

Chinese Bulletin of Botany ›› 2016, Vol. 51 ›› Issue (2): 194-201.DOI: 10.11983/CBB15081 cstr: 32102.14.CBB15081
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Lin Li, Kang Tan, Xiuguang Tang, Xiaoting Chao, Chenxi Wen, Zhuangdong Bai, Hualing Feng, Wenzhe Liu*( ), Hui Su*(
), Hui Su*( )
)
Received:2015-05-09
Accepted:2015-07-05
Online:2016-03-01
Published:2016-03-31
Contact:
E-mail: Lin Li, Kang Tan, Xiuguang Tang, Xiaoting Chao, Chenxi Wen, Zhuangdong Bai, Hualing Feng, Wenzhe Liu, Hui Su. Characterization of Programmed Cell Death During the Senescence of Root Hairs in Arabidopsis[J]. Chinese Bulletin of Botany, 2016, 51(2): 194-201.
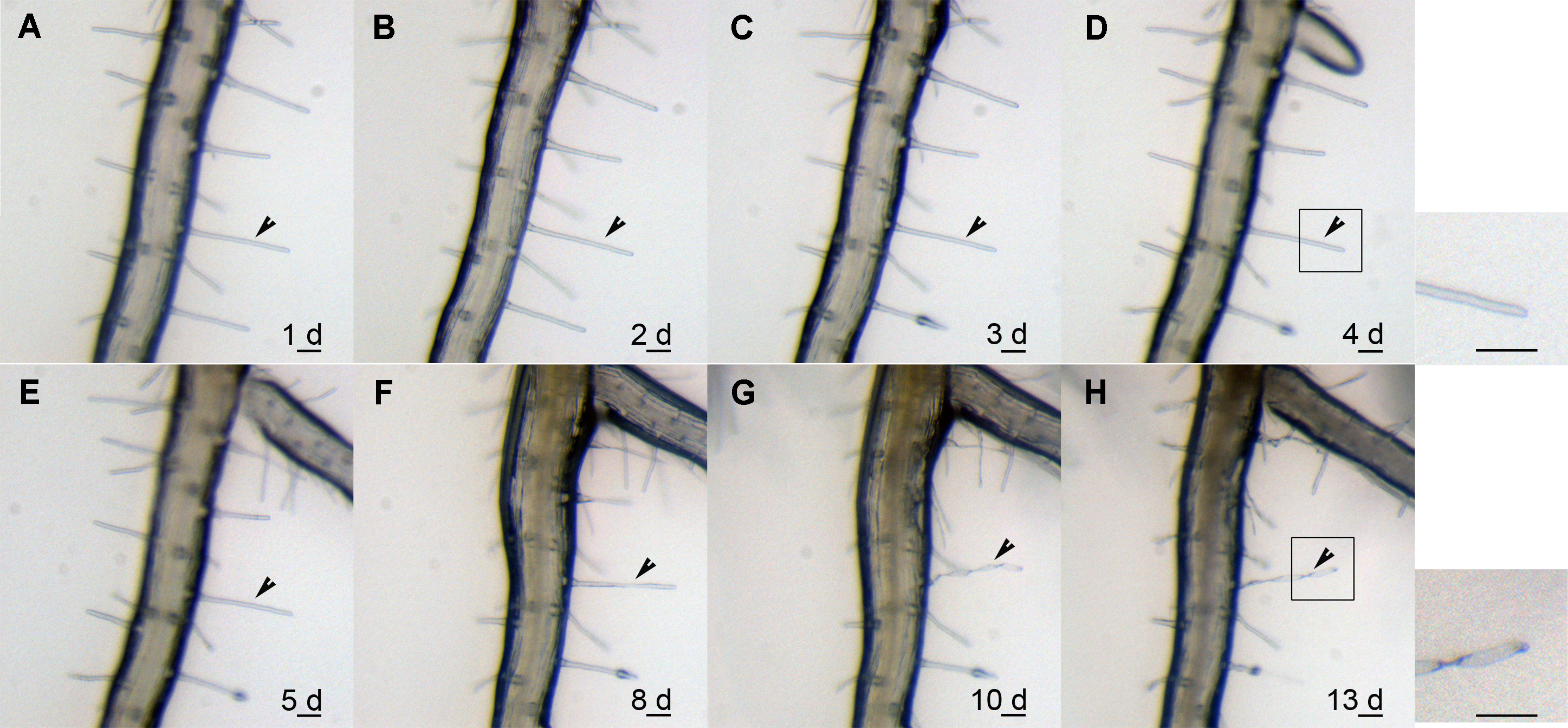
Figure 1 Time-lapse of root hairs in Arabidopsis (A)-(E) The morphology of the root hair indicated by the arrow head was observed for 1, 2, 3, 4 and 5 d, respectively; (F) The vary of the root hair was observed in the 8th day (indicating by the arrow head) ; (G) The root hair obviously twisted and curved in the 10th day (indicating by the arrow head), and at that time, root hair has been died); (H) Twisted and curved root hair in the 13th day (indicating by the arrow head). Bar=10 μm
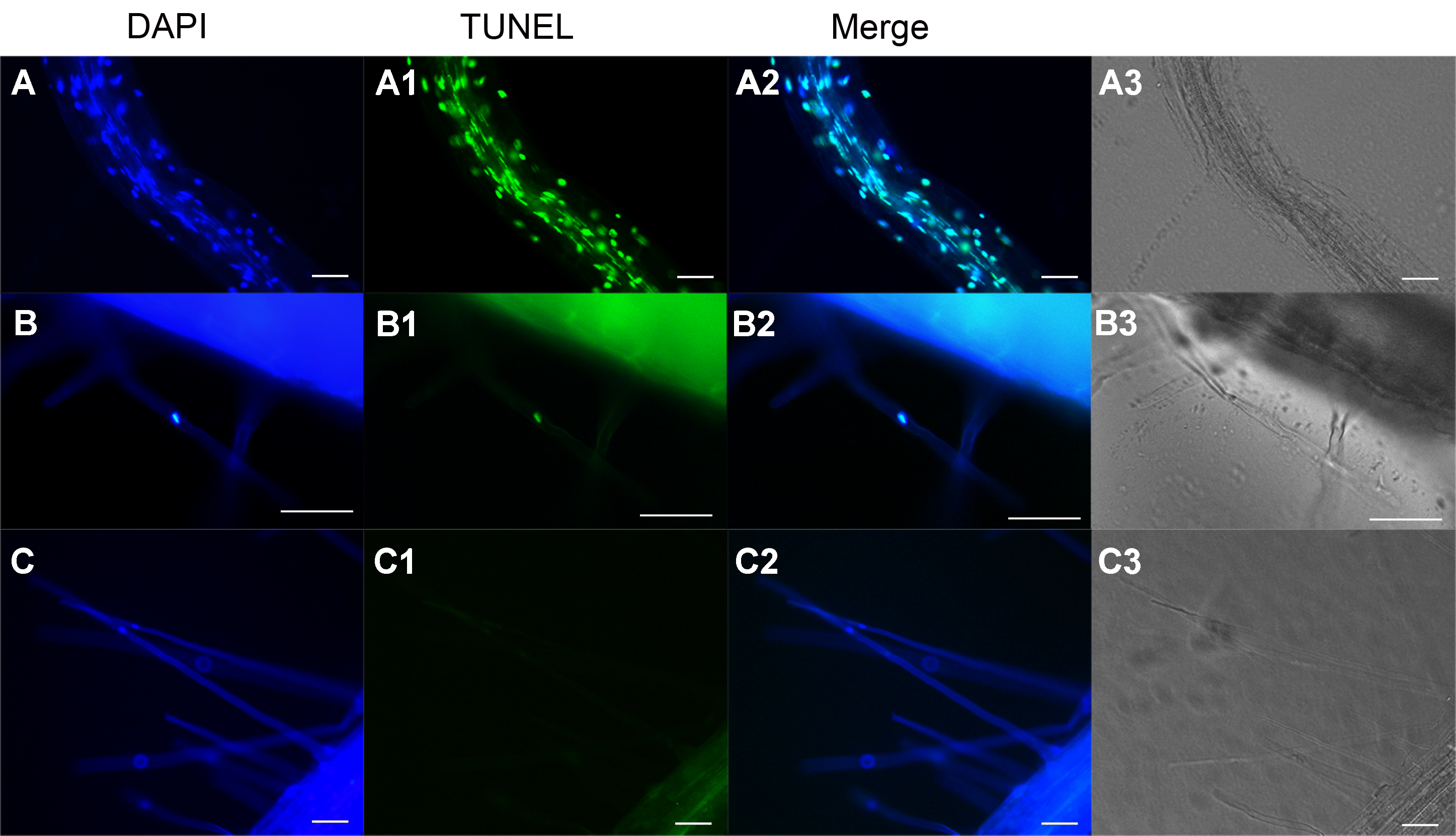
Figure 2 The TUNEL assay in roots and root hairs of Arabidopsis (A)-(A3) DNase I treated seedlings were fixed and subjected to TUNEL assay. The root and root hairs were TUNEL-positive; (B)-(B3) PCD root hair. A representative image shows TUNEL-positive root hair. (C)-(C3) Negative control. A representative image shows TUNEL-negative root hair. Bar=50 μm
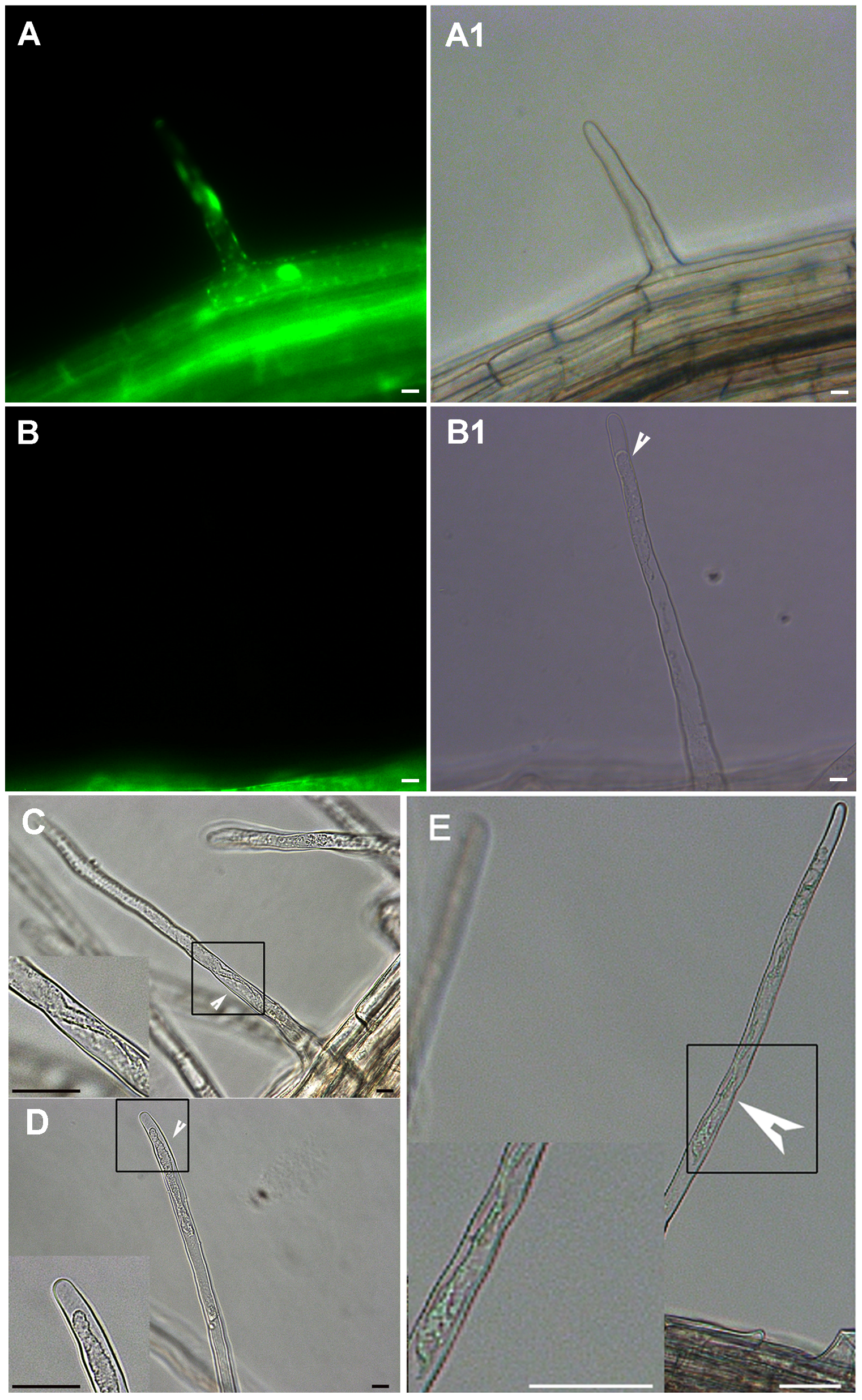
Figure 3 The plasmolysis of cell in Arabidopsis naturally dead root hair (A)-(A1) Normal root hairs stained with FDA and viewed under white (A1) or fluorescent light (A); (B)-(B1) Naturally dead root hair. The root hairs with no fluorescence after FDA staining shows obvious retraction of the cytoplasm; (C) and (D) The extent of protoplast shrinking varies depending on the sequential process of programmed cell death. (E) Root hair of Arabidopsis thaliana 6 h after a 10 minutes heat shock at 55°C. The root hair shows retraction of the cytoplasm indicating it has undergone AL-PCD (the positive control). Bar=10 μm
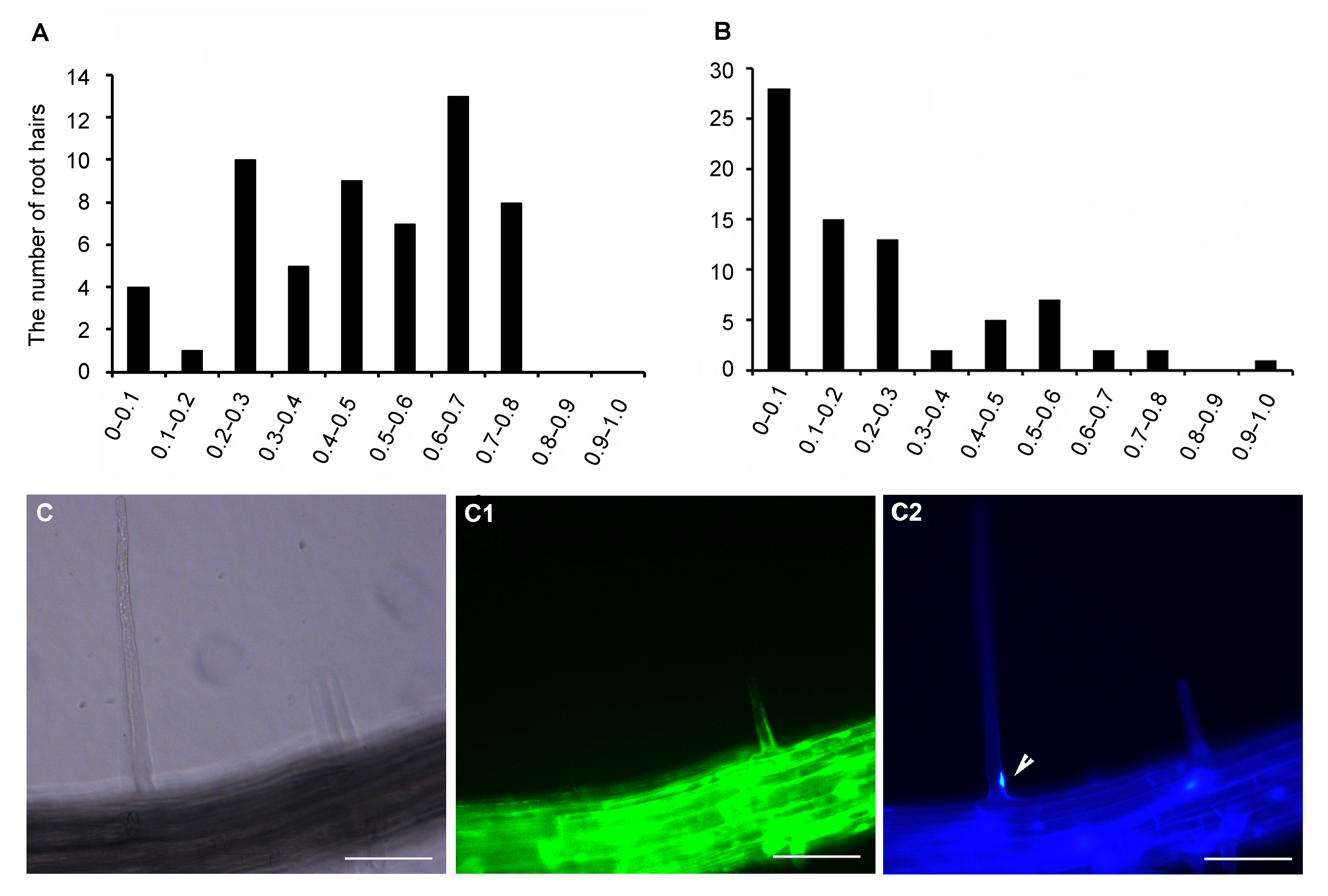
Figure 5 The position of nuclei in natural death root hairs is close to the basement of root hairs (A) Quantitative analysis of the position of nuclei in living root hairs; Abscissa represents ratio between the distance of the nucleus center from the basement of the root hair and root hair length; Ordinate represents the number of root hairs (n=57); (B) Quantitative analysis of the position of nuclei in dead root hairs; Abscissa represents ratio; Ordinate represents the number of root hairs (n=75); (C)-(C2) A representative image stained by FDA and DAPI shows that the nucleus in the dead root hair can be colored by DAPI (indicating by the arrow head) and the location of the nucleus is near the basement of the root hair. C: Bright field. Arrow head: Nucleus. Bar=50 μm
| [1] |
贺新强, 吴鸿 (2013). 植物发育性细胞程序死亡的发生机制. 植物学报 48, 357-370.
DOI |
| [2] | 李云霞, 程晓霞, 代小梅, 曾会明, 韩烈宝 (2009). 植物在逆境胁迫中的细胞程序性死亡. 生物技术通报 4, 711. |
| [3] |
Delorme VG, McCabe PF, Kim DJ, Leaver CJ (2000). A matrix metalloproteinase gene is expressed at the boun- dary of senescence and programmed cell death in cucumber. Plant Physiol 123, 917-928.
DOI PMID |
| [4] |
Doyle SM, Diamond M, McCabe PF (2010). Chloroplast and reactive oxygen species involvement in apoptotic-like programmed cell death in Arabidopsis suspension cultures. J Exp Bot 61, 473-482.
DOI PMID |
| [5] | Filonova LH, Bozhkov PV, Brukhin VB, Daniel G, Zhivo- tovsky B, Von Arnold S (2000). Two waves of program- med cell death occur during formation and development of somatic embryos in the gymnosperm, Norway spruce. J Cell Sci 113, 4399-4411. |
| [6] |
Filonova LH, Von Arnold S, Daniel G, Bozhkov PV (2002). Programmed cell death eliminates all but one embryo in a polyembryonic plant seed. Cell Death Differ 9, 1057-1062.
PMID |
| [7] | Fukuda H (1997). Tracheary element differentiation. Plant Cell 9, 1147. |
| [8] | Grierson C, Schiefelbein J (2002). Root Hairs. Rockville, MD: American Society of Plant Biologists. pp. 1-22 |
| [9] |
Groover A, Jones AM (1999). Tracheary element differentiation uses a novel mechanism coordinating programmed cell death and secondary cell wall synthesis. Plant Physiol 119, 375-384.
DOI PMID |
| [10] |
Gunawardena AH (2008). Programmed cell death and tissue remodelling in plants. J Exp Bot 59, 445-451.
DOI PMID |
| [11] |
Gunawardena AH, Greenwood JS, Dengler NG (2004). Programmed cell death remodels lace plant leaf shape during development. Plant Cell 16, 60-73.
DOI PMID |
| [12] |
Gunawardena AH, Pearce DM, Jackson MB, Hawes CR, Evans DE (2001). Characterisation of programmed cell death during aerenchyma formation induced by ethylene or hypoxia in roots of maize (Zea mays L.). Planta 212, 205-214.
DOI PMID |
| [13] | Hogg BV, Kacprzyk J, Molony EM, O’Reilly C, Gallagher TF, Gallois P, McCabe PF (2011). An in vivo root hair assay for determining rates of apoptotic-like programmed cell death in plants. Plant Methods 7: 45. |
| [14] |
Ketelaar T, Faivre-Moskalenko C, Esseling JJ, de Ruijter NC, Grierson CS, Dogterom M, Emons AMC (2002). Positioning of nuclei in Arabidopsis root hairs: an actin-regulated process of tip growth. Plant Cell 14, 2941-2955.
DOI PMID |
| [15] | Li D, Yang X, Cui K, Li Z (2003). Morphological changes in nucellar cells undergoing programmed cell death (PCD) during pollen chamber formation in Ginkgo biloba. Acta Botanica Sinica 45, 53-63. |
| [16] |
McCabe PF, Leaver CJ (2000). Programmed cell death in cell cultures. Plant Mol Biol 44, 359-368.
PMID |
| [17] | Miller DD, De Ruijter NC, Emons AMC (1997). From signal to form: aspects of the cytoskeleton-plasma membrane-cell wall continuum in root hair tips. J Exp Bot 48, 1881-1896. |
| [18] |
Mittler R, Lam E (1995). In situ detection of nDNA fragmentation during the differentiation of tracheary elements in higher plants. Plant Physiol 108, 489-493.
DOI PMID |
| [19] | Peterson RL, Farquhar ML (1996). Root hairs: specialized tubular cells extending root surface. Bot Rev 62, 1-40. |
| [20] |
Reape TJ, McCabe PF (2010). Apoptotic-like regulation of programmed cell death in plants. Apoptosis 15, 249-256.
DOI PMID |
| [21] |
Shishkova S, Dubrovsky JG (2005). Developmental programmed cell death in primary roots of Sonoran desert Cactaceae. Am J Bot 92, 1590-1594.
DOI PMID |
| [22] |
Van Doorn WG (2011). Classes of programmed cell death in plants, compared to those in animals. J Exp Bot 62, 4749-4761.
DOI PMID |
| [23] |
Van Doorn WG, Woltering EJ (2005). Many ways to exit? Cell death categories in plants. Trends Plant Sci 10, 117-122.
DOI PMID |
| [24] | Wang H, Li J, Bostock RM, Gilchrist DG (1996). Apoptosis: a functional paradigm for programmed plant cell death induced by a host-selective phytotoxin and invoked during development. Plant Cell 8, 375-391. |
| [25] |
Zuppini A, Navazio L, Mariani P (2004). Endoplasmic reticulum stress-induced programmed cell death in soybean cells. J Cell Sci 117, 2591-2598.
DOI PMID |
| [1] | Tiantian Zhi, Zhou Zhou, Chengyun Han, Chunmei Ren. PAD4 Mutation Accelerating Programmed Cell Death in Arabidopsis thaliana Tyrosine Degradation Deficient Mutant sscd1 [J]. Chinese Bulletin of Botany, 2022, 57(3): 288-298. |
| [2] | Tingting Shan,Xiaomei Chen,Shunxing Guo,Lixia Tian,Lin Yan,Xin Wang. Advances in Molecular Regulation of Sphingolipids in Plant-fungus Interactions [J]. Chinese Bulletin of Botany, 2019, 54(3): 396-404. |
| [3] | He Guangming, Deng Xingwang. Death Signal Transduction: Chloroplast-to-Mitochondrion Communication Regulates Programmed Cell Death in Plants [J]. Chinese Bulletin of Botany, 2018, 53(4): 441-444. |
| [4] | Zhao Xijuan, Qian Lichao, Liu Yule. Chinese Scientists Made Breakthrough Progresses in Plant Programmed Cell Death [J]. Chinese Bulletin of Botany, 2018, 53(4): 447-450. |
| [5] | Zhang Xian-sheng. Chinese Scientists Have Made a Great Breakthrough in the Mechanism of Programmed Cell Death [J]. Chinese Bulletin of Botany, 2018, 53(4): 445-446. |
| [6] | Xiao Huang, Faqiang Li. Roles of Autophagy in Plant Programmed Cell Death [J]. Chinese Bulletin of Botany, 2016, 51(6): 859-862. |
| [7] | Xinqiang He, Hong Wu. Mechanisms of Developmental Programmed Cell Death in Plants [J]. Chinese Bulletin of Botany, 2013, 48(4): 357-370. |
| [8] | Hongjuan Jing, Guangzhou Zhou, Xiaorong Tan, Kangkang Ping, Xuejian Ren. Research Progress in Regulation of Reactive Oxygen Species in Plant Autophagy [J]. Chinese Bulletin of Botany, 2012, 47(5): 534-542. |
| [9] | Cong Ma, Weiwen Kong. Research Progress in Plant Metacaspase [J]. Chinese Bulletin of Botany, 2012, 47(5): 543-549. |
| [10] | MA Huai-Yu, LÜ De-Guo, YANG Hong-Qiang. Characteristics of mitochondria and cell death in roots of Malus hupehensis var. pingyiensis under NaCl stress [J]. Chin J Plant Ecol, 2010, 34(12): 1448-1453. |
| [11] | LI Rong-Feng, CAI Miao-Zhen, LIU Peng, XU Gen-Di, CHEN Min-Yan, LIANG He. PHYTOECOLOGICAL EFFECT OF Al3+ ON THE INDUCTIVITY OF PROGRAMMED CELL DEATH OF BORDER CELLS IN SOYBEAN ROOT [J]. Chin J Plant Ecol, 2008, 32(3): 690-697. |
| [12] | Yuliang Chen;Feixiong Zhang;Guiyou Zhang*. Key Caspase-like Enzymes in Programmed Cell Death in Plants [J]. Chinese Bulletin of Botany, 2008, 25(05): 616-623. |
| [13] | Yu Kong;Zhong Wang*;Yunjie Gu;Yuexia Wang. Research Progress on Aerenchyma Formation in Plant Roots [J]. Chinese Bulletin of Botany, 2008, 25(02): 248-253. |
| [14] | . Developmental Programmed Cell Death in Plants [J]. Chinese Bulletin of Botany, 2005, 22(增刊): 22-28. |
| [15] | CHENG Hong-Yan SONG Song-Quan. Advances in Nitric Oxide Biology of Plants [J]. Chinese Bulletin of Botany, 2005, 22(06): 723-737. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||