

Chinese Bulletin of Botany ›› 2019, Vol. 54 ›› Issue (1): 72-80.DOI: 10.11983/CBB18118 cstr: 32102.14.CBB18118
• TECHNIQUE AND METHOD • Previous Articles Next Articles
Junhua Li1,2,3,†,Shiyu Liu1,†,Chenglong Li1,Linlin Han1,Yahui Dong1,Xiaoli Zhang1,2,3,Xiting Zhao1,2,3,Mingjun Li1,2,3,*( )
)
Received:2018-05-15
Accepted:2018-09-13
Online:2019-01-01
Published:2019-07-31
Contact:
Junhua Li,Shiyu Liu,Mingjun Li
Junhua Li,Shiyu Liu,Chenglong Li,Linlin Han,Yahui Dong,Xiaoli Zhang,Xiting Zhao,Mingjun Li. Establishment of a Genetic Transformation System for Dioscorea opposita Using Microtuber[J]. Chinese Bulletin of Botany, 2019, 54(1): 72-80.
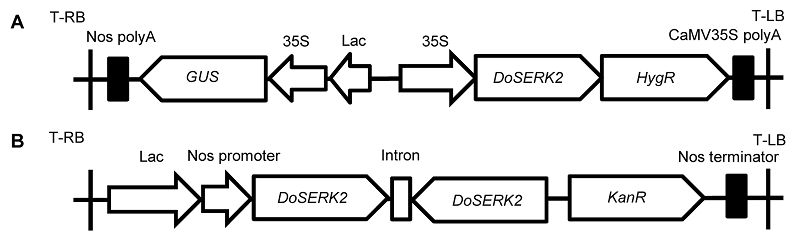
Figure 1 Schematic maps of the overexpression vector and silencing vector used in this study (A) The overexpression vector pCAMBIA1301-DoSERK2; (B) The silencing vector pART27-DoSERK2
| Media | Composition |
|---|---|
| MS0 | MS major salts, MS minor salts and MS vitamins +30 g·L-1 sucrose+6 g·L-1 fungible agar, pH5.8- 6.2 |
| MS0 1 | MS0+1 mg·L-1 6-BA+1 mg?L-1 IAA |
| MS0 2 | MS0+2 mg?L-1 PP333+0.05 mg?L-1 NAA |
| MS0 1-A | MS0 1+100 μmol?L-1 Acetosyringone (AS) |
| MS0 1-T | MS0 1+500 mg?L-1 Timentin (Tim) |
| MS0 1-TH | MS0 1+500 mg?L-1 Tim+15 mg?L-1 Hyg |
| MS0 1-TK | MS0 1+500 mg?L-1 Tim+120 mg?L-1 Kan |
| MS0 2-H | MS0 2+20 mg?L-1 Hyg |
| MS0 2-K | MS0 2+160 mg?L-1 Kan |
Table 1 Medium used for genetic transformation and plant regeneration of Dioscorea opposita cv. ‘Tiegun’
| Media | Composition |
|---|---|
| MS0 | MS major salts, MS minor salts and MS vitamins +30 g·L-1 sucrose+6 g·L-1 fungible agar, pH5.8- 6.2 |
| MS0 1 | MS0+1 mg·L-1 6-BA+1 mg?L-1 IAA |
| MS0 2 | MS0+2 mg?L-1 PP333+0.05 mg?L-1 NAA |
| MS0 1-A | MS0 1+100 μmol?L-1 Acetosyringone (AS) |
| MS0 1-T | MS0 1+500 mg?L-1 Timentin (Tim) |
| MS0 1-TH | MS0 1+500 mg?L-1 Tim+15 mg?L-1 Hyg |
| MS0 1-TK | MS0 1+500 mg?L-1 Tim+120 mg?L-1 Kan |
| MS0 2-H | MS0 2+20 mg?L-1 Hyg |
| MS0 2-K | MS0 2+160 mg?L-1 Kan |
| Primers | Sequences (5'-3') |
|---|---|
| DoSERK2-OE-F | ATGACGGCTTGGGTTTTC |
| DoSERK2-OE-R | TCACCTCGGACCAGATAGC |
| DoSERK2-RNAi-F | CAGATGATACAGAAAAGCACCG |
| DoSERK2-RNAi-R | TAACTTTCGGTAGAGCGGAC |
| DoActin-F | CTCATTGATCGGCATGGAAGC |
| DoActin-R | GGGGAACATAGTTGAACCACCAC |
| DoSERK2-qRT-F | TATCTGGACCAGTTCCATCC |
| DoSERK2-qRT-R | CTTCAGCAGGCACATCATAG |
Table 2 Sequences of primers
| Primers | Sequences (5'-3') |
|---|---|
| DoSERK2-OE-F | ATGACGGCTTGGGTTTTC |
| DoSERK2-OE-R | TCACCTCGGACCAGATAGC |
| DoSERK2-RNAi-F | CAGATGATACAGAAAAGCACCG |
| DoSERK2-RNAi-R | TAACTTTCGGTAGAGCGGAC |
| DoActin-F | CTCATTGATCGGCATGGAAGC |
| DoActin-R | GGGGAACATAGTTGAACCACCAC |
| DoSERK2-qRT-F | TATCTGGACCAGTTCCATCC |
| DoSERK2-qRT-R | CTTCAGCAGGCACATCATAG |
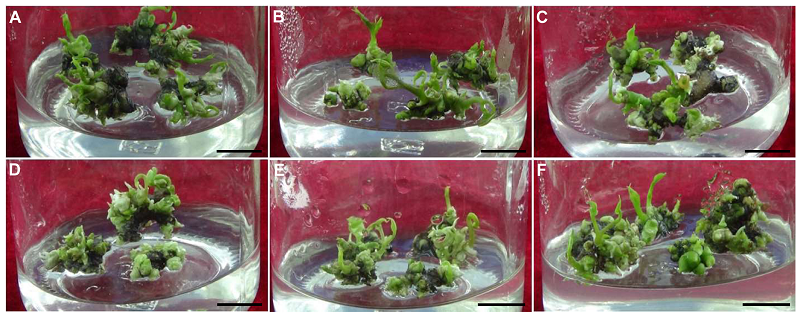
Figure 2 Effects of different concentrations of Tim on the proliferation and differentiation of protocorm-like bodies (PLBs) in Dioscorea opposita (A) 0 mg·L-1; (B) 100 mg·L-1; (C) 200 mg·L-1; (D) 300 mg·L-1; (E) 400 mg·L-1; (F) 500 mg·L-1. Bars=1 cm

Figure 3 Effects of antibiotic on microtuber regeneration and rooting of regenerated seedlings in Dioscorea opposita cv. ‘Tiegun’ (A)-(F) Effects of different concentrations of Hyg on microtuber regeneration, the concentrations of Hyg are 0, 5, 10, 15, 20, 25 mg·L-1, respectively; (G)-(L) Effects of different concentrations of Kan on microtuber regeneration, the concentrations of Kan are 0, 80, 100, 120, 140, 160 mg·L-1, respectively; (M) Effects of different concentrations of Hyg on rooting of regenerated plantlets; (N) Effects of different concentrations of Kan on rooting of regenerated plantlets. (A)-(L) Bars=0.5 cm; (M), (N) Bars=2 cm
| Concentration of Hyg (mg·L-1) | Regeneration (%) | Concentration of Kan (mg·L-1) | Regeneration (%) | Growth of seedlings |
|---|---|---|---|---|
| 0 | 60.00±2.00 | 0 | 65.56±2.52 | Strong growth vigor |
| 5 | 55.56±1.15 | 80 | 48.89±1.53 | Well |
| 10 | 33.33±2.00 | 100 | 34.44±1.53 | Slow, albefaction in some seedlings |
| 15 | 25.56±0.58 | 120 | 8.89±3.06 | Slow, albefaction in many seedlings |
| 20 | 2.22±1.15 | 140 | 1.11±0.58 | Growth stopped, albefaction seriously |
| 25 | 0 | 160 | 0 | All dead |
Table 3 Effects of antibiotic concentrations on regeneration of microtuber slices in Dioscorea opposita cv. ‘Tiegun’
| Concentration of Hyg (mg·L-1) | Regeneration (%) | Concentration of Kan (mg·L-1) | Regeneration (%) | Growth of seedlings |
|---|---|---|---|---|
| 0 | 60.00±2.00 | 0 | 65.56±2.52 | Strong growth vigor |
| 5 | 55.56±1.15 | 80 | 48.89±1.53 | Well |
| 10 | 33.33±2.00 | 100 | 34.44±1.53 | Slow, albefaction in some seedlings |
| 15 | 25.56±0.58 | 120 | 8.89±3.06 | Slow, albefaction in many seedlings |
| 20 | 2.22±1.15 | 140 | 1.11±0.58 | Growth stopped, albefaction seriously |
| 25 | 0 | 160 | 0 | All dead |
| Concentration of Hyg (mg·L-1) | Rooting (%) | Concentration of Kan (mg·L-1) | Rooting (%) | Growth of roots |
|---|---|---|---|---|
| 0 | 100 | 0 | 100 | Strong and flourishing |
| 5 | 100 | 120 | 100 | Well |
| 15 | 3±2.00 | 140 | 1±1.20 | Seldom rooting |
| 20 | 0 | 160 | 0 | Hardly rooting |
| 25 | 0 | 180 | 0 | Hardly rooting, albefaction |
Table 4 Effects of antibiotic concentrations on rooting of regenerated seedlings in Dioscorea opposita cv. ‘Tiegun’
| Concentration of Hyg (mg·L-1) | Rooting (%) | Concentration of Kan (mg·L-1) | Rooting (%) | Growth of roots |
|---|---|---|---|---|
| 0 | 100 | 0 | 100 | Strong and flourishing |
| 5 | 100 | 120 | 100 | Well |
| 15 | 3±2.00 | 140 | 1±1.20 | Seldom rooting |
| 20 | 0 | 160 | 0 | Hardly rooting |
| 25 | 0 | 180 | 0 | Hardly rooting, albefaction |
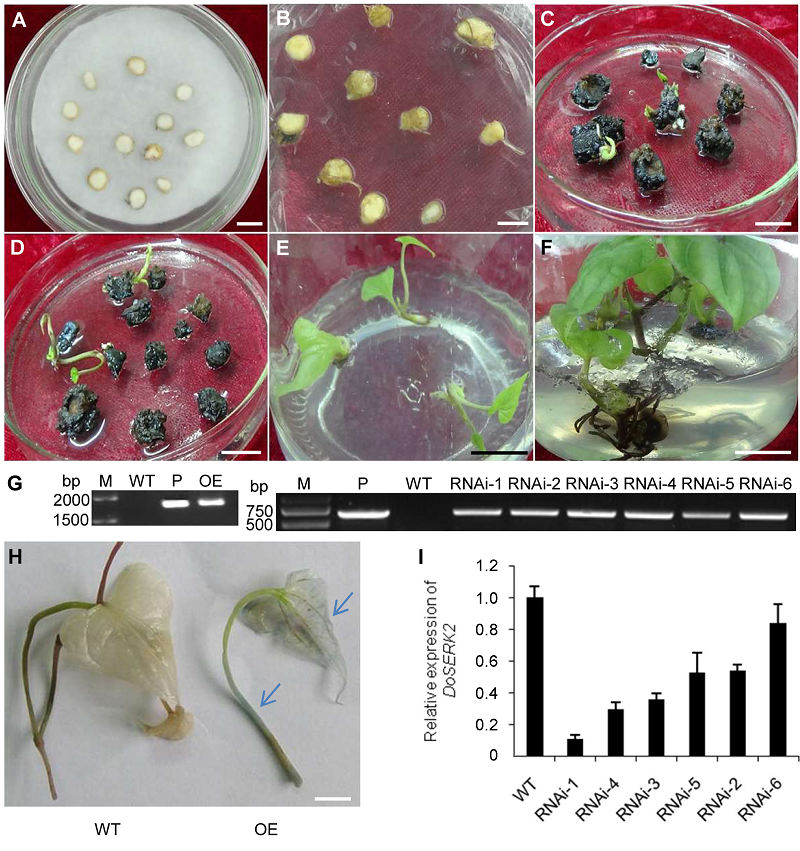
Figure 4 Genetic transformation and regeneration of Dioscorea opposita cv. ‘Tiegun’ microtuber slices and molecular verification of trans- genic plants (A) Microtuber slices infected by Agrobacterium; (B) Microtuber slices after co-cultivation with Agrobacterium for 3 days; (C) Tissues cultured on regeneration medium for 40 days; (D) Tissues cultured on regeneration medium for 70 days; (E) Antibiotic-resistant regenerated seedlings that had been transferred to rooting medium; (F) Regenerated seedlings that had been cultured on rooting medium for 30 days; (G) PCR assay of genome DNA from transgenic plants (M: DNA marker; P: Plasmid pCAMBIA1301-DoSERK2 (left panel) and pART27-DoSERK2 (right panel) as template; WT: Genome DNA of wild-type plants as template; OE: Genome DNA from overexpression transgenic plants as template; RNAi-1-6: Genome DNA from line 1-6 of transgenic plants of the silencing vector as template); (H) GUS activity assay in the leaf of an overexpression transformant, nodal or leaf segments of a wild-type plant (WT) or transgenic plant (OE) were stained with GUS staining solution; (I) RT-qPCR assay of the endogenous expression levels of DoSERK2 (WT: cDNA of wild-type plants as template; RNAi-1-6: cDNA from line 1-6 of transgenic plants of the silencing vector as template). (A)-(F), (H) Bars=1 cm
| 1 |
范俊强 ( 2008). 根癌农杆菌介导的盾叶薯蓣转化体系的建立. 硕士论文. 武汉: 华中农业大学. pp. 1-5.
DOI URL |
| 2 |
付洪冰, 崔崇士, 赵曦, 刘琦 ( 2010). 农杆菌介导南瓜遗传转化体系的建立. 植物学报 45, 472-478.
DOI URL |
| 3 |
郭利军, 曾炳山, 刘英 ( 2013). 农杆菌介导巨桉Eg5高效遗传转化. 植物学报 48, 87-93.
DOI URL |
| 4 |
韩林林, 李俊华, 赵喜亭, 张晓丽, 宋志辉, 刘世宇, 李明军 ( 2016). 农杆菌介导的怀山药叶片瞬时表达方法的建立. 河南师范大学学报(自然科学版) 44, 135-139.
DOI URL |
| 5 | 李明军 (2013).提高山药商品性栽培技术问答. 北京: 金盾出版社. pp. 36-50. |
| 6 |
李明军, 陈明霞, 洪森荣, 徐鑫, 张晓丽 ( 2004). NAA、IBA和PP333对怀山药试管苗生长发育的影响. 广西植物 24, 376-379.
DOI URL |
| 7 | 李明军, 刘世宇, 刘雯, 李俊华, 张晓丽, 赵喜亭 ( 2017). 怀山药微型块茎形成过程中的生理生化变化. 植物生理学报 53, 807-814. |
| 8 |
李明军, 张峰, 陈明霞, 于相丽 ( 2003). 怀山药病毒病的研究. 中草药 34, U003-U005.
DOI URL |
| 9 | 李瑞雪, 李纪强, 蒲腾飞, 张晓丽, 赵喜亭, 李俊华, 李明军 ( 2018). 怀山药类原球茎的诱导形成与植株再生. 植物学报 53, 334-340. |
| 10 |
李卫, 郭光沁, 郑国锠 ( 2000). 根癌农杆菌介导遗传转化研究的若干新进展. 科学通报 45, 798-807.
DOI URL |
| 11 |
廖华兰, 黎秀琼, 李可, 李伯凌, 熊茜, 苏童, 李春霞, 陈银华, 罗丽娟 ( 2016). 农杆菌介导的木薯遗传转化体系的优化. 热带生物学报 7, 427-434.
DOI URL |
| 12 | 王善平, 许智宏, 卫志明 ( 1990). 毛白杨叶外植体的遗传转化. 植物学报 32, 172-177. |
| 13 | 王运英 ( 2016). 怀山药脱毒微型块茎管内管外萌发条件及生理生化机制研究. 硕士论文. 新乡: 河南师范大学. pp. 9-19. |
| 14 | 许云 ( 2014). 大薯遗传多样性的AFLP分析和类原球茎遗传转化体系的研究. 硕士论文. 海口: 海南大学. pp. 61-66. |
| 15 | 杨亚萍, 李永兰, 梁月荣, 陆建良, 郑新强 ( 2015). 发根农杆菌抑菌剂的抑菌效果及对茶组培苗丛生芽的影响. 茶叶科学 35, 437-442. |
| 16 |
张宁, 司怀军, 李学才, 王蒂 ( 2004). 根癌农杆菌介导的马铃薯高效遗传转化体系的研究. 中国马铃薯 18, 132-135.
DOI URL |
| 17 |
赵喜亭, 蒋丽薇, 王苗, 朱玉婷, 张文芳, 李明军 ( 2016). 怀黄菊间接体胚受体再生体系的建立及CmTGA1的遗传转化. 植物学报 51, 525-532.
DOI URL |
| 18 |
Edwards K, Johnstone C, Thompson C ( 1991). A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res 19, 1349.
DOI URL PMID |
| 19 | Li MJ, Li JH, Wang YP, Liu W, Guo XB, Li SJ, Han LL, Song ZH, Zhao XT, Yang QX ( 2015). A simple method for microtuber production in Dioscorea opposita using single nodal segments . Pakistan J Bot 47, 665-668. |
| 20 | Mignouna HD, Abang MM, Asiedu R (2008). Genomics of yams, a common source of food and medicine in the tropics. In: Moore PH, Ming R, eds. Genomics of Tropical Crop Plants. Plant Genetics and Genomics: Crops and Models, Vol. 1. Berlin: Springer. pp. 549-570. |
| 21 | Nyaboga E, Tripathi JN, Manoharan R, Tripathi L ( 2014). Agrobacterium-mediated genetic transformation of yam ( Dioscorea rotundata): an important tool for functional study of genes and crop improvement.Front Plant Sci 5, 463. |
| 22 | Tör M, Ainsworth C, Mantell SH ( 1993). Stable transfor-mation of the food yam Dioscorea alata L.by particle bombardment. Plant Cell Rep 12, 468-473. |
| 23 |
Tör M, Twyford CT, Funes I, Boccon-Gibod J, Ainsworth CC, Mantell SH ( 1998). Isolation and culture of protoplasts from immature leaves and embryogenic cell suspensions of Dioscorea yams: tools for transient gene expression studies.Plant Cell Tissue Organ Cult 53, 113-126.
DOI URL |
| 24 |
Zhao XT, Zhang XL, Guo XB, Li SJ, Han LL, Song ZH, Wang YY, Li JH, Li MJ ( 2016). Identification and validation of reference genes for qRT-PCR studies of gene expression in Dioscorea opposita.Biomed Res Int 2016, 3089584.
DOI URL PMID |
| [1] | Jingjing Li, Yanfei Li, Anqi Wang, Jiaying Wang, Chengyan Deng, Min Lu, Jianying Ma, Silan Dai. Establishment of Regeneration and Genetic Transformation System for Chrysanthemum Cultivar ‘Wandai Fengguang’ [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [2] | Zeng Wendan, Yan Huabing, Wu Zhengdan, Shang Xiaohong, Cao Sheng, Lu Liuying, Xiao Liang, Shi Pingli, Cheng Dong, Long Ziyuan, Li Jieyu. Agrobacterium rhizogenes-mediated Transformation System of Pueraria lobata Hairy Roots [J]. Chinese Bulletin of Botany, 2025, 60(3): 425-434. |
| [3] | Yuchen Li, Haixia Zhao, Xiping Jiang, Xintian Huang, Yaling Liu, Zhenying Wu, Yan Zhao, Chunxiang Fu. Establishment of Agrobacterium-mediated Transformation System for Agropyron mongolicum [J]. Chinese Bulletin of Botany, 2024, 59(4): 600-612. |
| [4] | Yu Xiaomin, Wang Yaqin, Liu Yuhan, Yi Qingping, Cheng Wenhan, Zhu Yu, Duan Feng, Zhang Lixue, He Yanhong. Establishment of Agrobacterium tumefaciens-mediated Genetic Transformation System of Marigold (Tagetes erecta) [J]. Chinese Bulletin of Botany, 2023, 58(5): 760-769. |
| [5] | Lan Yang, Ya Liu, Yang Xiang, Xiujuan Sun, Jingwei Yan, Aying Zhang. Establishment and Optimization of a Shoot Tip-based Genetic Transformation System for Foxtail Millet [J]. Chinese Bulletin of Botany, 2021, 56(1): 71-79. |
| [6] | Lijun Guo, Bingshan Zeng, Ying Liu. Agrobacterium-mediated High-efficient Transformation of Eucalyptus grandis Clone Eg5 [J]. Chinese Bulletin of Botany, 2013, 48(1): 87-93. |
| [7] | Guimei Cui, Yi Sun, Yaoshan Hao, Jianzhong Du, Yixue Wang. The Improvement of Maize Pollen In Vitro Germination Method and Its Role in Pollen-mediated Plant Genetic Transformation [J]. Chinese Bulletin of Botany, 2012, 47(2): 155-161. |
| [8] | Xuanyu Liu, Qingyun Wang, Shujun Liu, Songquan Song. Advances in the Genetic Transformation of Sorghum bicolor [J]. Chinese Bulletin of Botany, 2011, 46(2): 216-223. |
| [9] | Hongbing Fu;Chongshi Cui;Xi Zhao;Qi Liu. Establishment of Cucurbita moschata Genetic Transformation System by Agrobacterium tumefaciens Transfection [J]. Chinese Bulletin of Botany, 2010, 45(04): 472-478. |
| [10] | Daojie Wang, Cuiling Yang, Ming Lu. Transformation of Brassica napus by Vacuum Infiltration [J]. Chinese Bulletin of Botany, 2009, 44(02): 216-222. |
| [11] | Kaifa Wei*;Yiping Liu;Ziying Lin;Yafang Yang;Zehong Zhang;Wensuo Jia. Problems and Solutions in Agrobacterium tumefaciens-mediated Genetic Transformation of Monocotyledons [J]. Chinese Bulletin of Botany, 2008, 25(04): 491-496. |
| [12] | Tingbo Jiang*;Xinhua Tang;Fengjuan Li;Baojian Ding;Hong Chen. Effects of Ferritin Gene Expression on Transgenic Tobacco for Low Iron Tolerance [J]. Chinese Bulletin of Botany, 2008, 25(02): 167-175. |
| [13] | Jianbin Hu*;Jun Liu. Progress in Tissue Culture and Genetic Transformation of Amorphophallus Blume [J]. Chinese Bulletin of Botany, 2008, 25(01): 14-19. |
| [14] | JI Feng_Yuan, WANG Ge_Liang, XU Yi_Nong. THE EFFECTS OF ANTIOXIDANTS ON THE TRANSIENT EXPRESSION OF GUS GENE IN SOYBEAN HYPOCOTYLS MEDIATED BY AGROBACTERIUM TUMEFACIENS [J]. Chin J Plant Ecol, 2006, 30(2): 330-334. |
| [15] | GAO Li-Ping BAO Man-Zhu. Advances in Plant Regeneration and Genetic Transformation of Roses [J]. Chinese Bulletin of Botany, 2005, 22(02): 231-237. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||