT5G56780 | 在成熟的种子胚中 表达 | 正向调控 种子休眠 | FUS3直接抑制AtET2的表达 | 拟南芥 | Ivanov et al., | ||||
| FUS3 | AT3G26790 | 在种子胚发育过程中持续表达, 在成熟种子中高表达 | 反向调控 种子休眠 | |||||||
| KYP/SUVH4 | AT5G13960 | 在吸胀种子中表达量最高 | 反向调控 种子休眠 | 突变体中, DOG1和ABI3的表达量升高 | 拟南芥 | Zheng et al., | ||||
| AMP1 | AT3G54720 | 在种子发育过程中表 达, 在开花后8天的种 子中表达量最高 | 反向调控 种子休眠 | 在不同背景的拟南芥中, 突变后的种子休眠表型不一致, 初步鉴定突变后影响ABA含量 | 拟南芥 | Griffiths et al., | ||||
| TaMFT | AB456688 | 在不成熟胚的盾片和 胚芽鞘中表达; 在种 子发育过程中保持高 表达 | 正向调控 种子休眠 | AtMFT的同源基因 | 小麦 | Nakamu- ra et al., | ||||
| NCED6 | AT3G24220 | 在发育中的种子胚乳 中表达 | 正向调控 种子休眠 | 在种子中诱导表达NCED6后, 种子中的ABA含量升高, 萌发率下降, 新鲜种子的休眠率升高 | 拟南芥 | Martínez-Andújar et al., | ||||
| DOG1 | AT5G45830 | 仅在种子中表达, 且 在开花后9天表达量 最高 | 正向调控 种子休眠 | - | 拟南芥 | Bentsink et al., | ||||
表1 可能作为long-lived mRNA参与调控种子休眠的基因
Table 1 The genes regulated seed dormancy may be long-lived mRNA
| 基因名称 | ID | 表达特点 | 功能 | 分子机制 | 物种 | 参考文献 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| SDR3.1 | LOC_Os03g-11550 | 开花后5天开始表达, 15天达到峰值 | 反向调控 种子休眠 | 与ABI5互作, 并抑制ABI5的表达 | 水稻 | Guo et al., | ||||
| SRO1 | TraesCS4A-01G321300 | 在叶片、根和种子中 表达 | 反向调控 种子休眠 | 与TaVP1互作, 抑制穗上萌发基因TaPHS1和TaSdr的表达, 且影响TaVP1与TaABI5的互作 | 小麦 | Liu et al., | ||||
| OsWSD1 | LOC_Os03g-24460 | 在种子发育过程中, 其表达量逐渐降低; 在种子吸胀前36小时 稳定表达 | 正向调控 种子休眠 | 抑制GA的合成和α-淀粉酶的活性; 突变后对ABA不敏感 | 水稻 | Huang et al., | ||||
| AtAIL6 | AT5G10510 | 在整个种子发育过 程中维持高表达 | 正向调控 种子休眠 | FUS3直接调控AtAIL6的表达 | 拟南芥 | Liu et al., | ||||
| WRKY36 | AT1G69810 | 在种子中高表达 | 反向调控 种子休眠 | WRKY36与AFP2互作, 复合体直接抑制DOG1的表达 | 拟南芥 | Deng et al., | ||||
| AFP2 | AT1G13740 | 在种子中高表达 | 反向调控 种子休眠 | |||||||
| OsNAC2 | LOC_Os04g-38720 | 在种子发育和萌发过程中高表达 | 正向调控 种子休眠 | 直接抑制OsABAox1和OsABAox2的表达 | 水稻 | Zhao et al., | ||||
| BG14 | AT2G27500 | 在整个种子发育过程中维持高表达 | 正向调控 种子休眠 | 降解胚细胞间的胼胝质, 调控发育种子中ABA的积累 | 拟南芥 | Wang et al., | ||||
| SPT | AT4G36930 | - | 存在正向 和反向两 种调控种 子休眠机制 | 在Landsberg erecta与Columbia中种子休眠的遗传表现相反。直接抑制RGA和MFT的表达, 直接调控ABI5的表达 | 拟南芥 | Vaistij et al., | ||||
| OsDOR1 | LOC_Os03g-20770 | 在种子胚中特异表达 | 正向调控 种子休眠 | OsDOR1与OsGID1互作, 破坏OsGID1-OsSLR1复合体的形成, 影响GA信号转导 | 水稻 | Kim et al., | ||||
| FIP1 | AT5G58040 | 在干种子中表达 | 正向调控 种子休眠 | FIP1是一个加工pre-mRNA 3'端的蛋白, Abi5、DOG1和PYL12在突变体fip1中表达量下降 | 拟南芥 | Li et al., | ||||
| OsNCED3 | LOC_Os03g-44380 | 在种子胚发育过程中高表达 | 反向调控 种子休眠 | 突变后, 种子胚的ABA含量降低, GA含量升高; 而超表达植株中, 种子胚的ABA/GA比例关系到种子的休眠性 | 水稻 | Chen et al., | ||||
| SFL1 | AT1G27461 | 在种子发育过程中表达量逐渐升高 | 反向调控 种子休眠 | OsSdr4的同源基因, 且功能相同 | 拟南芥 | Zheng et al., | ||||
| AtMLP329 | AT2G01530 | 在种子胚的胚根中表达 | 正向调控 种子的初 级休眠 | DOF6直接调控MLP329的表达; 突变后, GA合成酶基因GA1表达量升高, ABA合成酶基因ZEP表达量下降 | 拟南芥 | Chong et al., | ||||
| TaETR1 | TraesCS4A-02G274300 | 在所有组织中都有表达, 在根中表达量最高 | 正向调控 种子休眠 | 超表达植株的种子对乙烯不敏感 | 小麦 | Wei et al., | ||||
| AtAAH | AT4G20070 | 在发育中的角果、根和干种子及萌发期的种子中高表达 | 反向调控 种子休眠 | 外施硝酸钾可部分恢复ataah种子的高休眠性表型 | 拟南芥 | Yazdanpanah et al., | ||||
| OsABA8ox1 | LOC_Os02g-47470 | 在种子中表达 | 反向调控 种子休眠 | 降低ABA含量 | 水稻 | Fu et al., | ||||
| OsABA8ox2 | LOC_Os08g-36860 | |||||||||
| OsABA8ox3 | LOC_Os09g-28390 | |||||||||
| KCS12 | Mtr.49305.1.- S1_at | 在种皮中表达 | 正向调控 种子休眠 | 控制种皮细胞中长链脂肪酸的合成 | 蒺藜苜蓿 | Chai et al., | ||||
| FHY3 | AT3G22170 | 随着角果的发育, 表达量逐渐升高, 在干种子中表达量达到峰值 | 反向调控 种子休眠 | 白光促进FHY3蛋白积累; FHY3与phyB互作, 直接调控RVE2和RVE7的表达, 直接抑制SPT的表达 | 拟南芥 | Liu et al., | ||||
| FT | AT1G65480 | 正向调控 种子休眠 | 在种子中特异表达FT或者TFL1, GA含量降低 | 拟南芥 | Chen et al., | |||||
| TFL1 | AT5G03840 | |||||||||
| OsbZIP09 | LOC_Os01g-59760 | ABA处理15分钟可诱 导OsbZIP09的表达 | 反向调控 种子休眠 | 利用RNA-seq和DAP-seq分析了52个OsbZIP09直接调控的基因, 包括休眠相关基因OsLOX2和LEA家族 | 水稻 | Zhu et al., | ||||
| TaAMY2 | TraesCS7D-02G380400 | 开花后5天高表达, 之后表达量逐渐降低 | 反向调控 种子休眠 | 在超表达TaAmy2植株中, α-淀粉酶的活性升高, 导致可溶性糖含量升高, 新鲜种子没有休眠性, 对ABA不敏感 | 小麦 | Zhang et al., | ||||
| OsGLP2-1 | LOC_Os02g-29000 | 在种子盾片中特异表达 | 正向调控 种子休眠 | 受ABA诱导表达, GA抑制其表达; ABI5和GAMYB拮抗地调控GLP2-1的表达 | 水稻 | Wang et al., | ||||
| REF6 | AT3G48430 | 在发育中的角果表达 | 反向调控 种子休眠 | 在角果发育过程中, REF6结合CYP707A1和CYP707A3, 并负责这2个基因的H3K27me3修饰 | 拟南芥 | Chen et al., | ||||
| HSFA9 | Medtr4g126-070 | 在种子中特异表达 | 反向调控 种子休眠 | 调控ABA代谢和信号通路 | 蒺藜苜蓿 | Zinsmeis- ter et al., | ||||
| SD6 | LOC_Os06g-06900 | 在种子发育过程中表 达量逐渐降低 | 反向调控 种子休眠 | 与OsICE2互作, 直接促进ABAOX3的表达 | 水稻 | Xu et al., | ||||
| OsICE2 | LOC_Os01g-70310 | 在种子发育过程中表 达量逐渐升高 | 正向调控 种子休眠 | 与SD6互作, 直接抑制ABAOX3的表达 | 水稻 | |||||
| OsDOG1L-3 | LOC_Os01g-20030 | 在开花后15天的种子 中表达量达到最大值, 后逐渐下降 | 正向调控 种子休眠 | 正反馈影响ABA合成基因的表达, 增加种子中ABA含量 | 水稻 | Wang et al., | ||||
| OsbZIP75 | LOC_Os09g-34060 | 在种子中高表达 | 正向调控 种子休眠 | OsbZIP75直接调控OsDOG1L-3 | 水稻 | |||||
| OsbZIP78 | LOC_Os10g-38820 | 在种子中高表达 | 正向调控 种子休眠 | 同OsbZIP75 | 水稻 | |||||
| OsBT1 | LOC_Os02g-10800 | 在种子中特异表达, 开花后21天达到峰值; 在茎、叶、叶鞘和穗中几乎不表达 | 正向调控 种子休眠 | - | 水稻 | Song et al., | ||||
| AtPER1 | AT1G48130 | 在种子中特异表达 | 正向调控 种子的初 级休眠 | 清除种子中的活性氧, 抑制ABA降解和促进GA合成 | 拟南芥 | Chen et al., | ||||
| ETR1/RDO3 | AT1G66340 | - | 正向调控 种子休眠 | ERF12与TPL相互作用, 直接抑制DOG1的表达, 而ETR1是ERF12的上游基因, 参与调控ERF12的表达 | 拟南芥 | Li et al., | ||||
| DOGL4 | At4g18650 | 在新鲜种子和干种子中都有高表达; 在开花后6天胚乳中、开花后8天胚中开始表达 | 反向调控 种子休眠 | 在F1代种子的胚乳细胞中, 来自父本的基因甲基化DOG4的启动子, 降低其表达; 纯合突变体种子的休眠能力增强 | 拟南芥 | Zhu et al., | ||||
| ROS1 | AT2G36490 | 在干种子中表达 | 反向调控 种子休眠 | 促进DOG4的表达 | ||||||
| GHNAC83 | GlaUn057212 | 在叶片、花和根中高表 达; 在球茎中低表达 | 正向调控 种子休眠 | GHNAC83直接抑制GHPP2C1的表达, 影响ABA信号; 直接结合GHIPT的启动子, 负调控CK的合成 | 唐菖蒲 | Wu et al., | ||||
| GHPP2C | GlaUn078852 | 在根、叶、球茎、雄蕊、雌蕊和花瓣中都有表达 | 反向调控 种子休眠 | 识别ABA分子 | ||||||
| ASPG1 | AT3G18490 | 在圆锥花序及萌发的种子中表达 | 反向调控 种子休眠 | 可能通过影响GA信号通路, 影响种子休眠及萌发 | 拟南芥 | Shen et al., | ||||
| EBS | AT4G22140 | 在种子发育早期及吸胀前24小时的种子中表达 | 正向调控 种子休眠 | 植物特有的一类转录调节蛋白, 与SHL在调控种子休眠上功能冗余, 并且与AGL67互作 | 拟南芥 | Narro-Die- go et al., | ||||
| GATA12 | AT5G25830 | 在新鲜种子中表达量最高, 在干种子中也有表达 | 正向调控 种子休眠 | RGL12与DOF6互作, 直接调控GATA12的表达 | 拟南芥 | Ravindran et al., | ||||
| KNOX4 | Medtr5g011-070 | 在种皮细胞中显著表达 | 反向调控 种子休眠 | 突变体种子的种皮角质层发生变化, 直接调控角质层合成基因的表达 | 蒺藜苜蓿 | Chai et al., | ||||
| OsGA20ox2 | LOC_Os01g- 66100 | 在发育中的种子和胚细胞中表达 | 反向调控 种子休眠 | 影响发育中种子的GA合成基因表达 | 水稻 | Ye et al., | ||||
| RDO5 | AT4G11040 | 在种子中特异表达, 在干种子中表达量最高 | 正向调控 种子休眠 | RDO5编码一个PP2C磷酸酶, 通过抑制RNA结合蛋白APUM9的表达调控种子休眠 | 拟南芥 | Xiang et al., | ||||
| APUM9 | AT1G35730 | 在开花后16天的种子中高表达, 之后表达量逐渐降低 | 反向调控 种子休眠 | |||||||
| WRKY41 | AT4g11070 | 在成熟种子、种子胚、 叶脉和下胚轴中表达 | 正向调控 种子休眠 | WRKY14直接调控ABI3的表达, 但与ABA通路无关联 | 拟南芥 | Ding et al., | ||||
| HON | AT1g07430 | 在开花后9天的种子中 表达, 开花后12天表达量达到峰值 | 反向调控 种子休眠 | 是PP2C家族蛋白, 在ABA存在时, 与PYR1/RCAR11互作; 抑制ABA信号而激活GA信号 | 拟南芥 | Kim et al., | ||||
| ABI4 | AT2G40220 | 在种子中高表达 | 反向调控 种子休眠 | ABI4直接抑制CYP707A1和CYO- 707A2的表达; 突变体中ABA含量降低, GA含量升高, abi4突变体可以恢复ga1-t不发芽的表型 | 拟南芥 | Shu et al., | ||||
| SNL1 | AT3G01320 | 随着种子成熟, 表达量逐渐升高 | 正向调控 种子休眠 | SNL1与SNL2形成组蛋白去乙酰化复合体, SNL1与组蛋白去乙酰化酶HDA19互作, 调控乙烯合成基因及ABA合成、降解基因的乙酰化水平 | 拟南芥 | Wang et al., | ||||
| SNL2 | AT5G15020 | |||||||||
| AtDOF6 | AT3G45610 | 在新鲜种子中表达, 在后熟过程和吸胀时期逐渐降低 | 正向调控 种子休眠 | 与TCP14互作, TCP14反向调控种子休眠 | 拟南芥 | Rueda-Ro mero et al., | ||||
| AtET2 | AT5G56780 | 在成熟的种子胚中 表达 | 正向调控 种子休眠 | FUS3直接抑制AtET2的表达 | 拟南芥 | Ivanov et al., | ||||
| FUS3 | AT3G26790 | 在种子胚发育过程中持续表达, 在成熟种子中高表达 | 反向调控 种子休眠 | |||||||
| KYP/SUVH4 | AT5G13960 | 在吸胀种子中表达量最高 | 反向调控 种子休眠 | 突变体中, DOG1和ABI3的表达量升高 | 拟南芥 | Zheng et al., | ||||
| AMP1 | AT3G54720 | 在种子发育过程中表 达, 在开花后8天的种 子中表达量最高 | 反向调控 种子休眠 | 在不同背景的拟南芥中, 突变后的种子休眠表型不一致, 初步鉴定突变后影响ABA含量 | 拟南芥 | Griffiths et al., | ||||
| TaMFT | AB456688 | 在不成熟胚的盾片和 胚芽鞘中表达; 在种 子发育过程中保持高 表达 | 正向调控 种子休眠 | AtMFT的同源基因 | 小麦 | Nakamu- ra et al., | ||||
| NCED6 | AT3G24220 | 在发育中的种子胚乳 中表达 | 正向调控 种子休眠 | 在种子中诱导表达NCED6后, 种子中的ABA含量升高, 萌发率下降, 新鲜种子的休眠率升高 | 拟南芥 | Martínez-Andújar et al., | ||||
| DOG1 | AT5G45830 | 仅在种子中表达, 且 在开花后9天表达量 最高 | 正向调控 种子休眠 | - | 拟南芥 | Bentsink et al., | ||||
| [1] |
Aalen RB, Salehian Z, Steinum TM (2001). Stability of barley aleurone transcripts: dependence on protein synthesis, influence of the starchy endosperm and destabilization by GA3. Physiol Plant 112, 403-413.
PMID |
| [2] |
Almoguera C, Jordano J (1992). Developmental and environmental concurrent expression of sunflower dry-seed- stored low-molecular-weight heat-shock protein and Lea mRNAs. Plant Mol Biol 19, 781-792.
PMID |
| [3] |
Ariizumi T, Hauvermale AL, Nelson SK, Hanada A, Yamaguchi S, Steber CM (2013). Lifting DELLA repression of Arabidopsis seed germination by nonproteolytic gibberellin signaling. Plant Physiol 162, 2125-2139.
DOI PMID |
| [4] |
Ariizumi T, Steber CM (2007). Seed germination of GA- insensitive sleepy1 mutants does not require RGL2 protein disappearance in Arabidopsis. Plant Cell 19, 791-804.
PMID |
| [5] |
Aspart L, Meyer Y, Laroche M, Penon P (1984). Developmental regulation of the synthesis of proteins encoded by stored mRNA in radish embryos. Plant Physiol 76, 664-673.
DOI PMID |
| [6] | Bazin J, Langlade N, Vincourt P, Arribat S, Balzergue S, El-Maarouf-Bouteau H, Bailly C (2011). Targeted mRNA oxidation regulates sunflower seed dormancy alleviation during dry after-ripening. Plant Cell 23, 2196-2208. |
| [7] |
Beltrán-Peña E, Ortíz-López A, de Jiménez ES (1995). Synthesis of ribosomal proteins from stored mRNAs early in seed germination. Plant Mol Biol 28, 327-336.
PMID |
| [8] |
Bentsink L, Jowett J, Hanhart CJ, Koornneef M (2006). Cloning of DOG1, a quantitative trait locus controlling seed dormancy in Arabidopsis. Proc Natl Acad Sci USA 103, 17042-17047.
DOI PMID |
| [9] | Cadman CSC, Toorop PE, Hilhorst HWM, Finch-Savage WE (2006). Gene expression profiles of Arabidopsis Cvi seeds during dormancy cycling indicate a common underlying dormancy control mechanism. Plant J 46, 805-822. |
| [10] | Chai MF, Queralta Castillo I, Sonntag A, Wang SX, Zhao ZL, Liu W, Du J, Xie HL, Liao FQ, Yun JF, Jiang QZ, Sun J, Molina I, Wang ZY (2021). A seed coat-specific β-ketoacyl-CoA synthase, KCS12, is critical for preserving seed physical dormancy. Plant Physiol 186, 1606-1615. |
| [11] | Chai MF, Zhou C, Molina I, Fu CX, Nakashima J, Li GF, Zhang WZ, Park J, Tang YH, Jiang QZ, Wang ZY (2016). A class II KNOX gene, KNOX4, controls seed physical dormancy. Proc Natl Acad Sci USA 113, 6997-7002. |
| [12] | Chen FY, Li Y, Li XY, Li WL, Xu JM, Cao H, Wang Z, Li Y, Soppe WJJ, Liu YX (2021). Ectopic expression of the Arabidopsis florigen gene FLOWERING LOCUS T in seeds enhances seed dormancy via the GA and DOG1 pathways. Plant J 107, 909-924. |
| [13] | Chen HH, Ruan JX, Chu P, Fu W, Liang ZW, Li Y, Tong JH, Xiao LT, Liu J, Li CL, Huang SZ (2020a). AtPER1 enhances primary seed dormancy and reduces seed germination by suppressing the ABA catabolism and GA biosynthesis in Arabidopsis seeds. Plant J 101, 310-323. |
| [14] | Chen HH, Tong JH, Fu W, Liang ZW, Ruan JX, Yu YG, Song X, Yuan LB, Xiao LT, Liu J, Cui YH, Huang SZ, Li CL (2020b). The H3K27me3 demethylase RELATIVE OF EARLY FLOWERING6 suppresses seed dormancy by inducing abscisic acid catabolism. Plant Physiol 184, 1969-1978. |
| [15] |
Chen KG, An YQC (2006). Transcriptional responses to gibberellin and abscisic acid in barley aleurone. J Integr Plant Biol 48, 591-612.
DOI |
| [16] | Chen Y, Xiang ZP, Liu M, Wang SY, Zhang L, Cai D, Huang Y, Mao DD, Fu J, Chen LB (2023). ABA biosynthesis gene OsNCED3 contributes to preharvest sprouting resistance and grain development in rice. Plant Cell Environ 46, 1384-1401. |
| [17] |
Chong SN, Ravindran P, Kumar PP (2022). Regulation of primary seed dormancy by MAJOR LATEX PROTEIN- LIKE PROTEIN329 in Arabidopsis is dependent on DNA-BINDING ONE ZINC FINGER6. J Exp Bot 73, 6838-6852.
DOI PMID |
| [18] |
De Guzman MK, Parween S, Butardo VM, Alhambra CM, Anacleto R, Seiler C, Bird AR, Chow CP, Sreenivasulu N (2017). Investigating glycemic potential of rice by unraveling compositional variations in mature grain and starch mobilization patterns during seed germination. Sci Rep 7, 5854.
DOI PMID |
| [19] | Deng GL, Sun HQ, Hu YL, Yang YR, Li P, Chen YL, Zhu Y, Zhou Y, Huang JL, Neill SJ, Hu XY (2023). A transcription factor WRKY36 interacts with AFP2 to break primary seed dormancy by progressively silencing DOG1 in Arabidopsis. New Phytol 238, 688-704. |
| [20] | Ding ZJ, Yan JY, Li GX, Wu ZC, Zhang SQ, Zheng SJ (2014). WRKY41 controls Arabidopsis seed dormancy via direct regulation of ABI3 transcript levels not downstream of ABA. Plant J 79, 810-823. |
| [21] |
Dure L, Waters L (1965). Long-lived messenger RNA: evidence from cotton seed germination. Science 147, 410-412.
PMID |
| [22] |
Fleming MB, Patterson EL, Reeves PA, Richards CM, Gaines TA, Walters C (2018). Exploring the fate of mRNA in aging seeds: protection, destruction, or slow decay? J Exp Bot 69, 4309-4321.
DOI PMID |
| [23] | Fu K, Song WH, Chen C, Mou CL, Huang YS, Zhang FL, Hao QX, Wang P, Ma TF, Chen YP, Zhu ZY, Zhang M, Tong QK, Liu X, Jiang L, Wan JM (2022). Improving pre-harvest sprouting resistance in rice by editing OsABA8ox using CRISPR/Cas9. Plant Cell Rep 41, 2107-2110. |
| [24] | Gao F, Rampitsch C, Chitnis VR, Humphreys GD, Jordan MC, Ayele BT (2013). Integrated analysis of seed proteome and mRNA oxidation reveals distinct post-transcriptional features regulating dormancy in wheat (Triticum aestivum L.). Plant Biotechnol J 11, 921-932. |
| [25] | Griffiths J, Barrero JM, Taylor J, Helliwell CA, Gubler F (2011). ALTERED MERISTEM PROGRAM 1 is involved in development of seed dormancy in Arabidopsis. PLoS One 6, e20408. |
| [26] | Guan YJ, Hu J (2000). Seed Science (Condensed edition). Beijing: China Agriculture Press. pp. 64. (in Chinese) |
| 关亚静, 胡晋 (2000). 种子学(精编版). 北京: 中国农业出版社. pp. 64. | |
| [27] |
Guo NH, Tang SJ, Wang YK, Chen W, An RH, Ren ZL, Hu SK, Tang SQ, Wei XJ, Shao GN, Jiao GA, Xie LH, Wang L, Chen Y, Zhao FL, Sheng ZH, Hu PS (2024). A mediator of OsbZIP46 deactivation and degradation negatively regulates seed dormancy in rice. Nat Commun 15, 1134.
DOI PMID |
| [28] |
Harris B, Dure L 3rd (1978). Developmental regulation in cotton seed germination: polyadenylation of stored messenger RNA. Biochemistry 17, 3250-3256.
PMID |
| [29] | Hazra A, Varshney V, Verma P, Kamble NU, Ghosh S, Achary RK, Gautam S, Majee M (2022). Methionine sulfoxide reductase B5 plays a key role in preserving seed vigor and longevity in rice (Oryza sativa). New Phytol 236, 1042-1060. |
| [30] |
He DL, Han C, Yao JL, Shen SH, Yang PF (2011). Constructing the metabolic and regulatory pathways in germinating rice seeds through proteomic approach. Proteomics 11, 2693-2713.
DOI PMID |
| [31] | He WP, Wang R, Zhang Q, Fan MX, Lyu Y, Chen S, Chen DF, Chen XW (2023). E3 ligase ATL5 positively regulates seed longevity by mediating the degradation of ABT1 in Arabidopsis. New Phytol 239, 1754-1770. |
| [32] |
Howell KA, Narsai R, Carroll A, Ivanova A, Lohse M, Usadel B, Millar AH, Whelan J (2009). Mapping metabolic and transcript temporal switches during germination in rice highlights specific transcription factors and the role of RNA instability in the germination process. Plant Physiol 149, 961-980.
DOI PMID |
| [33] | Huang YS, Song JW, Hao QX, Mou CL, Wu HM, Zhang FL, Zhu ZY, Wang P, Ma TF, Fu K, Chen YP, Nguyen T, Liu SJ, Jiang L, Wan JM (2023). WEAK SEED DORMANCY 1, an aminotransferase protein, regulates seed dormancy in rice through the GA and ABA pathways. Plant Physiol Biochem 202, 107923. |
| [34] |
Huh JH, Bauer MJ, Hsieh TF, Fischer R (2007). Endosperm gene imprinting and seed development. Curr Opin Genet Dev 17, 480-485.
DOI PMID |
| [35] |
Hundertmark M, Hincha DK (2008). LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genomics 9, 118.
DOI PMID |
| [36] |
Ingle J, Hageman RH (1965). Metabolic changes associated with the germination of corn. II. Nucleic acid metabolism. Plant Physiol 40, 48-53.
PMID |
| [37] |
Ishibashi N, Yamauchi D, Minamikawa T (1990). Stored mRNA in cotyledons of Vigna unguiculata seeds: nucleotide sequence of cloned cDNA for a stored mRNA and induction of its synthesis by precocious germination. Plant Mol Biol 15, 59-64.
PMID |
| [38] |
Isono K, Shimizu M, Yoshimoto K, Niwa Y, Satoh K, Yokota A, Kobayashi H (1997). Leaf-specifically expressed genes for polypeptides destined for chloroplasts with domains of σ70 factors of bacterial RNA polymerases in Arabidopsis thaliana. Proc Natl Acad Sci USA 94, 14948-14953.
DOI PMID |
| [39] | Itoh JI, Nonomura KI, Ikeda K, Yamaki S, Inukai Y, Yamagishi H, Kitano H, Nagato Y (2005). Rice plant development: from zygote to spikelet. Plant Cell Physiol 46, 23-47. |
| [40] | Ivanov R, Tiedemann J, Czihal A, Baumlein H (2012). Transcriptional regulator AtET2 is required for the induction of dormancy during late seed development. J Plant Physiol 169, 501-508. |
| [41] | Iwasaki M, Hyvärinen L, Piskurewicz U, Lopez-Molina L (2019). Non-canonical RNA-directed DNA methylation participates in maternal and environmental control of seed dormancy. eLife 8, e37434. |
| [42] |
Jacobsen JV, Pearce DW, Poole AT, Pharis RP, Mander LN (2002). Abscisic acid, phaseic acid and gibberellin contents associated with dormancy and germination in barley. Physiol Plant 115, 428-441.
DOI PMID |
| [43] | Kearly A, Nelson ADL, Skirycz A, Chodasiewicz M (2024). Composition and function of stress granules and P-bodies in plants. Semin Cell Dev Biol 156, 167-175. |
| [44] | Kim S, Huh SM, Han HJ, Lee GS, Hwang YS, Cho MH, Kim BG, Song JS, Chung JH, Nam MH, Ji H, Kim KH, Yoon IS (2023). A rice seed-specific glycine-rich protein OsDOR1 interacts with GID1 to repress GA signaling and regulates seed dormancy. Plant Mol Biol 111, 523-539. |
| [45] | Kim W, Lee Y, Park J, Lee N, Choi G (2013). HONSU, a protein phosphatase 2C, regulates seed dormancy by inhibiting ABA signaling in Arabidopsis. Plant Cell Physiol 54, 555-572. |
| [46] | Kimura M, Nambara E (2010). Stored and neosynthesized mRNA in Arabidopsis seeds: effects of cycloheximide and controlled deterioration treatment on the resumption of transcription during imbibition. Plant Mol Biol 73, 119-129. |
| [47] |
Kong QM, Lin CLG (2010). Oxidative damage to RNA: mechanisms, consequences, and diseases. Cell Mol Life Sci 67, 1817-1829.
DOI PMID |
| [48] | Li XY, Chen TT, Li Y, Wang Z, Cao H, Chen FY, Li Y, Soppe WJJ, Li WL, Liu YX (2019). ETR1/RDO3 regulates seed dormancy by relieving the inhibitory effect of the ERF12-TPL complex on DELAY OF GERMINATION1 expression. Plant Cell 31, 832-847. |
| [49] | Li Y, Chen FY, Yang Y, Han Y, Ren ZY, Li XY, Soppe WJJ, Cao H, Liu YX (2023). The Arabidopsis pre-mRNA 3' end processing related protein FIP1 promotes seed dormancy via the DOG1 and ABA pathways. Plant J 115, 494-509. |
| [50] |
Liu SP, Li L, Wang WL, Xia GM, Liu SW (2024). TaSRO1 interacts with TaVP1 to modulate seed dormancy and pre-harvest sprouting resistance in wheat. J Integr Plant Biol 66, 36-53.
DOI |
| [51] |
Liu SR, Yang LW, Li JL, Tang WJ, Li JG, Lin RC (2021). FHY3 interacts with phytochrome B and regulates seed dormancy and germination. Plant Physiol 187, 289-302.
DOI PMID |
| [52] |
Liu XL, Li N, Chen AY, Saleem N, Jia QL, Zhao CZ, Li WQ, Zhang M (2023). FUSCA3-induced AINTEGUMENTA-like 6 manages seed dormancy and lipid metabolism. Plant Physiol 193, 1091-1108.
DOI PMID |
| [53] |
Martinet W, De Meyer GRY, Herman AG, Kockx MM (2005). RNA damage in human atherosclerosis: pathophysiological significance and implications for gene expression studies. RNA Biol 2, 4-7.
PMID |
| [54] |
Martínez-Andújar C, Ordiz MI, Huang ZL, Nonogaki M, Beachy RN, Nonogaki H (2011). Induction of 9-cis-epoxycarotenoid dioxygenase in Arabidopsis thaliana seeds enhances seed dormancy. Proc Natl Acad Sci USA 108, 17225-17229.
DOI PMID |
| [55] |
Masaki S, Yamada T, Hirasawa T, Todaka D, Kanekatsu M (2008). Proteomic analysis of RNA-binding proteins in dry seeds of rice after fractionation by ssDNA affinity column chromatography. Biotechnol Lett 30, 955-960.
PMID |
| [56] | Matilla AJ (2022). Exploring breakthroughs in three traits belonging to seed life. Plants (Basel) 11, 490. |
| [57] | Mertens J, Aliyu H, Cowan DA (2018). LEA proteins and the evolution of the WHy domain. Appl Environ Microbiol 84, e00539-18. |
| [58] |
Montoya-García L, Muñoz-Ocotero V, Aguilar R, Sánchez de Jiménez E (2002). Regulation of acidic ribosomal protein expression and phosphorylation in maize. Biochemistry 41, 10166-10172.
PMID |
| [59] | Müller K, Carstens AC, Linkies A, Torres MA, Leubner-Metzger G (2009). The NADPH-oxidase AtrbohB plays a role in Arabidopsis seed after-ripening. New Phytol 184, 885-897. |
| [60] | Nakabayashi K, Bartsch M, Xiang Y, Miatton E, Pellengahr S, Yano R, Seo M, Soppe WJJ (2012). The time required for dormancy release in Arabidopsis is determined by DELAY OF GERMINATION1 protein levels in freshly harvested seeds. Plant Cell 24, 2826-2838. |
| [61] |
Nakabayashi K, Okamoto M, Koshiba T, Kamiya Y, Nambara E (2005). Genome-wide profiling of stored mRNA in Arabidopsis thaliana seed germination: epigenetic and genetic regulation of transcription in seed. Plant J 41, 697-709.
DOI PMID |
| [62] | Nakamura S, Abe F, Kawahigashi H, Nakazono K, Tagiri A, Matsumoto T, Utsugi S, Ogawa T, Handa H, Ishida H, Mori M, Kawaura K, Ogihara Y, Miura H (2011). A wheat homolog of MOTHER OF FT AND TFL1 acts in the regulation of germination. Plant Cell 23, 3215-3229. |
| [63] | Narro-Diego L, López-González L, Jarillo JA, Piñeiro M (2017). The PHD-containing protein EARLY BOLTING IN SHORT DAYS regulates seed dormancy in Arabidopsis. Plant Cell Environ 40, 2393-2405. |
| [64] |
Narsai R, Howell KA, Millar AH, O'Toole N, Small I, Whelan J (2007). Genome-wide analysis of mRNA decay rates and their determinants in Arabidopsis thaliana. Plant Cell 19, 3418-3436.
DOI PMID |
| [65] |
Nelson SK, Ariizumi T, Steber CM (2017). Biology in the dry seed: transcriptome changes associated with dry seed dormancy and dormancy loss in the Arabidopsis GA-insensitive sleepy1-2 mutant. Front Plant Sci 8, 2158.
DOI PMID |
| [66] | Niñoles R, Planes D, Arjona P, Ruiz-Pastor C, Chazarra R, Renard J, Bueso E, Forment J, Serrano R, Kranner I, Roach T, Gadea J (2022). Comparative analysis of wild-type accessions reveals novel determinants of Arabidopsis seed longevity. Plant Cell Environ 45, 2708-2728. |
| [67] |
Nomura T, Ueno M, Yamada Y, Takatsuto S, Takeuchi Y, Yokota T (2007). Roles of brassinosteroids and related mRNAs in pea seed growth and germination. Plant Physiol 143, 1680-1688.
DOI PMID |
| [68] | Okamoto M, Tatematsu K, Matsui A, Morosawa T, Ishida J, Tanaka M, Endo TA, Mochizuki Y, Toyoda T, Kamiya Y, Shinozaki K, Nambara E, Seki M (2010). Genome- wide analysis of endogenous abscisic acid-mediated transcription in dry and imbibed seeds of Arabidopsis using tiling arrays. Plant J 62, 39-51. |
| [69] |
Oracz K, El-Maarouf Bouteau H, Farrant JM, Cooper K, Belghazi M, Job C, Job D, Corbineau F, Bailly C (2007). ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation. Plant J 50, 452-465.
PMID |
| [70] | Qin P, Zhang GH, Hu BH, Wu J, Chen WL, Ren ZJ, Liu YL, Xie J, Yuan H, Tu BT, Ma B, Wang YP, Ye LM, Li LG, Xiang CB, Li SG (2021). Leaf-derived ABA regulates rice seed development via a transporter-mediated and temperature-sensitive mechanism. Sci Adv 7, eabc8873. |
| [71] | Rajjou L, Debeaujon I (2008). Seed longevity: survival and maintenance of high germination ability of dry seeds. C R Biol 331, 796-805. |
| [72] |
Rajjou L, Gallardo K, Debeaujon I, Vandekerckhove J, Job C, Job D (2004). The effect of α-amanitin on the Arabidopsis seed proteome highlights the distinct roles of stored and neosynthesized mRNAs during germination. Plant Physiol 134, 1598-1613.
DOI PMID |
| [73] |
Ravindran P, Verma V, Stamm P, Kumar PP (2017). A novel RGL2-DOF6 complex contributes to primary seed dormancy in Arabidopsis thaliana by regulating a GATA transcription factor. Mol Plant 10, 1307-1320.
DOI PMID |
| [74] |
Rueda-Romero P, Barrero-Sicilia C, Gómez-Cadenas A, Carbonero P, Oñate-Sánchez L (2012). Arabidopsis thaliana DOF6 negatively affects germination in non-after- ripened seeds and interacts with TCP14. J Exp Bot 63, 1937-1949.
DOI PMID |
| [75] |
Sajeev N, Bai B, Bentsink L (2019). Seeds: a unique system to study translational regulation. Trends Plant Sci 24, 487-495.
DOI PMID |
| [76] | Sajeev N, Baral A, America AHP, Willems LAJ, Merret R, Bentsink L (2022). The mRNA-binding proteome of a critical phase transition during Arabidopsis seed germination. New Phytol 233, 251-264. |
| [77] |
Sánchez-de-Jiménez E, Aguilar R, Dinkova T (1997). S6 ribosomal protein phosphorylation and translation of stored mRNA in maize. Biochimie 79, 187-194.
PMID |
| [78] |
Sano N, Masaki S, Tanabata T, Yamada T, Hirasawa T, Kashiwagi M, Kanekatsu M (2013). RNA-binding proteins associated with desiccation during seed development in rice. Biotechnol Lett 35, 1945-1952.
DOI PMID |
| [79] |
Sano N, Ono H, Murata K, Yamada T, Hirasawa T, Kanekatsu M (2015). Accumulation of long-lived mRNAs associated with germination in embryos during seed development of rice. J Exp Bot 66, 4035-4046.
DOI PMID |
| [80] | Sano N, Permana H, Kumada R, Shinozaki Y, Tanabata T, Yamada T, Hirasawa T, Kanekatsu M (2012). Proteomic analysis of embryonic proteins synthesized from long- lived mRNAs during germination of rice seeds. Plant Cell Physiol 53, 687-698. |
| [81] | Sano N, Takebayashi Y, To A, Mhiri C, Rajjou L, Nakagami H, Kanekatsu M (2019). Shotgun proteomic analysis highlights the roles of long-lived mRNAs and de novo transcribed mRNAs in rice seeds upon imbibition. Plant Cell Physiol 60, 2584-2596. |
| [82] | Sharma SN, Maheshwari A, Sharma C, Shukla N (2018). Gene expression patterns regulating the seed metabolism in relation to deterioration/ageing of primed mung bean (Vigna radiata L.) seeds. Plant Physiol Biochem 124, 40-49. |
| [83] | Shen WZ, Yao X, Ye TT, Ma S, Liu X, Yin XM, Wu Y (2018). Arabidopsis aspartic protease ASPG1 affects seed dormancy, seed longevity and seed germination. Plant Cell Physiol 59, 1415-1431. |
| [84] | Shu K, Zhang HW, Wang SF, Chen ML, Wu YR, Tang SY, Liu CY, Feng YQ, Cao XF, Xie Q (2013). ABI4 regulates primary seed dormancy by regulating the biogenesis of abscisic acid and gibberellins in Arabidopsis. PLoS Genet 9, e1003577. |
| [85] | Siloto RMP, Findlay K, Lopez-Villalobos A, Yeung EC, Nykiforuk CL, Moloney MM (2006). The accumulation of oleosins determines the size of seed oilbodies in Arabidopsis. Plant Cell 18, 1961-1974. |
| [86] | Song WH, Hao QX, Cai MY, Wang YH, Zhu XJ, Liu X, Huang YS, Nguyen T, Yang CY, Yu JF, Wu HM, Chen LM, Tian YL, Jiang L, Wan JM (2020). Rice OsBT1 regulates seed dormancy through the glycometabolism pathway. Plant Physiol Biochem 151, 469-476. |
| [87] |
Sreenivasulu N, Usadel B, Winter A, Radchuk V, Scholz U, Stein N, Weschke W, Strickert M, Close TJ, Stitt M, Graner A, Wobus U (2008). Barley grain maturation and germination: metabolic pathway and regulatory network commonalities and differences highlighted by new MapMan/PageMan profiling tools. Plant Physiol 146, 1738-1758.
DOI PMID |
| [88] |
Standart N, Weil D (2018). P-bodies: cytosolic droplets for coordinated mRNA storage. Trends Genet 34, 612-626.
DOI PMID |
| [89] |
Sugimoto M, Oono Y, Kawahara Y, Gusev O, Maekawa M, Matsumoto T, Levinskikh M, Sychev V, Novikova N, Grigoriev A (2016). Gene expression of rice seeds surviving 13- and 20-month exposure to space environment. Life Sci Space Res (Amst) 11, 10-17.
DOI PMID |
| [90] | Suriyasak C, Oyama Y, Ishida T, Mashiguchi K, Yamaguchi S, Hamaoka N, Iwaya-Inoue M, Ishibashi Y (2020). Mechanism of delayed seed germination caused by high temperature during grain filling in rice (Oryza sativa L.). Sci Rep 10, 17378. |
| [91] |
Suzuki Y, Minamikawa T (1985). On the role of stored mRNA in protein synthesis in embryonic axes of germinating Vigna unguiculata seeds. Plant Physiol 79, 327-331.
DOI PMID |
| [92] | Taylor RE, Waterworth W, West CE, Foyer CH (2023). WHIRLY proteins maintain seed longevity by effects on seed oxygen signaling during imbibition. Biochem J 480, 941-956. |
| [93] |
Tiller K, Eisermann A, Link G (1991). The chloroplast transcription apparatus from mustard (Sinapis alba L.). Evidence for three different transcription factors which resemble bacterial sigma factors. Eur J Biochem 198, 93-99.
PMID |
| [94] |
Vaistij FE, Gan YB, Penfield S, Gilday AD, Dave A, He ZS, Josse EM, Choi G, Halliday KJ, Graham IA (2013). Differential control of seed primary dormancy in Arabidopsis ecotypes by the transcription factor SPATULA. Proc Natl Acad Sci USA 110, 10866-10871.
DOI PMID |
| [95] |
Villa-Hernández JM, Dinkova TD, Aguilar-Caballero R, Rivera-Cabrera F, Sánchez de Jiménez E, Pérez-Flores LJ (2013). Regulation of ribosome biogenesis in maize embryonic axes during germination. Biochimie 95, 1871-1879.
DOI PMID |
| [96] | Wang BQ, Wang SY, Tang YQ, Jiang LL, He W, Lin QL, Yu F, Wang L (2022). Transcriptome-wide characterization of seed aging in rice: identification of specific long-lived mRNAs for seed longevity. Front Plant Sci 13, 857390. |
| [97] | Wang CL, Lyu Y, Zhang Q, Guo HY, Chen DF, Chen XW (2023). Disruption of BG14 results in enhanced callose deposition in developing seeds and decreases seed longevity and seed dormancy in Arabidopsis. Plant J 113, 1080-1094. |
| [98] | Wang HT, Zhang YM, Xiao N, Zhang G, Wang F, Chen XY, Fang RX (2020a). Rice GERMIN-LIKE PROTEIN 2-1 functions in seed dormancy under the control of abscisic acid and gibberellic acid signaling pathways. Plant Physiol 183, 1157-1170. |
| [99] | Wang Q, Lin QB, Wu T, Duan EC, Huang YS, Yang CY, Mou CL, Lan J, Zhou CL, Xie K, Liu X, Zhang X, Guo XP, Wang J, Jiang L, Wan JM (2020b). OsDOG1L-3 regulates seed dormancy through the abscisic acid pathway in rice. Plant Sci 298, 110570. |
| [100] | Wang Z, Cao H, Sun YZ, Li XY, Chen FY, Carles A, Li Y, Ding M, Zhang C, Deng X, Soppe WJJ, Liu YX (2013). Arabidopsis paired amphipathic helix proteins SNL1 and SNL2 redundantly regulate primary seed dormancy via abscisic acid-ethylene antagonism mediated by histone deacetylation. Plant Cell 25, 149-166. |
| [101] |
Wei J, Wu XT, Li XY, Soppe WJJ, Cao H, Liu YX (2023). Overexpression of Taetr1-1 promotes enhanced seed dormancy and ethylene insensitivity in wheat. Planta 258, 56.
DOI PMID |
| [102] |
Wise MJ (2003). LEAping to conclusions: a computational reanalysis of late embryogenesis abundant proteins and their possible roles. BMC Bioinformatics 4, 52.
PMID |
| [103] | Wu J, Jin YJ, Liu C, Vonapartis E, Liang JH, Wu WJ, Gazzarrini S, He JN, Yi MF (2019). GhNAC83 inhibits corm dormancy release by regulating ABA signaling and cytokinin biosynthesis in Gladiolus hybridus. J Exp Bot 70, 1221-1237. |
| [104] | Xiang Y, Nakabayashi K, Ding J, He F, Bentsink L, Soppe WJJ (2014). REDUCED DORMANCY5 encodes a protein phosphatase 2C that is required for seed dormancy in Arabidopsis. Plant Cell 26, 4362-4375. |
| [105] |
Xu F, Tang JY, Wang SX, Cheng X, Wang HR, Ou SJ, Gao SP, Li BS, Qian YW, Gao CX, Chu CC (2022). Antagonistic control of seed dormancy in rice by two bHLH transcription factors. Nat Genet 54, 1972-1982.
DOI PMID |
| [106] | Yazdanpanah F, Willems LAJ, He HZ, Hilhorst HWM, Bentsink L (2022). A role for allantoate amidohydrolase (AtAAH) in the germination of Arabidopsis thaliana seeds. Plant Cell Physiol 63, 1298-1308. |
| [107] | Ye H, Feng JH, Zhang LH, Zhang JF, Mispan MS, Cao ZQ, Beighley DH, Yang JC, Gu XY (2015). Map-based cloning of seed Dormancy1-2 identified a gibberellin synthesis gene regulating the development of endosperm-imposed dormancy in rice. Plant Physiol 169, 2152-2165. |
| [108] |
Yoshiyama K, Conklin PA, Huefner ND, Britt AB (2009). Suppressor of gamma response 1 ( SOG1) encodes a putative transcription factor governing multiple responses to DNA damage. Proc Natl Acad Sci USA 106, 12843-12848.
DOI PMID |
| [109] | Zhang Q, Pritchard J, Mieog J, Byrne K, Colgrave ML, Wang JR, Ral JPF (2021). Overexpression of a wheat α-amylase type 2 impact on starch metabolism and abscisic acid sensitivity during grain germination. Plant J 108, 378-393. |
| [110] | Zhao FK, Ma Q, Li YJ, Jiang MH, Zhou ZJ, Meng S, Peng Y, Zhang JH, Ye NH, Liu BH (2023). OsNAC2 regulates seed dormancy and germination in rice by inhibiting ABA catabolism. Biochem Biophys Res Commun 682, 335-342. |
| [111] | Zhao L, Wang H, Fu YB (2020a). Analysis of stored mRNA degradation in acceleratedly aged seeds of wheat and canola in comparison to Arabidopsis. Plants (Basel) 9, 1707. |
| [112] | Zhao L, Wang S, Fu YB, Wang H (2020b). Arabidopsis seed stored mRNAs are degraded constantly over aging time, as revealed by new quantification methods. Front Plant Sci 10, 1764. |
| [113] |
Zheng J, Chen FY, Wang Z, Cao H, Li XY, Deng X, Soppe WJJ, Li Y, Liu YX (2012). A novel role for histone methyltransferase KYP/SUVH4 in the control of Arabidopsis primary seed dormancy. New Phytol 193, 605-616.
DOI PMID |
| [114] | Zheng LP, Otani M, Kanno Y, Seo M, Yoshitake Y, Yoshimoto K, Sugimoto K, Kawakami N (2022). Seed dormancy 4 like1 of Arabidopsis is a key regulator of phase transition from embryo to vegetative development. Plant J 112, 460-475. |
| [115] | Zhu CC, Wang CX, Lu CY, Wang JD, Zhou Y, Xiong M, Zhang CQ, Liu QQ, Li QF (2021). Genome-wide identification and expression analysis of OsbZIP09 target genes in rice reveal its mechanism of controlling seed germination. Int J Mol Sci 22, 1661. |
| [116] | Zhu HF, Xie WX, Xu DC, Miki D, Tang K, Huang CF, Zhu JK (2018). DNA demethylase ROS1 negatively regulates the imprinting of DOGL4 and seed dormancy in Arabidopsis thaliana. Proc Natl Acad Sci USA 115, E9962- E9970. |
| [117] | Zinsmeister J, Berriri S, Basso DP, Ly-Vu B, Dang TT, Lalanne D, da Silva EAA, Leprince O, Buitink J (2020). The seed-specific heat shock factor A9 regulates the depth of dormancy in Medicago truncatula seeds via ABA signaling. Plant Cell Environ 43, 2508-2522. |
| [1] | 周鑫宇, 刘会良, 高贝, 卢妤婷, 陶玲庆, 文晓虎, 张岚, 张元明. 新疆特有濒危植物雪白睡莲繁殖生物学研究[J]. , 2025, 49(濒危植物的保护与恢复): 0-. |
| [2] | 孙龙, 李文博, 娄虎, 于澄, 韩宇, 胡同欣. 火干扰对兴安落叶松种子萌发的影响[J]. 植物生态学报, 2024, 48(6): 770-779. |
| [3] | 袁涵, 钟爱文, 刘送平, 彭焱松, 徐磊. 水毛花种子萌发特性的差异及休眠解除方法[J]. 植物生态学报, 2024, 48(5): 638-650. |
| [4] | 罗燕, 刘奇源, 吕元兵, 吴越, 田耀宇, 安田, 李振华. 拟南芥光敏色素突变体种子萌发的光温敏感性[J]. 植物学报, 2024, 59(5): 752-762. |
| [5] | 蔡淑钰, 刘建新, 王国夫, 吴丽元, 宋江平. 褪黑素促进镉胁迫下番茄种子萌发的调控机理[J]. 植物学报, 2023, 58(5): 720-732. |
| [6] | 宋松泉, 刘军, 杨华, 张文虎, 张琪, 高家东. 细胞分裂素调控种子发育、休眠与萌发的研究进展[J]. 植物学报, 2021, 56(2): 218-231. |
| [7] | 李绍阳, 马红媛, 赵丹丹, 马梦谣, 亓雯雯. 火烧信号对种子萌发影响的研究进展[J]. 植物生态学报, 2021, 45(11): 1177-1190. |
| [8] | 张楠,刘自广,孙世臣,刘圣怡,林建辉,彭疑芳,张晓旭,杨贺,岑曦,吴娟. 拟南芥AtR8 lncRNA对盐胁迫响应及其对种子萌发的调节作用[J]. 植物学报, 2020, 55(4): 421-429. |
| [9] | 曹栋栋,陈珊宇,秦叶波,吴华平,阮关海,黄玉韬. 水杨酸调控盐胁迫下羽衣甘蓝种子萌发的机理[J]. 植物学报, 2020, 55(1): 49-61. |
| [10] | 杨立文,刘双荣,林荣呈. 光信号与激素调控种子休眠和萌发研究进展[J]. 植物学报, 2019, 54(5): 569-581. |
| [11] | 艾沙江•阿不都沙拉木, 迪丽娜尔•阿布拉, 张凯, 买热也木古•吐尔逊, 卡迪尔•阿布都热西提, 李玲. 喀什霸王的结实和种子萌发特性[J]. 植物生态学报, 2019, 43(5): 437-446. |
| [12] | 吴小琪, 杨圣贺, 黄力, 李笑寒, 杨超, 钱深华, 杨永川. 常绿阔叶林林冠环境对栲幼苗建成的影响[J]. 植物生态学报, 2019, 43(1): 55-64. |
| [13] | 伍静辉, 谢楚萍, 田长恩, 周玉萍. 脱落酸调控种子休眠和萌发的分子机制[J]. 植物学报, 2018, 53(4): 542-555. |
| [14] | 薛婷婷, 沈永宝, 刘嘉, 史锋厚. 种子物理休眠研究进展[J]. 植物学报, 2016, 51(6): 863-871. |
| [15] | 刘波, 吕宪国, 姜明, 张文广, 武海涛. 光照、水深交互作用对松嫩湿地芦苇种子萌发的影响[J]. 植物生态学报, 2015, 39(6): 616-620. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||