

Chinese Bulletin of Botany ›› 2022, Vol. 57 ›› Issue (5): 596-610.DOI: 10.11983/CBB22022 cstr: 32102.14.CBB22022
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Liu Xiaolong1,2,*( ), Ji Ping1, Yang Hongtao1,2, Ding Yongdian1,2, Fu Jialing1, Liang Jiangxia1, Yu Congcong1
), Ji Ping1, Yang Hongtao1,2, Ding Yongdian1,2, Fu Jialing1, Liang Jiangxia1, Yu Congcong1
Received:2022-01-28
Accepted:2022-04-20
Online:2022-09-01
Published:2022-09-09
Contact:
Liu Xiaolong
About author:*E-mail: lxl032202@163.comLiu Xiaolong, Ji Ping, Yang Hongtao, Ding Yongdian, Fu Jialing, Liang Jiangxia, Yu Congcong. Priming Effect of Abscisic Acid on High Temperature Stress During Rice Heading-flowering Stage[J]. Chinese Bulletin of Botany, 2022, 57(5): 596-610.
| Primer name | Primer sequence (5'-3') |
|---|---|
| SalTF | CGAAATAATGTTCCATGGTGTT |
| SalTR | TGTACTACGGATCGGTGCAA |
| OsWsi18F | TGTGACTCGATCCAGCGTAG |
| OsWsi18R | GTTCCTGCTGAGAAGCCATC |
| OsACT1F | TTCCAGCCTTCCTTCATA |
| OsACT1R | AACGATGTTGCCATATAGAT |
| OsCATBF | GCTGGTGAGAGATACCGGTCA |
| OsCATBR | TCAACCCACCGCTGGAGA |
| OsAPX6F | CCCCAAGATCCCCATGATCTA |
| OsAPX6R | CCTCTGGCGGGCATTG |
| OsFe-SODF | CGACGCCGAGGAATTTCTAG |
| OsFe-SODR | AGGTGGTGTAAGTGTCTCTCATGC |
| OsCu/Zn-SODF | TGTGACGGGACTTACTCCTGG |
| OsCu/Zn-SODR | CACCCATTCGTAGTATCGCCA |
| Ghd7F | AATCCGGTACGCGTCCAGA |
| Ghd7R | CCAAGCTCAAGCCTACTAGG |
| WxF | TTGGGATACCAGCGTTGTGG |
| WxR | CGGTCTTTCCCCAAACCTTCT |
| OsAGPL2F | AAGCCGAGAGCGTGGTAAAA |
| OsAGPL2R | CAGGAGCGCTAGGGTTTTCA |
| OsAGPL3F | TGCCGATGAGCAACTGCATA |
| OsAGPL3R | TTATACGCCCGGGAAAGGTG |
Table 1 Primers used in this study
| Primer name | Primer sequence (5'-3') |
|---|---|
| SalTF | CGAAATAATGTTCCATGGTGTT |
| SalTR | TGTACTACGGATCGGTGCAA |
| OsWsi18F | TGTGACTCGATCCAGCGTAG |
| OsWsi18R | GTTCCTGCTGAGAAGCCATC |
| OsACT1F | TTCCAGCCTTCCTTCATA |
| OsACT1R | AACGATGTTGCCATATAGAT |
| OsCATBF | GCTGGTGAGAGATACCGGTCA |
| OsCATBR | TCAACCCACCGCTGGAGA |
| OsAPX6F | CCCCAAGATCCCCATGATCTA |
| OsAPX6R | CCTCTGGCGGGCATTG |
| OsFe-SODF | CGACGCCGAGGAATTTCTAG |
| OsFe-SODR | AGGTGGTGTAAGTGTCTCTCATGC |
| OsCu/Zn-SODF | TGTGACGGGACTTACTCCTGG |
| OsCu/Zn-SODR | CACCCATTCGTAGTATCGCCA |
| Ghd7F | AATCCGGTACGCGTCCAGA |
| Ghd7R | CCAAGCTCAAGCCTACTAGG |
| WxF | TTGGGATACCAGCGTTGTGG |
| WxR | CGGTCTTTCCCCAAACCTTCT |
| OsAGPL2F | AAGCCGAGAGCGTGGTAAAA |
| OsAGPL2R | CAGGAGCGCTAGGGTTTTCA |
| OsAGPL3F | TGCCGATGAGCAACTGCATA |
| OsAGPL3R | TTATACGCCCGGGAAAGGTG |
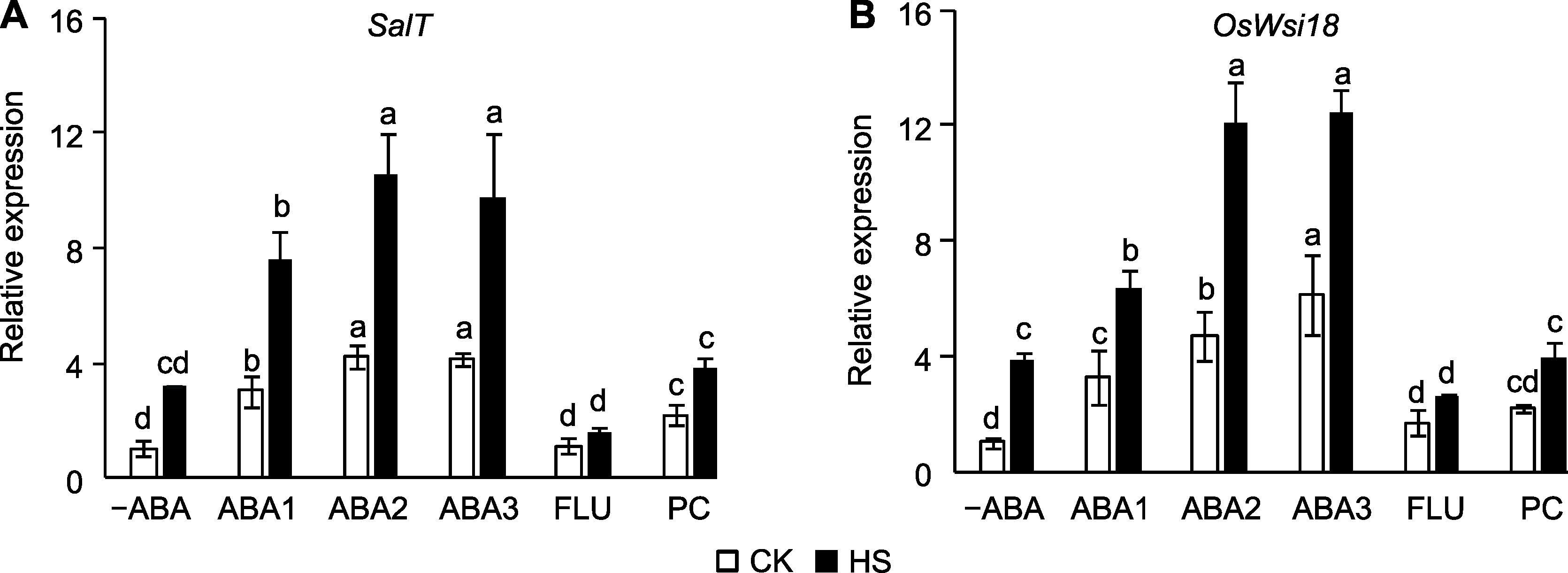
Figure 1 Effect of spraying ABA solution at booting stage on the expression of ABA-responsive genes in rice under high temperature stress (A) SalT; (B) OsWsi18. -ABA: Distilled water; ABA1: 10 μmol?L-1 ABA; ABA2: 50 μmol?L-1 ABA; ABA3: 100 μmol?L-1 ABA; FLU: 10 μmol?L-1 fluridone; PC: 1% proanthocyanidins; CK: Control; HS: High temperature stress. Different lowercase letters indicate significance differences among different treatments under control and high temperature stress (P<0.05).
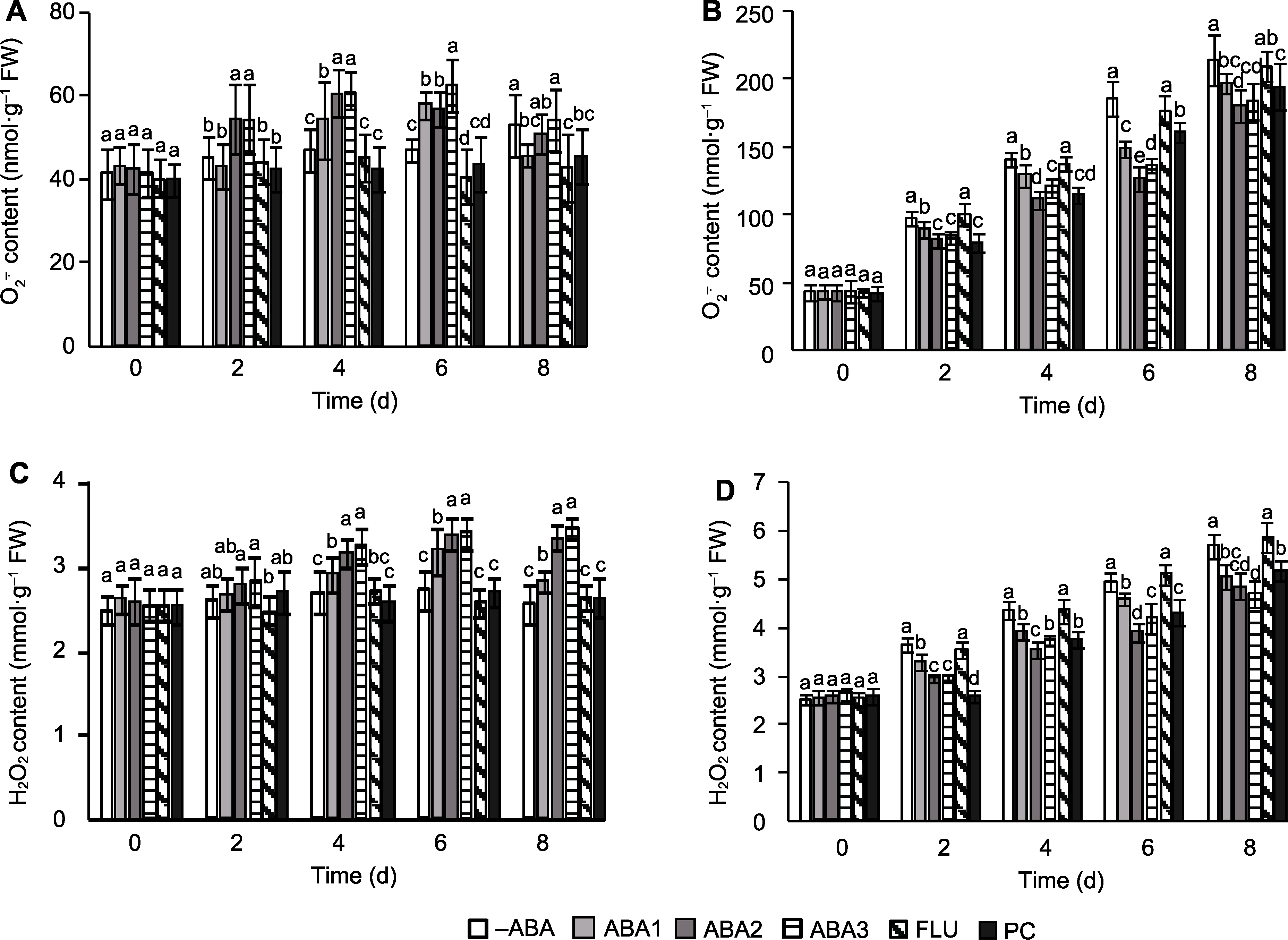
Figure 2 Effect of spraying ABA solution at booting stage on reactive oxygen species (ROS) accumulation in rice spikelets under high temperature stress (A) O2-. content of CK; (B) O2-. content of HS; (C) H2O2 content of CK; (D) H2O2 content of HS. -ABA, ABA1, ABA2, ABA3, FLU, PC, CK, and HS are the same as shown in Figure 1. Different lowercase letters indicate significance differences among different treatments under control and high temperature stress (P<0.05).
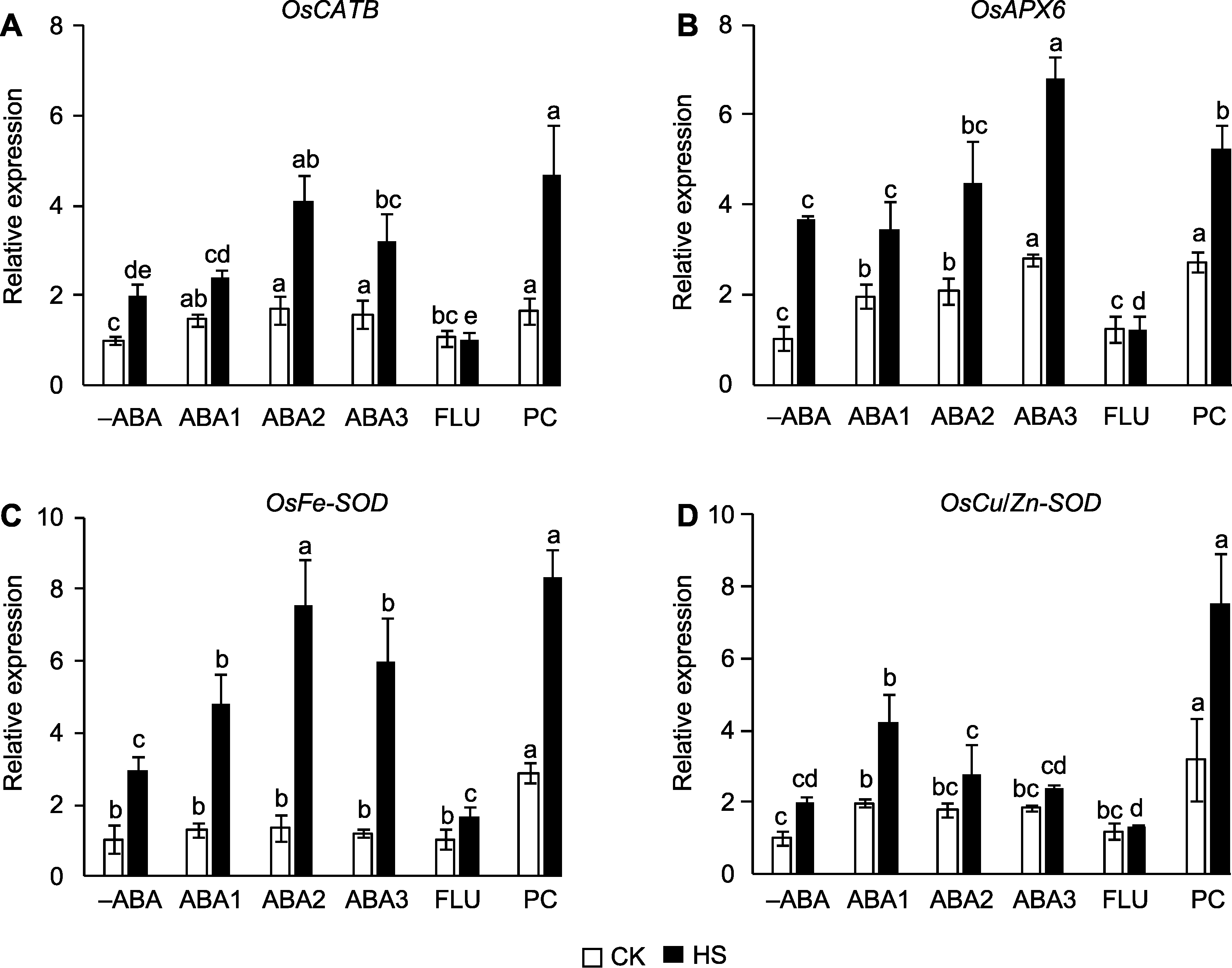
Figure 3 Effect of spraying ABA solution at booting stage on the expression of reactive oxygen species (ROS)-scavenging genes in rice under high temperature stress (A) OsCATB; (B) OsAPX6; (C) OsFe-SOD; (D) OsCu/Zn-SOD. -ABA, ABA1, ABA2, ABA3, FLU, PC, CK, and HS are the same as shown in Figure 1. Different lowercase letters indicate significance differences among different treatments under control and high temperature stress (P<0.05).
| Treatments | Shoot length (cm) | Stem dry weight (g) | Panicle length (cm) | Panicle weight (g) | |
|---|---|---|---|---|---|
| CK | -ABA | 85.54±5.46 a | 37.27±5.10 a | 20.96±0.92 b | 3.01±0.18 ab |
| ABA1 | 86.70±5.38 a | 38.19±3.92 a | 21.41±0.94 ab | 3.05±0.38 ab | |
| ABA2 | 86.54±3.47 a | 39.90±4.39 a | 21.64±0.90 ab | 3.03±0.41 ab | |
| ABA3 | 87.88±3.74 a | 41.52±2.66 a | 21.79±0.61 a | 3.12±0.42 a | |
| FLU | 84.10±4.24 a | 37.28±5.77 a | 20.92±0.67 b | 2.75±0.29 b | |
| PC | 87.30±5.50 a | 41.61±2.77 a | 22.03±0.73 a | 3.09±0.18 ab | |
| HS | -ABA | 82.64±3.23 a | 34.79±4.08 a | 19.96±0.86 b | 2.42±0.29 b |
| ABA1 | 85.01±6.15 a | 36.08±3.69 a | 20.42±0.66 ab | 2.73±0.32 a | |
| ABA2 | 85.09±6.20 a | 37.74±5.74 a | 20.94±1.96 ab | 2.86±0.21 a | |
| ABA3 | 87.02±3.37 a | 38.55±5.34 a | 20.85±0.44 ab | 2.81±0.23 a | |
| FLU | 83.00±4.00 a | 36.96±3.34 a | 19.84±0.73 b | 2.36±0.19 b | |
| PC | 85.03±3.98 a | 37.56±2.99 a | 21.28±1.03 a | 2.89±0.40 a | |
Table 2 Effect of spraying ABA solution at booting stage on the growth and development of rice plants under high temperature stress
| Treatments | Shoot length (cm) | Stem dry weight (g) | Panicle length (cm) | Panicle weight (g) | |
|---|---|---|---|---|---|
| CK | -ABA | 85.54±5.46 a | 37.27±5.10 a | 20.96±0.92 b | 3.01±0.18 ab |
| ABA1 | 86.70±5.38 a | 38.19±3.92 a | 21.41±0.94 ab | 3.05±0.38 ab | |
| ABA2 | 86.54±3.47 a | 39.90±4.39 a | 21.64±0.90 ab | 3.03±0.41 ab | |
| ABA3 | 87.88±3.74 a | 41.52±2.66 a | 21.79±0.61 a | 3.12±0.42 a | |
| FLU | 84.10±4.24 a | 37.28±5.77 a | 20.92±0.67 b | 2.75±0.29 b | |
| PC | 87.30±5.50 a | 41.61±2.77 a | 22.03±0.73 a | 3.09±0.18 ab | |
| HS | -ABA | 82.64±3.23 a | 34.79±4.08 a | 19.96±0.86 b | 2.42±0.29 b |
| ABA1 | 85.01±6.15 a | 36.08±3.69 a | 20.42±0.66 ab | 2.73±0.32 a | |
| ABA2 | 85.09±6.20 a | 37.74±5.74 a | 20.94±1.96 ab | 2.86±0.21 a | |
| ABA3 | 87.02±3.37 a | 38.55±5.34 a | 20.85±0.44 ab | 2.81±0.23 a | |
| FLU | 83.00±4.00 a | 36.96±3.34 a | 19.84±0.73 b | 2.36±0.19 b | |
| PC | 85.03±3.98 a | 37.56±2.99 a | 21.28±1.03 a | 2.89±0.40 a | |
| Treatments | Panicle number | Spikelets per panicle | Percentage of filled spikelets (%) | 1000-kernel weight (g) | Harvest index (%) | Grain yield per hole (g) | |
|---|---|---|---|---|---|---|---|
| CK | -ABA | 14.22±1.86 a | 124.73±14.91 a | 84.89±2.11 a | 22.97±1.21 a | 49.41±1.79 a | 34.31±4.49 a |
| ABA1 | 14.44±1.74 a | 121.55±12.92 a | 85.21±1.76 a | 22.80±1.45 a | 48.77±1.99 a | 33.90±4.47 a | |
| ABA2 | 14.78±1.64 a | 124.81±13.85 a | 84.63±2.78 a | 22.43±1.06 a | 48.39±1.72 a | 34.73±3.50 a | |
| ABA3 | 15.33±1.32 a | 128.67±13.16 a | 84.00±2.63 a | 21.85±0.91 a | 48.53±1.23 a | 35.92±1.81 a | |
| FLU | 14.44±1.42 a | 123.27±14.02 a | 83.02±3.71 a | 22.61±0.91 a | 49.43±3.72 a | 33.31±4.41 a | |
| PC | 15.78±1.56 a | 124.53±12.76 a | 85.26±2.58 a | 21.93±0.58 a | 48.58±2.21 a | 36.46±2.65 a | |
| HS | -ABA | 13.56±1.67 a | 119.76±16.90 a | 78.02±1.96 b | 19.76±0.54 c | 44.83±2.73 a | 24.84±3.69 bc |
| ABA1 | 14.22±1.39 a | 119.04±11.58 a | 81.93±1.63 a | 20.72±0.81 abc | 46.70±2.03 a | 28.59±2.84 a | |
| ABA2 | 14.56±1.88 a | 121.99±8.02 a | 81.12±2.48 a | 20.98±1.45 a | 47.04±2.78 a | 30.00±3.14 a | |
| ABA3 | 14.44±2.07 a | 115.09±18.40 a | 81.10±3.45 a | 21.19±0.93 a | 44.76±3.97 a | 28.01±2.41 ab | |
| FLU | 14.22±1.99 a | 113.38±16.50 a | 75.43±3.33 b | 19.81±0.50 bc | 42.69±2.39 a | 23.74±2.29 c | |
| PC | 14.78±1.30 a | 116.31±14.12 a | 81.89±4.24 a | 20.79±1.32 ab | 46.01±3.50 a | 29.08±3.47 a | |
Table 3 Effect of spraying ABA at booting stage on yield components and grain yield of rice under high temperature stress
| Treatments | Panicle number | Spikelets per panicle | Percentage of filled spikelets (%) | 1000-kernel weight (g) | Harvest index (%) | Grain yield per hole (g) | |
|---|---|---|---|---|---|---|---|
| CK | -ABA | 14.22±1.86 a | 124.73±14.91 a | 84.89±2.11 a | 22.97±1.21 a | 49.41±1.79 a | 34.31±4.49 a |
| ABA1 | 14.44±1.74 a | 121.55±12.92 a | 85.21±1.76 a | 22.80±1.45 a | 48.77±1.99 a | 33.90±4.47 a | |
| ABA2 | 14.78±1.64 a | 124.81±13.85 a | 84.63±2.78 a | 22.43±1.06 a | 48.39±1.72 a | 34.73±3.50 a | |
| ABA3 | 15.33±1.32 a | 128.67±13.16 a | 84.00±2.63 a | 21.85±0.91 a | 48.53±1.23 a | 35.92±1.81 a | |
| FLU | 14.44±1.42 a | 123.27±14.02 a | 83.02±3.71 a | 22.61±0.91 a | 49.43±3.72 a | 33.31±4.41 a | |
| PC | 15.78±1.56 a | 124.53±12.76 a | 85.26±2.58 a | 21.93±0.58 a | 48.58±2.21 a | 36.46±2.65 a | |
| HS | -ABA | 13.56±1.67 a | 119.76±16.90 a | 78.02±1.96 b | 19.76±0.54 c | 44.83±2.73 a | 24.84±3.69 bc |
| ABA1 | 14.22±1.39 a | 119.04±11.58 a | 81.93±1.63 a | 20.72±0.81 abc | 46.70±2.03 a | 28.59±2.84 a | |
| ABA2 | 14.56±1.88 a | 121.99±8.02 a | 81.12±2.48 a | 20.98±1.45 a | 47.04±2.78 a | 30.00±3.14 a | |
| ABA3 | 14.44±2.07 a | 115.09±18.40 a | 81.10±3.45 a | 21.19±0.93 a | 44.76±3.97 a | 28.01±2.41 ab | |
| FLU | 14.22±1.99 a | 113.38±16.50 a | 75.43±3.33 b | 19.81±0.50 bc | 42.69±2.39 a | 23.74±2.29 c | |
| PC | 14.78±1.30 a | 116.31±14.12 a | 81.89±4.24 a | 20.79±1.32 ab | 46.01±3.50 a | 29.08±3.47 a | |
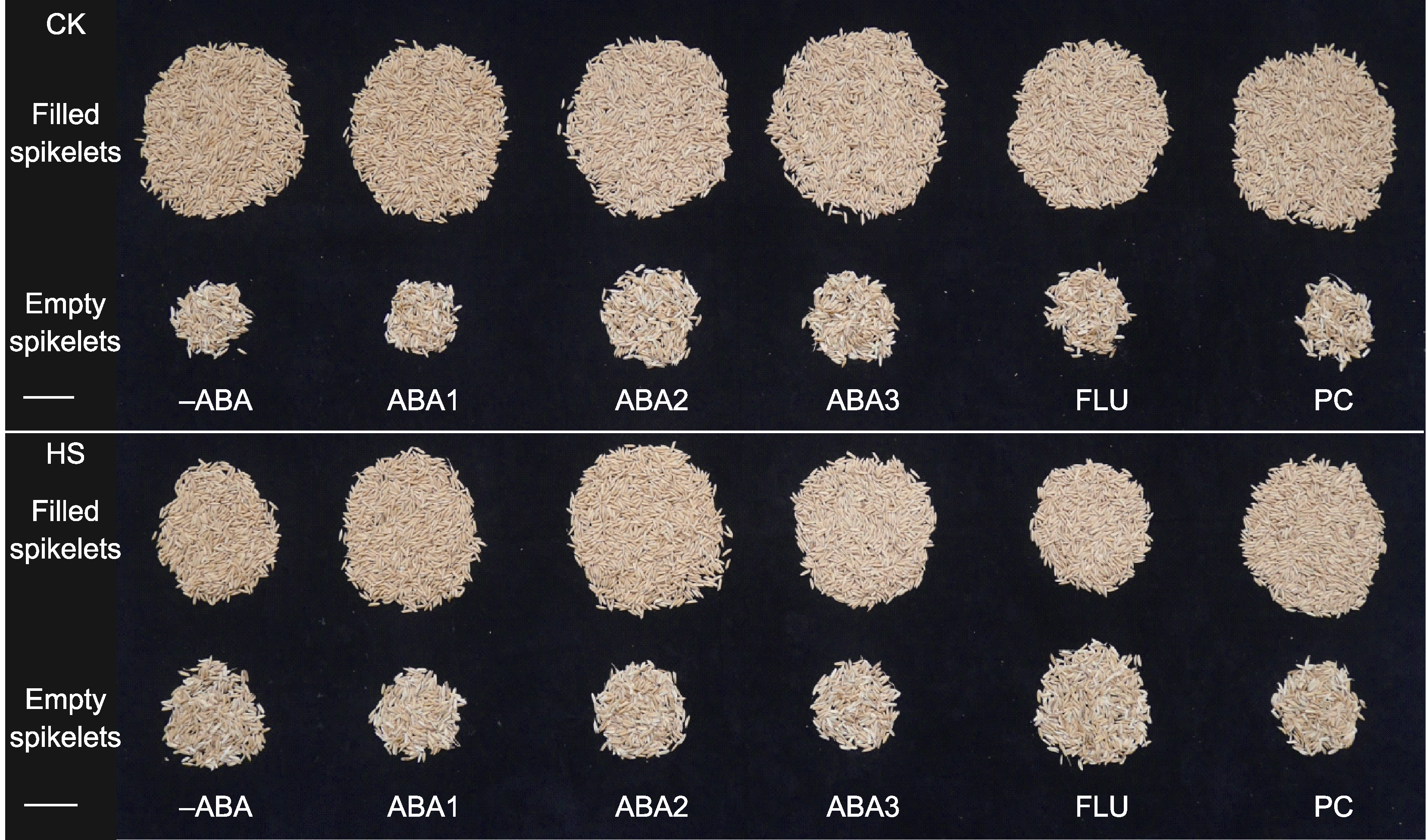
Figure 4 Rice grains at mature stage under different treatments -ABA, ABA1, ABA2, ABA3, FLU, PC, CK, and HS are the same as shown in Figure 1. Bars=3 cm
| Indexes | Treatment time (d) | Shoot length | Stem dry weight | Panicle length | Panicle weight | Panicle number | Spikelets per panicle | Percentage of filled spikelets | 1000-kernel weight | Harvest index | Yield | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contents of O2- . | CK | 0 | 0.059 | 0.135 | 0.173 | 0.063 | 0.161 | -0.115 | 0.006 | 0.135 | -0.023 | 0.128 |
| 2 | -0.028 | -0.071 | -0.081 | -0.053 | -0.143 | 0.134 | -0.233 | -0.138 | -0.033 | -0.151 | ||
| 4 | 0.097 | -0.02 | -0.083 | 0.030 | -0.176 | 0.196 | -0.062 | -0.181 | -0.379* | -0.102 | ||
| 6 | 0.209 | 0.150 | 0.152 | 0.257 | 0.040 | 0.053 | 0.019 | -0.329* | -0.107 | 0.069 | ||
| 8 | -0.049 | 0.052 | -0.066 | 0.061 | -0.05 | 0.052 | 0.002 | -0.104 | -0.147 | -0.072 | ||
| HS | 0 | -0.075 | -0.348** | -0.359** | -0.117 | -0.331* | 0.076 | 0.004 | 0.055 | -0.047 | -0.203 | |
| 2 | -0.099 | -0.093 | -0.463** | -0.510** | -0.138 | -0.173 | -0.448** | -0.421** | -0.413** | -0.600** | ||
| 4 | -0.059 | -0.195 | -0.373** | -0.441** | -0.142 | -0.029 | -0.384** | -0.390** | -0.174 | -0.435** | ||
| 6 | -0.215 | -0.249 | -0.339* | -0.487** | -0.140 | -0.070 | -0.408** | -0.404** | -0.205 | -0.450** | ||
| 8 | 0.101 | -0.191 | -0.257 | -0.393** | -0.138 | -0.106 | -0.241 | -0.330* | -0.275* | -0.375** | ||
| Contents of H2O2 | CK | 0 | -0.181 | -0.021 | 0.086 | 0.035 | 0.047 | -0.163 | 0.222 | -0.070 | -0.109 | -0.061 |
| 2 | 0.070 | 0.024 | 0.134 | 0.171 | 0.030 | 0.061 | -0.045 | -0.066 | 0.028 | 0.048 | ||
| 4 | 0.077 | 0.194 | 0.374* | 0.353* | 0.089 | -0.046 | -0.160 | 0.066 | -0.188 | 0.018 | ||
| 6 | 0.196 | 0.228 | 0.174 | 0.339* | 0.070 | 0.034 | 0.068 | -0.329* | -0.322* | 0.050 | ||
| 8 | 0.225 | 0.195 | 0.221 | 0.135 | 0.093 | 0.070 | -0.107 | -0.152 | -0.123 | 0.065 | ||
| HS | 0 | 0.097 | 0.168 | 0.130 | 0.290* | 0.227 | -0.251 | 0.151 | 0.166 | -0.174 | 0.071 | |
| 2 | -0.208 | -0.210 | -0.439** | -0.569** | -0.206 | -0.033 | -0.477** | -0.400** | -0.201 | -0.502** | ||
| 4 | -0.160 | -0.051 | -0.317* | -0.400** | -0.076 | -0.063 | -0.499** | -0.412** | -0.182 | -0.435** | ||
| 6 | -0.196 | -0.252 | -0.372** | -0.642** | -0.250 | 0.009 | -0.493** | -0.505** | -0.169 | -0.531** | ||
| 8 | -0.120 | -0.231 | -0.346* | -0.510** | -0.121 | -0.090 | -0.500** | -0.533** | -0.212 | -0.500** | ||
Table 4 Correlation index between the growth indexes, yield component indexes and contents of O2-. and H2O2 in rice
| Indexes | Treatment time (d) | Shoot length | Stem dry weight | Panicle length | Panicle weight | Panicle number | Spikelets per panicle | Percentage of filled spikelets | 1000-kernel weight | Harvest index | Yield | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contents of O2- . | CK | 0 | 0.059 | 0.135 | 0.173 | 0.063 | 0.161 | -0.115 | 0.006 | 0.135 | -0.023 | 0.128 |
| 2 | -0.028 | -0.071 | -0.081 | -0.053 | -0.143 | 0.134 | -0.233 | -0.138 | -0.033 | -0.151 | ||
| 4 | 0.097 | -0.02 | -0.083 | 0.030 | -0.176 | 0.196 | -0.062 | -0.181 | -0.379* | -0.102 | ||
| 6 | 0.209 | 0.150 | 0.152 | 0.257 | 0.040 | 0.053 | 0.019 | -0.329* | -0.107 | 0.069 | ||
| 8 | -0.049 | 0.052 | -0.066 | 0.061 | -0.05 | 0.052 | 0.002 | -0.104 | -0.147 | -0.072 | ||
| HS | 0 | -0.075 | -0.348** | -0.359** | -0.117 | -0.331* | 0.076 | 0.004 | 0.055 | -0.047 | -0.203 | |
| 2 | -0.099 | -0.093 | -0.463** | -0.510** | -0.138 | -0.173 | -0.448** | -0.421** | -0.413** | -0.600** | ||
| 4 | -0.059 | -0.195 | -0.373** | -0.441** | -0.142 | -0.029 | -0.384** | -0.390** | -0.174 | -0.435** | ||
| 6 | -0.215 | -0.249 | -0.339* | -0.487** | -0.140 | -0.070 | -0.408** | -0.404** | -0.205 | -0.450** | ||
| 8 | 0.101 | -0.191 | -0.257 | -0.393** | -0.138 | -0.106 | -0.241 | -0.330* | -0.275* | -0.375** | ||
| Contents of H2O2 | CK | 0 | -0.181 | -0.021 | 0.086 | 0.035 | 0.047 | -0.163 | 0.222 | -0.070 | -0.109 | -0.061 |
| 2 | 0.070 | 0.024 | 0.134 | 0.171 | 0.030 | 0.061 | -0.045 | -0.066 | 0.028 | 0.048 | ||
| 4 | 0.077 | 0.194 | 0.374* | 0.353* | 0.089 | -0.046 | -0.160 | 0.066 | -0.188 | 0.018 | ||
| 6 | 0.196 | 0.228 | 0.174 | 0.339* | 0.070 | 0.034 | 0.068 | -0.329* | -0.322* | 0.050 | ||
| 8 | 0.225 | 0.195 | 0.221 | 0.135 | 0.093 | 0.070 | -0.107 | -0.152 | -0.123 | 0.065 | ||
| HS | 0 | 0.097 | 0.168 | 0.130 | 0.290* | 0.227 | -0.251 | 0.151 | 0.166 | -0.174 | 0.071 | |
| 2 | -0.208 | -0.210 | -0.439** | -0.569** | -0.206 | -0.033 | -0.477** | -0.400** | -0.201 | -0.502** | ||
| 4 | -0.160 | -0.051 | -0.317* | -0.400** | -0.076 | -0.063 | -0.499** | -0.412** | -0.182 | -0.435** | ||
| 6 | -0.196 | -0.252 | -0.372** | -0.642** | -0.250 | 0.009 | -0.493** | -0.505** | -0.169 | -0.531** | ||
| 8 | -0.120 | -0.231 | -0.346* | -0.510** | -0.121 | -0.090 | -0.500** | -0.533** | -0.212 | -0.500** | ||
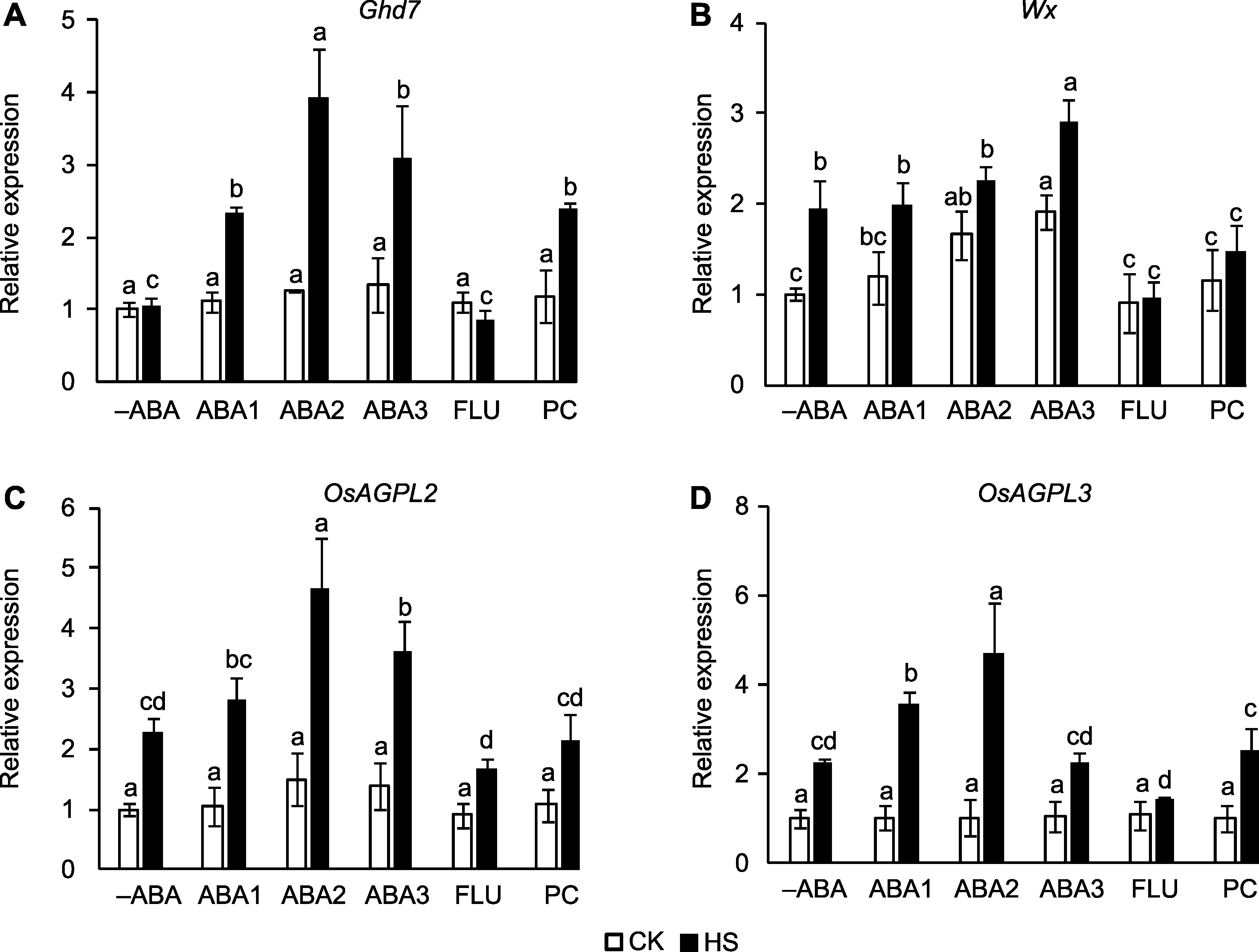
Figure 5 Effect of spraying ABA solution at booting stage on the expression of yield related genes in rice under high temperature stress (A) Ghd7; (B) Wx; (C) OsAGPL2; (D) OsAGPL3. -ABA, ABA1, ABA2, ABA3, FLU, PC, CK, and HS are the same as shown in Figure 1. Different lowercase letters indicate significance differences among different treatments under control and high temperature stress (P<0.05).
| [1] | 陈唯, 曾晓贤, 谢楚萍, 田长恩, 周玉萍 (2019). 植物内源ABA水平的动态调控机制. 植物学报 54, 677-687. |
| [2] | 段骅, 傅亮, 剧成欣, 刘立军, 杨建昌 (2013). 氮素穗肥对高温胁迫下水稻结实和稻米品质的影响. 中国水稻科学 27, 591-602. |
| [3] | 郭贵华, 刘海艳, 李刚华, 刘明, 李岩, 王绍华, 刘正辉, 唐设, 丁艳锋 (2014). ABA缓解水稻孕穗期干旱胁迫生理特性的分析. 中国农业科学 47, 4380-4391. |
| [4] | 雷娅伟, 白小明, 王婷, 吕优伟, 雷舒涵 (2015). 脱落酸对3个野生草地早熟禾种质高温胁迫的缓解效应. 草地学报 23, 89-94, 100. |
| [5] | 李迎春, 帅细强, 杨蓉, 刘丹 (2019). 高温热害对江西省早稻产量影响的定量分析. 湖北农业科学 58(20), 79-83. |
| [6] | 刘晓龙, 季平, 杨洪涛, 邵勤, 任珺怡, 祝佳滢, 张超毅, 陆晨旭 (2021). 高温胁迫对水稻内源脱落酸含量及相关基因表达的影响. 分子植物育种 1-12. [2021-12-20].https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CAPJ&dbname=CAPJLAST&filename=FZZW20211215000&uniplatform=NZKPT&v=_MB1PS_Ch2BhePRwLHu5Uh4VVaM4GNe8EyhVd2rEe7ln_AJgZxEiEWU6WlC2bx-z. |
| [7] | 隆春艳, 古洪辉, 汪正香, 蒋雄, 杨翠芹, 秦耀国 (2017). 外源脱落酸对高温胁迫下菠菜光合与叶绿素荧光参数的影响. 四川农业大学学报 35, 24-30. |
| [8] |
王强, 陈雷, 张晓丽, 唐茂艳, 吕荣华, 陶伟, 梁天锋 (2015). 化学调控对水稻高温热害的缓解作用研究. 中国稻米 21 (4), 80-82.
DOI |
| [9] | 杨建莹, 霍治国, 王培娟, 邬定荣 (2020). 江西早稻高温热害等级动态判识及时空变化特征. 应用生态学报 31, 199-207. |
| [10] | 杨卫丽, 黄福灯, 曹珍珍, 雷炳婷, 胡东维, 程方民 (2013). 高温胁迫对水稻光合PSII系统伤害及其与叶绿体D1蛋白间关系. 作物学报 39, 1060-1068. |
| [11] |
杨雲雲, 陈鑫, 陈启洲, 卢芳, 徐晨, 杨洪涛, 苏佩佩, 刘晓龙 (2021). 脱落酸对水稻种子萌发期耐高温胁迫的诱抗效应. 华北农学报 36(3), 185-194.
DOI |
| [12] | 张桂莲, 陈立云, 张顺堂, 肖应辉, 贺治洲, 雷东阳 (2006). 高温胁迫对水稻剑叶保护酶活性和膜透性的影响. 作物学报 32, 1306-1310. |
| [13] | Brennan T, Frenkel C (1977). Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol 59, 411-416. |
| [14] | Choudhury FK, Rivero RM, Blumwald E, Mittler R (2017). Reactive oxygen species, abiotic stress and stress combination. Plant J 90, 856-867. |
| [15] | Dar NA, Amin I, Wani W, Wani SA, Shikari AB, Wani SH, Masoodi KZ (2017). Abscisic acid: a key regulator of abiotic stress tolerance in plants. Plant Gene 11, 106-111. |
| [16] | Dwivedi SK, Basu S, Kumar S, Kumari S, Kumar A, Jha S, Mishra JS, Bhatt BP, Kumar G (2019). Enhanced antioxidant enzyme activities in developing anther contributes to heat stress alleviation and sustains grain yield in wheat. Funct Plant Biol 46, 1090-1102. |
| [17] | Elstner EF, Heupel A (1976). Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem 70, 616-620. |
| [18] | Feng BH, Zhang CX, Chen TT, Zhang XF, Tao LX, Fu GF (2018). Salicylic acid reverses pollen abortion of rice caused by heat stress. BMC Plant Biol 18, 245. |
| [19] | Fu GF, Feng BH, Zhang CX, Yang YJ, Yang XQ, Chen TT, Zhao X, Zhang XF, Jin QY, Tao LX (2016). Heat stress is more damaging to superior spikelets than inferiors of rice (Oryza sativa L.) due to their different organ temperatures. Front Plant Sci 7, 1637. |
| [20] | Hirose T, Ohdan T, Nakamura Y, Terao T (2006). Expression profiling of genes related to starch synthesis in rice leaf sheaths during the heading period. Physiol Plant 128, 425-435. |
| [21] | Huang J, Zhang FM, Xue Y, Lin J (2017). Recent changes of rice heat stress in Jiangxi province, southeast China. Int J Biometeorol 61, 623-633. |
| [22] | Huang YC, Niu CY, Yang CR, Jinn TL (2016). The heat stress factor HSFA6b connects ABA signaling and ABA-mediated heat responses. Plant Physiol 172, 1182-1199. |
| [23] | Janni M, Gullì M, Maestri E, Marmiroli M, Valliyodan B, Nguyen HT, Marmiroli N (2020). Molecular and genetic bases of heat stress responses in crop plants and breeding for increased resilience and productivity. J Exp Bot 71, 3780-3802. |
| [24] | Jiang CJ, Liu XL, Liu XQ, Zhang H, Yu YJ, Liang ZW (2017). Stunted growth caused by blast disease in rice seedlings is associated with changes in phytohormone signaling pathways. Front Plant Sci 8, 1558. |
| [25] | Joshee N, Kisaka H, Kitagawa Y (1998). Isolation and characterization of a water stress-specific genomic gene, pwsi 18, from rice. Plant Cell Physiol 39, 64-72. |
| [26] | Li N, Euring D, Cha JY, Lin Z, Lu MZ, Huang LJ, Kim WY (2021). Plant hormone-mediated regulation of heat tolerance in response to global climate change. Front Plant Sci 11, 627969. |
| [27] | Liu XL, Xie XZ, Zheng CK, Wei LX, Li XW, Jin YY, Zhang GH, Jiang CJ, Liang ZW (2022). RNAi-mediated suppression of the abscisic acid catabolism gene OsABA- 8ox1 increases abscisic acid content and tolerance to saline-alkaline stress in rice (Oryza sativa L.). Crop J 10, 354-367. |
| [28] | Liu XL, Zhang H, Jin YY, Wang MM, Yang HY, Ma HY, Jiang CJ, Liang ZW (2019). Abscisic acid primes rice seedlings for enhanced tolerance to alkaline stress by upregulating antioxidant defense and stress tolerance- related genes. Plant Soil 438, 39-55. |
| [29] | Livak KJ, Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402-408. |
| [30] | Qiao B, Zhang Q, Liu DL, Wang HQ, Yin JY, Wang R, He ML, Cui M, Shang ZL, Wang DK, Zhu ZG (2015). A calcium-binding protein, rice annexin OsANN1, enhances heat stress tolerance by modulating the production of H2O2. J Exp Bot 66, 5853-5866. |
| [31] | Qin P, Zhang GH, Hu BH, Wu J, Chen WL, Ren ZJ, Liu YL, Xie J, Yuan H, Tu B, Ma BT, Wang YP, Ye LM, Li LG, Xiang CB, Li SG (2021). Leaf-derived ABA regulates rice seed development via a transporter-mediated and temperature-sensitive mechanism. Sci Adv 7, eabc8873. |
| [32] | Qiu ZN, Zhu L, He L, Chen DD, Zeng DL, Chen G, Hu J, Zhang GH, Ren DY, Dong GJ, Gao ZY, Shen L, Zhang Q, Guo LB, Qian Q (2019). DNA damage and reactive oxygen species cause cell death in the rice local lesions 1 mutant under high light and high temperature. New Phytol 222, 349-365. |
| [33] | Rabbani MA, Maruyama K, Abe H, Khadri MA, Katsura K, Ito Y, Yoshiwara K, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003). Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiol 133, 1755-1767. |
| [34] | Rezaul IM, Feng BH, Chen TT, Fu WM, Zhang CX, Tao LX, Fu GF (2019). Abscisic acid prevents pollen abortion under high-temperature stress by mediating sugar metabolism in rice spikelets. Physiol Plant 165, 644-663. |
| [35] | Sato H, Suzuki Y, Sakai M, Imbe T (2002). Molecular characterization of Wx-mq, a novel mutant gene for low- amylose content in endosperm of rice (Oryza sativa L.). Breed Sci 52, 131-135. |
| [36] | Shi WJ, Yin XY, Struik PC, Solis C, Xie FM, Schmidt RC, Huang M, Zou YB, Ye CR, Jagadish SVK (2017). High day- and night-time temperatures affect grain growth dynamics in contrasting rice genotypes. J Exp Bot 68, 5233-5245. |
| [37] | Suriyasak C, Harano K, Tanamachi K, Matsuo K, Tamada A, Iwaya-Inoue M, Ishibashi Y (2017). Reactive oxygen species induced by heat stress during grain filling of rice (Oryza sativa L.) are involved in occurrence of grain chalkiness. J Plant Physiol 216, 52-57. |
| [38] | Tang RS, Zheng JC, Jin ZQ, Zhang DD, Huang YH (2008). Possible correlation between high temperature-induced floret sterility and endogenous levels of IAA, GAs and ABA in rice (Oryza sativa L.). Plant Growth Regul 54, 37-43. |
| [39] | Vishwakarma K, Upadhyay N, Kumar N, Yadav G, Singh J, Mishra RK, Kumar V, Verma R, Upadhyay RG, Pandey M, Sharma S (2017). Abscisic acid signaling and abiotic stress tolerance in plants: a review on current knowledge and future prospects. Front Plant Sci 8, 161. |
| [40] | Wang YL, Wang L, Zhou JX, Hu SB, Chen HZ, Xiang J, Zhang YK, Zeng YJ, Shi QH, Zhu DF, Zhang YP (2019). Research progress on heat stress of rice at flowering stage. Rice Sci 26, 1-10. |
| [41] | Wei LX, Lv BS, Li XW, Wang MM, Ma HY, Yang HY, Yang RF, Piao ZZ, Wang ZH, Lou JH, Jiang CJ, Liang ZW (2017a). Priming of rice (Oryza sativa L.) seedlings with abscisic acid enhances seedling survival, plant growth, and grain yield in saline-alkaline paddy fields. Field Crop Res 203, 86-93. |
| [42] | Wei LX, Lv BS, Wang MM, Ma HY, Yang HY, Liu XL, Jiang CJ, Liang ZW (2015). Priming effect of abscisic acid on alkaline stress tolerance in rice (Oryza sativa L.) seedlings. Plant Physiol Biochem 90, 50-57. |
| [43] | Wei XJ, Jiao GA, Lin HY, Sheng ZH, Shao GN, Xie LH, Tang SQ, Xu QG, Hu PS (2017b). GRAIN INCOMPLETE FILLING 2 regulates grain filling and starch synthesis during rice caryopsis development. J Integr Plant Biol 59, 134-153. |
| [44] | Xu YF, Chu CC, Yao SG (2021). The impact of high-temperature stress on rice: challenges and solutions. Crop J 9, 963-976. |
| [45] | Xu YY, Ramanathan V, Victor DG (2018). Global warming will happen faster than we think. Nature 564, 30-32. |
| [46] | Ye NH, Jia LG, Zhang JH (2012). ABA signal in rice under stress conditions. Rice 5, 1. |
| [47] | Zhang CX, Feng BH, Chen TT, Fu WM, Li HB, Li GY, Jin QY, Tao LX, Fu GF (2018). Heat stress-reduced kernel weight in rice at anthesis is associated with impaired source-sink relationship and sugars allocation. Environ Exp Bot 155, 718-733. |
| [48] | Zhang CX, Fu GF, Yang XQ, Yang YJ, Zhao X, Chen TT, Zhang XF, Jin QY, Tao LX (2016). Heat stress effects are stronger on spikelets than on flag leaves in rice due to differences in dissipation capacity. J Agron Crop Sci 202, 394-408. |
| [49] | Zhao C, Liu B, Piao SL, Wang XH, Lobell DB, Huang Y, Huang MT, Yao YT, Bassu S, Ciais P, Durand JL, Elliott J, Ewert F, Janssens IA, Li T, Lin ED, Liu Q, Martre P, Müller C, Peng SS, Peñuelas J, Ruane AC, Wallach D, Wang T, Wu DH, Liu Z, Zhu Y, Zhu ZC, Asseng S (2017). Temperature increase reduces global yields of major crops in four independent estimates. Proc Natl Acad Sci USA 114, 9326-9331. |
| [50] | Zhao Q, Zhou LJ, Liu JC, Du XX, Asad MAU, Huang FD, Pan G, Cheng FM (2018). Relationship of ROS accumulation and superoxide dismutase isozymes in developing anther with floret fertility of rice under heat stress. Plant Physiol Biochem 122, 90-101. |
| [51] | Zong WB, Ren D, Huang MH, Sun KL, Feng JL, Zhao J, Xiao DD, Xie WH, Liu SQ, Zhang H, Qiu R, Tang WJ, Yang RQ, Chen HY, Xie XR, Chen LT, Liu YG, Guo JX (2021). Strong photoperiod sensitivity is controlled by cooperation and competition among Hd1, Ghd7 and DTH8 in rice heading. New Phytol 229, 1635-1649. |
| [1] | TANG Yuan-Xiang, XIONG Shi-Chen, ZHU Hong-Feng, ZHANG Xin-Sheng, YOU Cheng-Ming, LIU Si-Ning, TAN Bo, XU Zhen-Feng. Effects of long-term nitrogen addition on leaf litter production and carbon, nitrogen and phosphorus return of the dominant tree species in broadleaf evergreen forests on the western margin of Sichuan Basin [J]. Chin J Plant Ecol, 2025, 49(5): 720-731. |
| [2] | Xu Tingyang, Liu Yuchen, Wang Wanpeng, Su Hang, Su Kunlong, Wu Zhenying, Lϋ Ming, Li Fuli, Wang Xiaoshan, Fu Chunxiang. Effects of Different Plant Growth Regulators on Wheat Growth and Development in the Saline-alkali Land [J]. Chinese Bulletin of Botany, 2025, 60(3): 354-362. |
| [3] | Chaoyu Zhu, Chengxiang Hu, Zhenan Zhu, Zhining Zhang, Lihai Wang, Jun Chen, Sanfeng Li, Jinjin Lian, Luyao Tang, Qianqian Zhong, Wenjing Yin, Yuexing Wang, Yuchun Rao. Mapping of QTLs Associated with Rice Panicle Traits and Candidate Gene Analysis [J]. Chinese Bulletin of Botany, 2024, 59(2): 217-230. |
| [4] | Yuejing Zhang, Hetian Sang, Hanqi Wang, Zhenzhen Shi, Li Li, Xin Wang, Kun Sun, Ji Zhang, Hanqing Feng. Research Progress of Plant Signaling in Systemic Responses to Abiotic Stresses [J]. Chinese Bulletin of Botany, 2024, 59(1): 122-133. |
| [5] | LIU Jian-Xin, LIU Rui-Rui, LIU Xiu-Li, JIA Hai-Yan, BU Ting, LI Na. Regulation of exogenous hydrogen sulfide on photosynthetic carbon metabolism in Avena nude under saline-alkaline stress [J]. Chin J Plant Ecol, 2023, 47(3): 374-388. |
| [6] | Yongjiang Sun, Qi Wang, Qiwen Shao, Zhiming Xin, Huijie Xiao, Jin Cheng. Research Advances on the Effect of High Temperature Stress on Plant Photosynthesis [J]. Chinese Bulletin of Botany, 2023, 58(3): 486-498. |
| [7] | Yuping Yan, Xiaoqi Yu, Deyong Ren, Qian Qian. Genetic Mechanisms and Breeding Utilization of Grain Number Per Panicle in Rice [J]. Chinese Bulletin of Botany, 2023, 58(3): 359-372. |
| [8] | Weijun Ye, Yin Zhang, Peiran Wang, Lingling Zhang, Dongfeng Tian, Zejiang Wu, Bin Zhou. QTLs Analysis for Five Yield-related Traits in Mungbean [J]. Chinese Bulletin of Botany, 2023, 58(1): 150-158. |
| [9] | YU Shui-Jin, WANG Juan, ZHANG Chun-Yu, ZHAO Xiu-Hai. Impact and mechanism of maintaining biomass stability in a temperate coniferous and broadleaved mixed forest [J]. Chin J Plant Ecol, 2022, 46(6): 632-641. |
| [10] | Wang Lei, Chong Kang. Choice of both Ways: Variations of Reverted Repeats Balance Environmental Adaptation and Yield in Maize [J]. Chinese Bulletin of Botany, 2022, 57(5): 555-558. |
| [11] | Zhou Yuping, Yan Jiahao, Tian Chang’en. Research Progress on the Regulatory Mechanisms of ABA Signal Transduction in Guard Cells [J]. Chinese Bulletin of Botany, 2022, 57(5): 684-696. |
| [12] | Haitao Hu, Tingting Qian, Ling Yang. Detection of Reactive Oxygen Species Using H2DCFDA Probe in Plant [J]. Chinese Bulletin of Botany, 2022, 57(3): 320-326. |
| [13] | XIONG Shu-Ping, CAO Wen-Bo, CAO Rui, ZHANG Zhi-Yong, FU Xin-Lu, XU Sai-Jun, PAN Hu-Qiang, WANG Xiao-Chun, MA Xin-Ming. Effects of horizontal structure on canopy vertical structure, microenvironment and yield of Triticum aestivum [J]. Chin J Plant Ecol, 2022, 46(2): 188-196. |
| [14] | LIN Yong, CHEN Zhi, YANG Meng, CHEN Shi-Ping, GAO Yan-Hong, LIU Ran, HAO Yan-Bin, XIN Xiao-Ping, ZHOU Li, YU Gui-Rui. Temporal and spatial variations of ecosystem photosynthetic parameters in arid and semi-arid areas of China and its influencing factors [J]. Chin J Plant Ecol, 2022, 46(12): 1461-1472. |
| [15] | Jian-Min Zhou. A Ca2+-ROS Signaling Axis in Rice Provides Clues to Rice-pathogen Coevolution and Crop Improvements [J]. Chinese Bulletin of Botany, 2021, 56(5): 513-515. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||