

Chinese Bulletin of Botany ›› 2022, Vol. 57 ›› Issue (5): 649-660.DOI: 10.11983/CBB22049 cstr: 32102.14.CBB22049
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Zhang Hechen, Wang Huijuan, Li Yanmin, Gao Jie, Yuan Xin, Wang Limin, Wang Xiaochen, Zhao Yinge, Fu Zhenzhu*( )
)
Received:2022-03-12
Accepted:2022-06-23
Online:2022-09-01
Published:2022-09-09
Contact:
Fu Zhenzhu
About author:*E-mail: pearlgh2005@163.comZhang Hechen, Wang Huijuan, Li Yanmin, Gao Jie, Yuan Xin, Wang Limin, Wang Xiaochen, Zhao Yinge, Fu Zhenzhu. The Chemical Composition and Transcriptome Analysis Reveal the Mechanism of Color Formation in Rosa hybrida cv. ‘Double delight’[J]. Chinese Bulletin of Botany, 2022, 57(5): 649-660.
| Gene name | Pri- mers | Sequences (5′-3′) |
|---|---|---|
| CHI | F | GCAATACTCGGAGAAGGTTTCA |
| (LOC112182551) | R | CAATCACCGCATTTCCAAC |
| ANS | F | TAGAAGAAGGGAGGCTGGAG |
| (LOC112179310) | R | TGTGGAGGATGAAGGTGAGT |
| UFGT | F | TTGTAACACACTGCGGGTG |
| (LOC112172868) | R | GAACATCTCTGAGCATTCGTG |
| PSY | F | GCTGTTGCTCACCCATCAAG |
| (LOC112190337) | R | CAAACCTCACCACACCTATCG |
| LYCB | F | ACACAGACCCTTCCCTCCAA |
| (LOC112188432) | R | TGGTTCTTCCACAACGGTTT |
| ZDE | F | CCTGCCTGTCAATCTTGTAGAC |
| (LOC112189356) | R | TCCCACTATCACCACATCCTC |
| AN2-like1 | F | GCTGTAGACTGAGGTGGCTAAA |
| (LOC112185634) | R | GTGAAAGGACGATGGGCTA |
| AN2-like2 | F | GGACGAACTGGAAACGATG |
| (LOC112193894) | R | GTGATGCTTGTGTTGAGCG |
| AN2-like3 | F | GGAAGATGGCACAAGGTTC |
| (LOC112186121) | R | GCCGAGCACTCCAATAGTTT |
| UVR8 | F | GGCAGAGTTCTTTCTTGACAGAC |
| (LOC112182836) | R | GGCAATGCTGAGAGAGTTTCA |
| PIF3 | F | TGATGAGAAGATTGACCGAGG |
| (LOC112192990) | R | AGAAGACGGCGAAAGGCTA |
| HY5 | F | GGCATACTTGAGTGACTTGGAA |
| (LOC112172411) | R | CGGCTTGCTGTTGTGTTCT |
| GAPDH | F | TATGACCAGATCAAGGCTGCT |
| (JN399220) | R | ACCAATGAAGTCGGTTGACAC |
Table 1 The primers used for qRT-PCR
| Gene name | Pri- mers | Sequences (5′-3′) |
|---|---|---|
| CHI | F | GCAATACTCGGAGAAGGTTTCA |
| (LOC112182551) | R | CAATCACCGCATTTCCAAC |
| ANS | F | TAGAAGAAGGGAGGCTGGAG |
| (LOC112179310) | R | TGTGGAGGATGAAGGTGAGT |
| UFGT | F | TTGTAACACACTGCGGGTG |
| (LOC112172868) | R | GAACATCTCTGAGCATTCGTG |
| PSY | F | GCTGTTGCTCACCCATCAAG |
| (LOC112190337) | R | CAAACCTCACCACACCTATCG |
| LYCB | F | ACACAGACCCTTCCCTCCAA |
| (LOC112188432) | R | TGGTTCTTCCACAACGGTTT |
| ZDE | F | CCTGCCTGTCAATCTTGTAGAC |
| (LOC112189356) | R | TCCCACTATCACCACATCCTC |
| AN2-like1 | F | GCTGTAGACTGAGGTGGCTAAA |
| (LOC112185634) | R | GTGAAAGGACGATGGGCTA |
| AN2-like2 | F | GGACGAACTGGAAACGATG |
| (LOC112193894) | R | GTGATGCTTGTGTTGAGCG |
| AN2-like3 | F | GGAAGATGGCACAAGGTTC |
| (LOC112186121) | R | GCCGAGCACTCCAATAGTTT |
| UVR8 | F | GGCAGAGTTCTTTCTTGACAGAC |
| (LOC112182836) | R | GGCAATGCTGAGAGAGTTTCA |
| PIF3 | F | TGATGAGAAGATTGACCGAGG |
| (LOC112192990) | R | AGAAGACGGCGAAAGGCTA |
| HY5 | F | GGCATACTTGAGTGACTTGGAA |
| (LOC112172411) | R | CGGCTTGCTGTTGTGTTCT |
| GAPDH | F | TATGACCAGATCAAGGCTGCT |
| (JN399220) | R | ACCAATGAAGTCGGTTGACAC |
| No. | Type of carotenoid | Y1 (μg·g-1) | Y2 (μg·g-1) | R1 (μg·g-1) | R2 (μg·g-1) | Log2FC | Regulated |
|---|---|---|---|---|---|---|---|
| Carotenoid_22 | Neochrome palmitate | 2.2016998 | 2.3834704 | 1.0197834 | 1.0475690 | -1.1491910 | Down |
| Carotenoid_24 | Rubixanthin laurate | 0.1569250 | 0.1481073 | 0.0527210 | 0.0507537 | -1.5596845 | Down |
| Carotenoid_32 | Violaxanthin dilaurate | 3.9240426 | 4.7154605 | 2.9313100 | 2.8637581 | -0.5761227 | Unchanged |
| Carotenoid_33 | Violaxanthin-myristate-caprate | 21.7732951 | 22.4958882 | 12.8019824 | 12.7492289 | -0.7929111 | Unchanged |
| Carotenoid_34 | Violaxanthin-myristate-laurate | 5.6980954 | 6.7643503 | 4.7010832 | 5.0801542 | -0.3494983 | Unchanged |
| Carotenoid_39 | Violaxanthin dioleate | 0.7922281 | 0.8738322 | 0.4032495 | 0.3662500 | -1.11444831 | Down |
| Carotenoid_41 | Zeaxanthin palmitate | 0.1189156 | 0.1116267 | 0.3534273 | 0.3530580 | 1.61562841 | Up |
| Carotenoid_51 | β-cryptoxanthin laurate | 0.7270018 | 0.7903577 | 0.3395545 | 0.3316843 | -1.1766652 | Down |
| Carotenoid_54 | β-cryptoxanthin oleate | 0.1793999 | 0.1468413 | 0.0389599 | 0.0467119 | -1.9290470 | Down |
| Carotenoid_56 | Zeaxanthin | 5.9183084 | 6.1692434 | 17.8138771 | 17.0472808 | 1.5280984 | Up |
| Carotenoid_57 | Violaxanthin | 4.9150727 | 4.8171053 | 3.8840384 | 3.8675731 | -0.3282664 | Unchanged |
| Carotenoid_58 | Neoxanthin | 2.0484948 | 2.2917558 | 1.9106499 | 1.9435004 | -0.1713655 | Unchanged |
| Carotenoid_59 | Lutein | 7.8872005 | 8.3871299 | 18.9305539 | 18.6268263 | 1.2064982 | Up |
| Carotenoid_66 | Canthaxanthin | 0.0005338 | 0.0004385 | 0 | 0 | -Inf | Down |
Table 2 Differential carotenoid components in different colored petals of Rosa hybrida cv. ‘Double delight’
| No. | Type of carotenoid | Y1 (μg·g-1) | Y2 (μg·g-1) | R1 (μg·g-1) | R2 (μg·g-1) | Log2FC | Regulated |
|---|---|---|---|---|---|---|---|
| Carotenoid_22 | Neochrome palmitate | 2.2016998 | 2.3834704 | 1.0197834 | 1.0475690 | -1.1491910 | Down |
| Carotenoid_24 | Rubixanthin laurate | 0.1569250 | 0.1481073 | 0.0527210 | 0.0507537 | -1.5596845 | Down |
| Carotenoid_32 | Violaxanthin dilaurate | 3.9240426 | 4.7154605 | 2.9313100 | 2.8637581 | -0.5761227 | Unchanged |
| Carotenoid_33 | Violaxanthin-myristate-caprate | 21.7732951 | 22.4958882 | 12.8019824 | 12.7492289 | -0.7929111 | Unchanged |
| Carotenoid_34 | Violaxanthin-myristate-laurate | 5.6980954 | 6.7643503 | 4.7010832 | 5.0801542 | -0.3494983 | Unchanged |
| Carotenoid_39 | Violaxanthin dioleate | 0.7922281 | 0.8738322 | 0.4032495 | 0.3662500 | -1.11444831 | Down |
| Carotenoid_41 | Zeaxanthin palmitate | 0.1189156 | 0.1116267 | 0.3534273 | 0.3530580 | 1.61562841 | Up |
| Carotenoid_51 | β-cryptoxanthin laurate | 0.7270018 | 0.7903577 | 0.3395545 | 0.3316843 | -1.1766652 | Down |
| Carotenoid_54 | β-cryptoxanthin oleate | 0.1793999 | 0.1468413 | 0.0389599 | 0.0467119 | -1.9290470 | Down |
| Carotenoid_56 | Zeaxanthin | 5.9183084 | 6.1692434 | 17.8138771 | 17.0472808 | 1.5280984 | Up |
| Carotenoid_57 | Violaxanthin | 4.9150727 | 4.8171053 | 3.8840384 | 3.8675731 | -0.3282664 | Unchanged |
| Carotenoid_58 | Neoxanthin | 2.0484948 | 2.2917558 | 1.9106499 | 1.9435004 | -0.1713655 | Unchanged |
| Carotenoid_59 | Lutein | 7.8872005 | 8.3871299 | 18.9305539 | 18.6268263 | 1.2064982 | Up |
| Carotenoid_66 | Canthaxanthin | 0.0005338 | 0.0004385 | 0 | 0 | -Inf | Down |
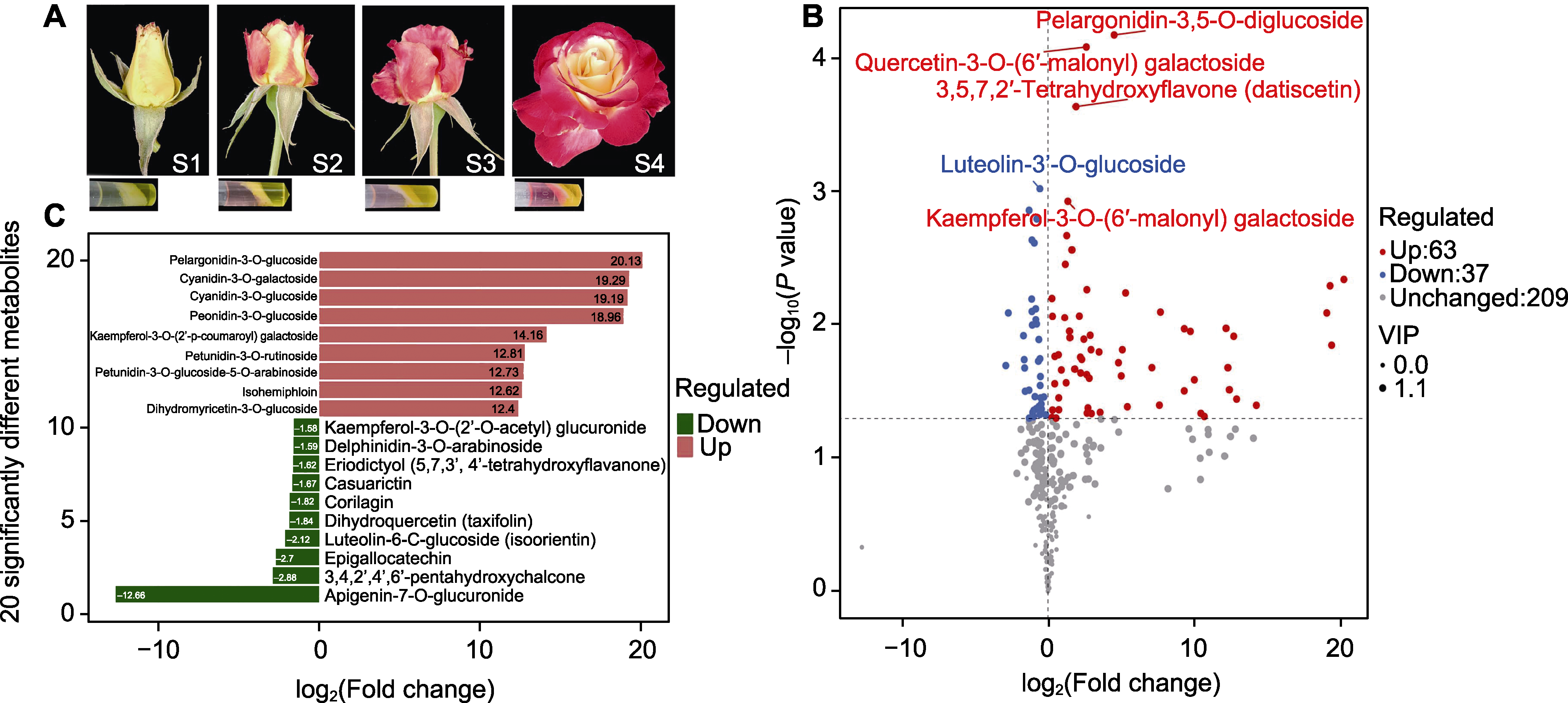
Figure 1 The phenotype of the petal coloration (A), the difference of flavonoids (B), and the main different components (C) in petals of Rosa hybrida cv. ‘Double delight’ S1: The development stage when sepals are initially unfolded; S2: The development stage when petals are being colored; S3: The development stage when petals are just opened; S4: The development stage when petals are in full bloom
| Type | Total transcripts | Differentially expressed transcripts | Up regu- lated | Down regu- lated |
|---|---|---|---|---|
| mRNA | 36193 | 2250 | 1371 | 879 |
| miRNA | 7845 | 22 | 21 | 1 |
| LncRNA | 146605 | 51 | 24 | 27 |
Table 3 Transcripts of mRNA, miRNA and LncRNA in different colored petals of Rosa hybrida cv. ‘Double delight’
| Type | Total transcripts | Differentially expressed transcripts | Up regu- lated | Down regu- lated |
|---|---|---|---|---|
| mRNA | 36193 | 2250 | 1371 | 879 |
| miRNA | 7845 | 22 | 21 | 1 |
| LncRNA | 146605 | 51 | 24 | 27 |
| Comparison set | Total DEG | COG | GO | KEGG | KOG | NR | Pfam | Swiss-Prot | eggNOG |
|---|---|---|---|---|---|---|---|---|---|
| Y_vs_R | 2177 | 965 | 1904 | 1596 | 1034 | 2177 | 1923 | 1733 | 1858 |
Table 4 Functional annotation of differentially expressed genes in different colored petals of Rosa hybrida cv. ‘Double delight’
| Comparison set | Total DEG | COG | GO | KEGG | KOG | NR | Pfam | Swiss-Prot | eggNOG |
|---|---|---|---|---|---|---|---|---|---|
| Y_vs_R | 2177 | 965 | 1904 | 1596 | 1034 | 2177 | 1923 | 1733 | 1858 |
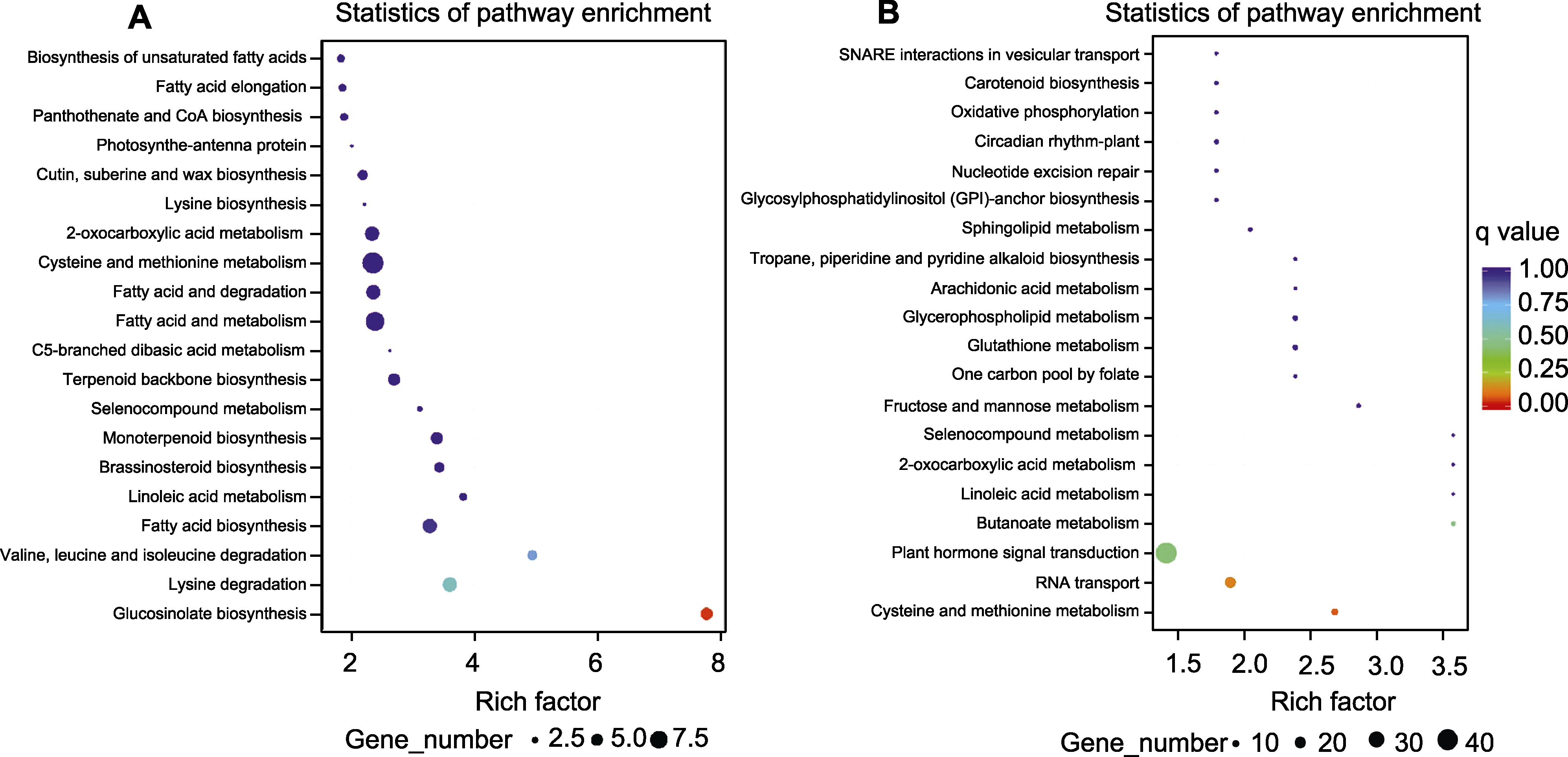
Figure 2 Analysis of differential LncRNA target genes (A) and siRNA target genes (B) clustering in different colored petals of Rosa hybrida cv. ‘Double delight’
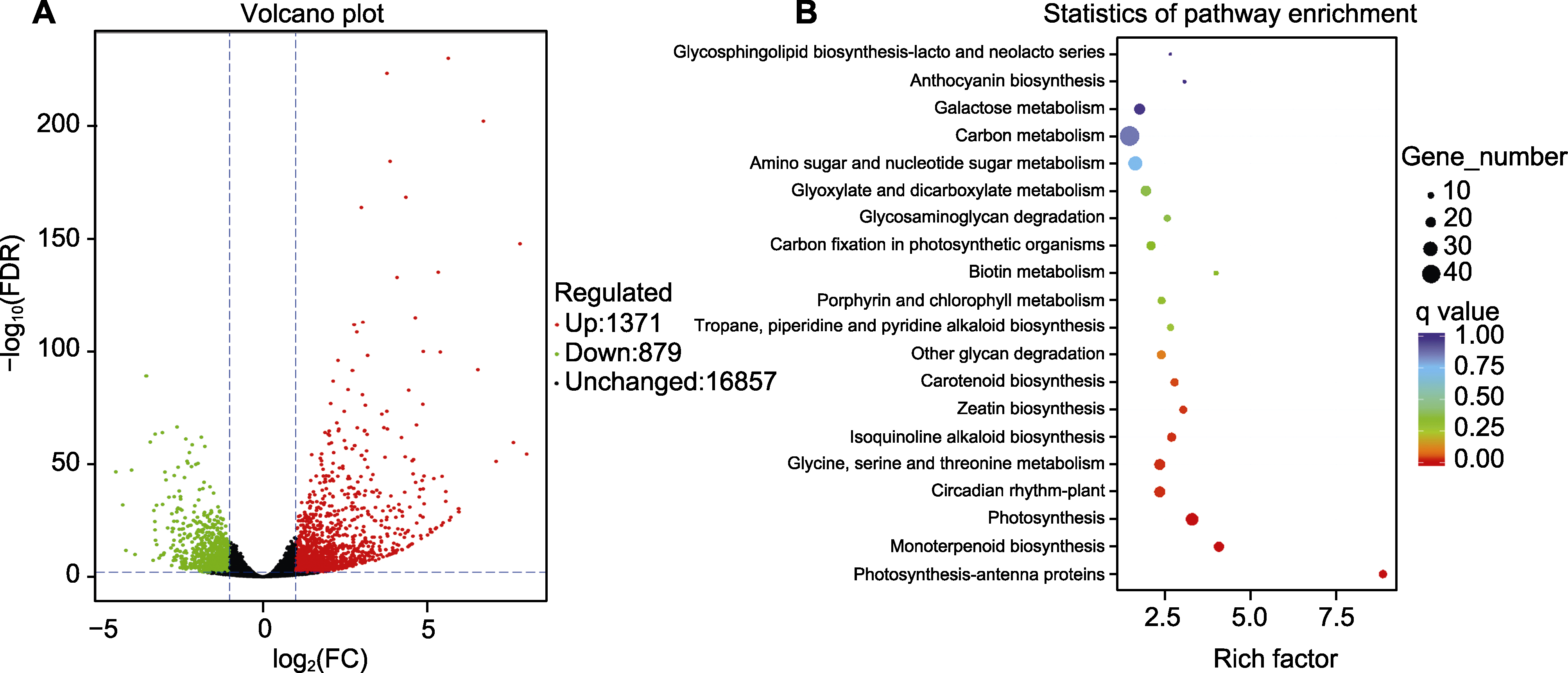
Figure 3 Characteristics of gene expression (A) and KEGG clustering of DEGs (B) in different colored petals of Rosa hybrida cv. ‘Double delight’ FDR: False discovery rate
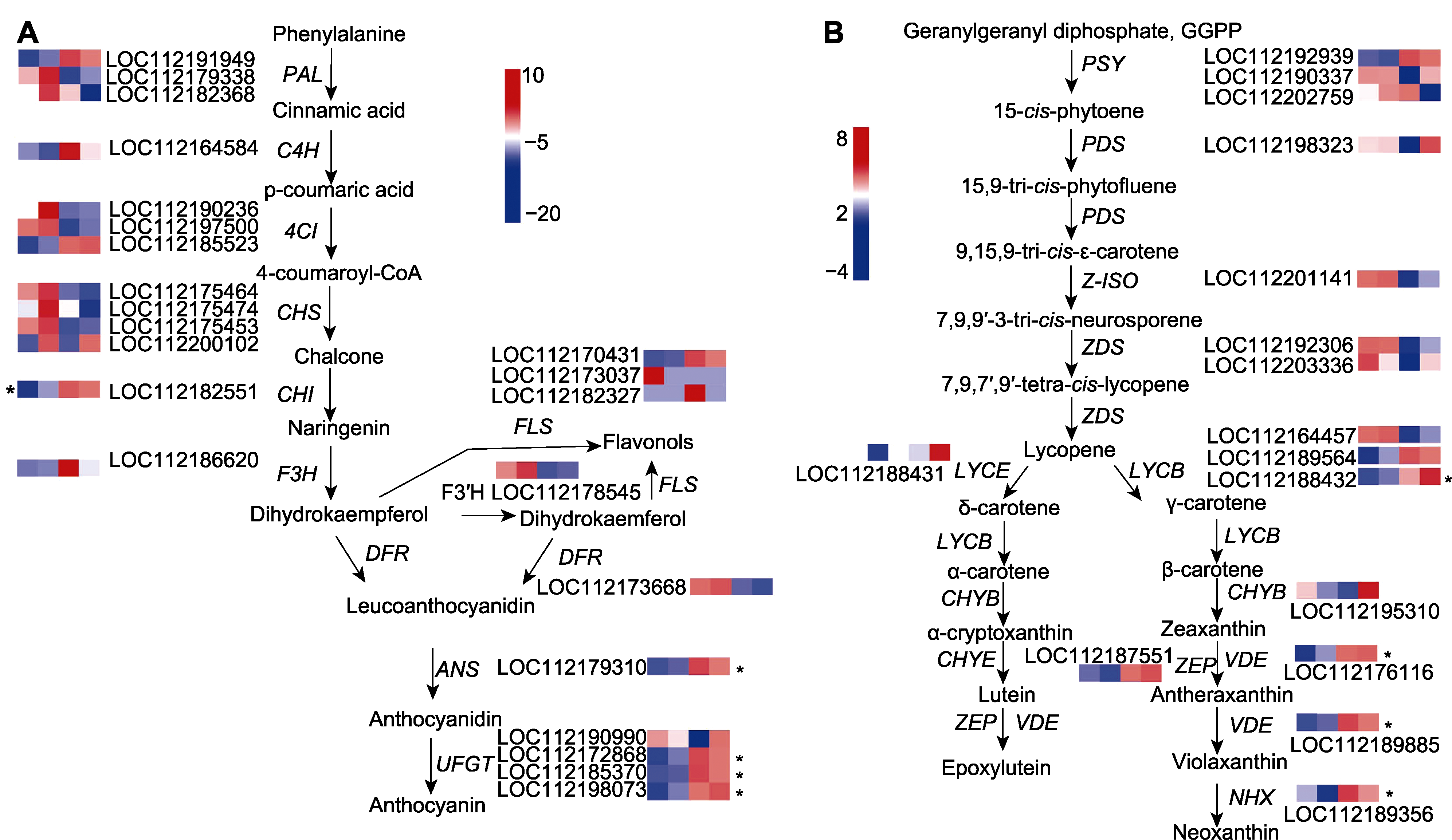
Figure 4 Expression pattern analysis of anthocyanin and carotenoid synthesis-related genes in the petals of Rosa hybrida cv. ‘Double delight’ The blue to red color represents gene expression from weak to strong (the color version is shown in online). *represent genes with significant difference.
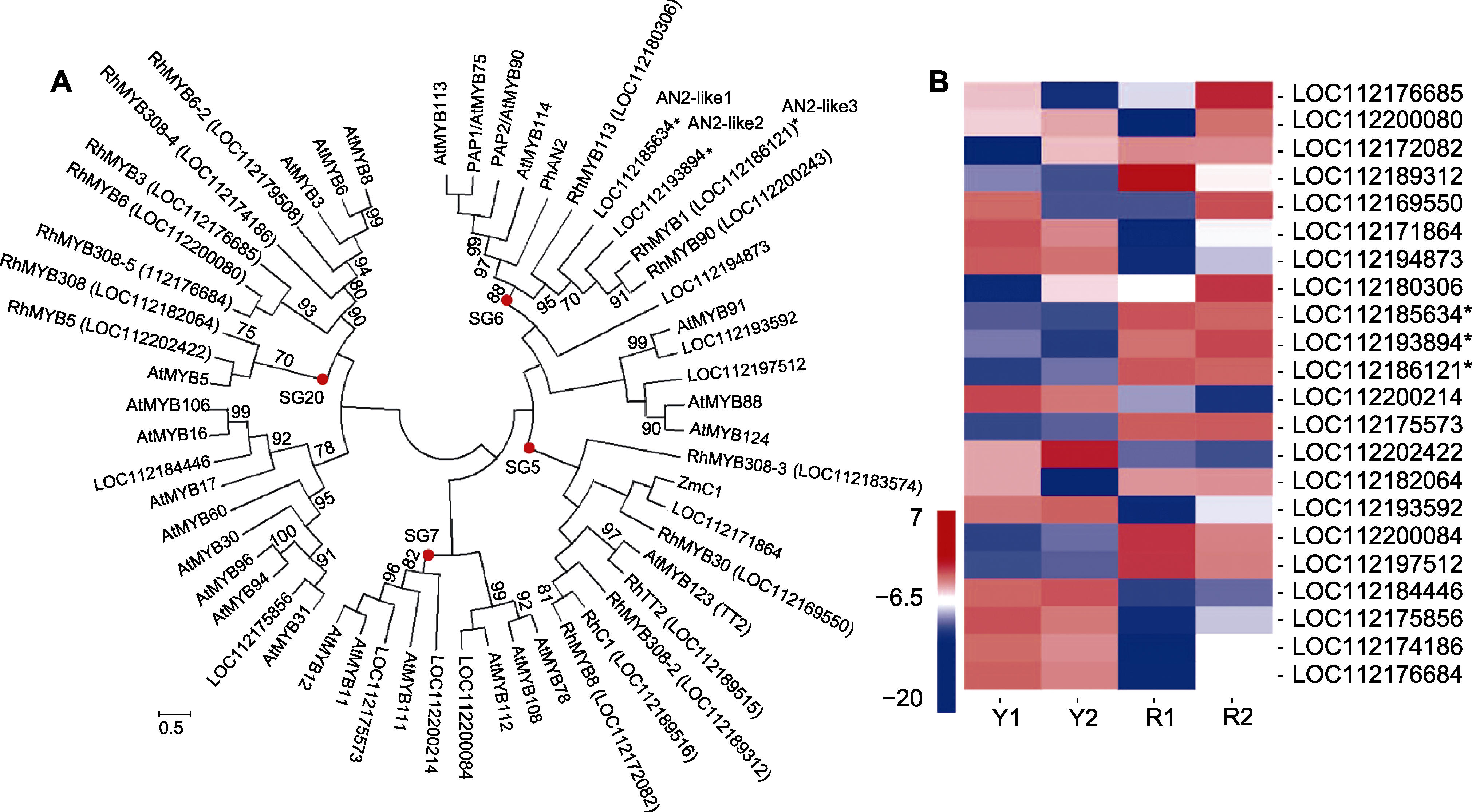
Figure 5 Phylogenic analysis of differentially expressed R2R3-MYB and flavonoid regulation related genes (A), and heat map of the corresponding R2R3-MYB members in different colored petals of Rosa hybrida cv. ‘Double delight’ (B) Y and R see Table 2. * represent R2R3-MYB genes related to the regulation of anthocyanin biosynthesis.
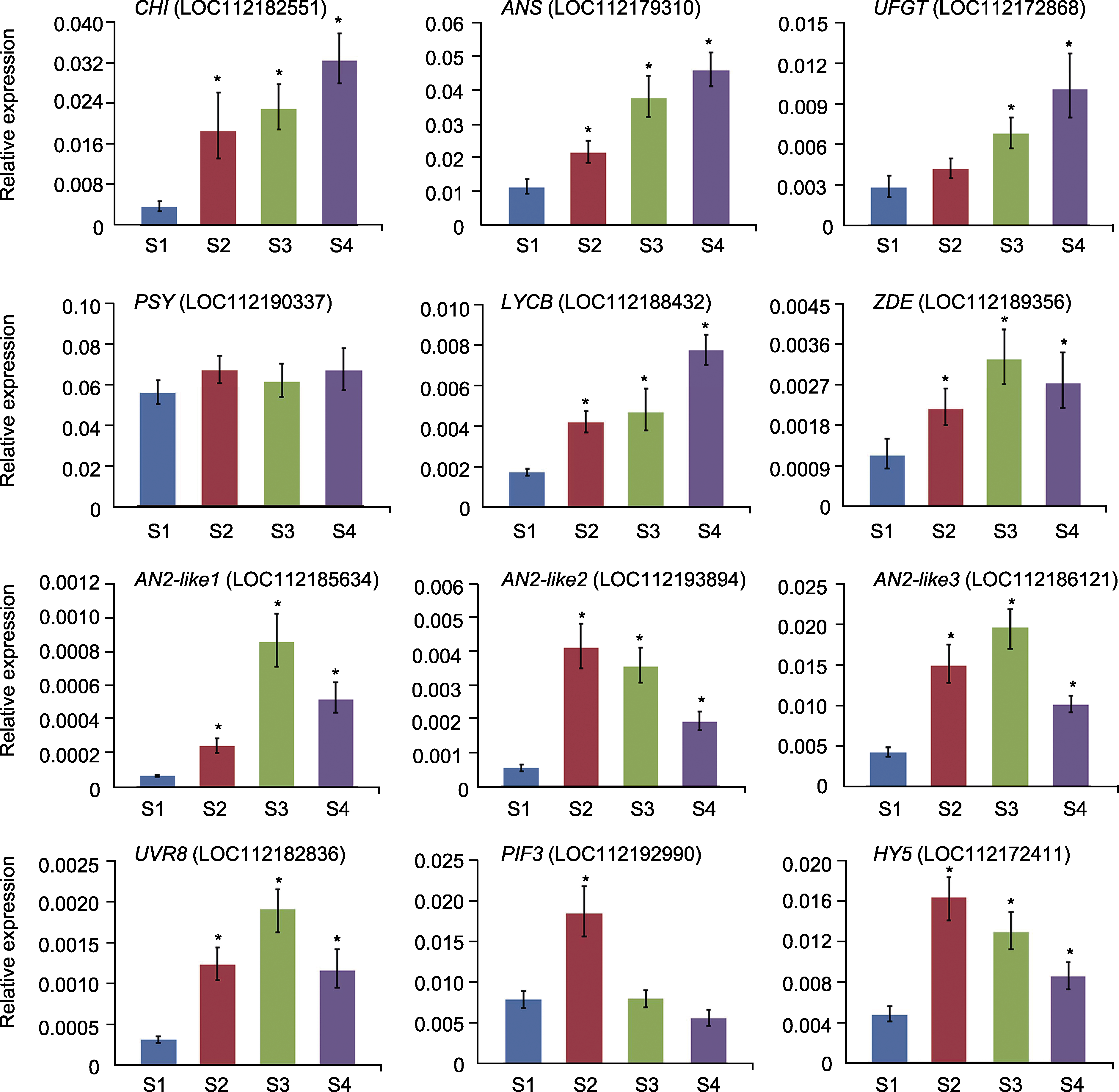
Figure 6 qRT-PCR analysis of the expression pattern of the corresponding genes in the Rosa hybrida cv. ‘Double delight’ petals at different developmental stages S1-S4 see Figure 1. * mean significant differences compared with S1 stage.
| [1] | 李茂福, 杨媛, 王华, 范又维, 孙佩, 金万梅 (2022). 月季中 R2R3-MYB基因RhMYB113c调控花青素苷合成. 园艺学报 49, 1957-1966. |
| [2] | 王峰, 杨树华, 刘新艳, 崔娇鹏, 常智慧, 葛红 (2017). 月季种质资源花色多样性及其与花青苷的关系. 园艺学报 44, 1125-1134. |
| [3] | 温佳辛, 王超林, 冯慧, 李珊珊, 王亮生, 武荣花, 赵世伟 (2021). 月季花色研究进展. 园艺学报 48, 2044-2056. |
| [4] | 张泰然, 张和臣, 武荣花 (2020). 蓝色花形成分子机理研究进展. 植物学报 55, 216-227. |
| [5] | 朱满兰, 王亮生, 张会金, 徐彦军, 郑绪辰, 王丽金 (2012). 耐寒睡莲花瓣中花青素苷组成及其与花色的关系. 植物学报 47, 437-453. |
| [6] | 邹红竹, 周琳, 韩璐璐, 吕纪杭, 王雁 (2021). 滇牡丹花瓣着色过程中类胡萝卜素成分变化和相关基因表达分析. 园艺学报 48, 1934-1944. |
| [7] | Bradley D, Xu P, Mohorianu II, Whibley A, Field D, Tavares H, Couchman M, Copsey L, Carpenter R, Li MM, Li Q, Xue YB, Dalmay T, Coen E (2017). Evolution of flower color pattern through selection on regulatory small RNAs. Science 358, 925-928. |
| [8] | Fu ZZ, Jiang H, Chao YC, Dong XY, Yuan X, Wang LM, Zhang J, Xu ML, Wang HJ, Li YM, Gao J, Zhang HC (2021). Three paralogous R2R3-MYB genes contribute to delphinidin-related anthocyanins synthesis in Petunia hybrida. J Plant Growth Regul 40, 1687-1700. |
| [9] | Fu ZZ, Shang HQ, Jiang H, Gao J, Dong XY, Wang HJ, Li YM, Wang LM, Zhang J, Shu QY, Chao YC, Xu ML, Wang R, Wang LS, Zhang HC (2020). Systematic identification of the light-quality responding anthocyanin synthesis-related transcripts in Petunia petals. Hortic Plant J 6, 428-438. |
| [10] | González-Villagra J, Kurepin LV, Reyes-Díaz MM (2017). Evaluating the involvement and interaction of abscisic acid and miRNA156 in the induction of anthocyanin biosynthesis in drought-stressed plants. Planta 246, 299-312. |
| [11] | Grotewold E (2006). The genetics and biochemistry of floral pigments. Annu Rev Plant Biol 57, 761-780. |
| [12] | Katsumoto Y, Fukuchi-Mizutani M, Fukui Y, Brugliera F, Holton TA, Karan M, Nakamura N, Yonekura-Sakakibara K, Togami J, Pigeaire A, Tao GQ, Nehra NS, Lu CY, Dyson BK, Tsuda S, Ashikari T, Kusumi T, Mason JG, Tanaka Y (2007). Engineering of the rose flavonoid biosynthetic pathway successfully generated blue-hued flowers accumulating delphinidin. Plant Cell Physiol 48, 1589-1600. |
| [13] | Klie M, Debener T (2011). Identification of superior reference genes for data normalisation of expression studies via quantitative PCR in hybrid roses (Rosa hybrida). BMC Res Notes 4, 518. |
| [14] | Koes R, Verweij W, Quattrocchio F (2005). Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci 10, 236-242. |
| [15] | Li ZJ, Zhao MY, Jin JF, Zhao LY, Xu ZD (2018). Anthocyanins and their biosynthetic genes in three novel-colored Rosa rugosa cultivars and their parents. Plant Physiol Biochem 129, 421-428. |
| [16] | Liu CC, Chi C, Jin LJ, Zhu JH, Yu JQ, Zhou YH (2018). The bZip transcription factor HY5 mediates CRY1a-induced anthocyanin biosynthesis in tomato. Plant Cell Environ 41, 1762-1775. |
| [17] | Liu ZJ, Zhang YQ, Wang JF, Li P, Zhao CZ, Chen YD, Bi YR (2015). Phytochrome-interacting factors PIF4 and PIF5 negatively regulate anthocyanin biosynthesis under red light in Arabidopsis seedlings. Plant Sci 238, 64-72. |
| [18] | Llorente B, Martinez-Garcia JF, Stange C, Rodriguez- Concepcion M (2017). Illuminating colors: regulation of carotenoid biosynthesis and accumulation by light. Curr Opin Plant Biol 37, 49-55. |
| [19] | Moehs CP, Tian L, Osteryoung KW, DellaPenna D (2001). Analysis of carotenoid biosynthetic gene expression during marigold petal development. Plant Mol Biol 45, 281-293. |
| [20] | Podolec R, Ulm R (2018). Photoreceptor-mediated regulation of the COP1/SPA E3 ubiquitin ligase. Curr Opin Plant Biol 45, 18-25. |
| [21] | Provenzano S, Spelt C, Hosokawa S, Nakamura N, Brugliera F, Demelis L, Geerke DP, Schubert A, Tanaka Y, Quattrocchio F, Koes R (2014). Genetic control and evolution of anthocyanin methylation. Plant Physiol 165, 962-977. |
| [22] | Sasaki N, Nakayama T (2015). Achievements and perspectives in biochemistry concerning anthocyanin modification for blue flower coloration. Plant Cell Physiol 56, 28-40. |
| [23] | Sui X, Zhao MY, Han X, Zhao LY, Xu ZD (2019). RrGT1, a key gene associated with anthocyanin biosynthesis, was isolated from Rosa rugosa and identified via overexpression and VIGS. Plant Physiol Biochem 135, 19-29. |
| [24] | Tanaka Y, Ohmiya A (2008). Seeing is believing: engineering anthocyanin and carotenoid biosynthetic pathways. Curr Opin Biotechnol 19, 190-197. |
| [25] | Tu ZH, Xia H, Yang LC, Zhai XY, Shen YF, Li HG (2022). The roles of microRNA-long non-coding RNA-mRNA networks in the regulation of leaf and flower development in Liriodendron chinense. Front Plant Sci 13, 816875. |
| [26] | Watkins JL, Pogson BJ (2020). Prospects for carotenoid biofortification targeting retention and catabolism. Trends Plant Sci 25, 501-512. |
| [27] | Xu WJ, Dubos C, Lepiniec L (2015). Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci 20, 176-185. |
| [28] | Yamagishi M (2011). Oriental hybrid lily Sorbonne homo-logue of LhMYB12regulates anthocyanin biosyntheses in flower tepals and tepal spots. Mol Breed 28, 381-389. |
| [29] | Zhang HC, Koes R, Shang HQ, Fu ZZ, Wang LM, Dong XY, Zhang J, Passeri V, Li YB, Jiang H, Gao J, Li YM, Wang HJ, Quattrocchio FM (2019). Identification and functional analysis of three new anthocyanin R2R3-MYB genes in Petunia. Plant Direct 3, e00114. |
| [30] | Zimmermann IM, Heim MA, Weisshaar B, Uhrig JF (2004). Comprehensive identification of Arabidopsis thaliana MYB transcription factors interacting with R/B-like BHLH proteins. Plant J 40, 22-34. |
| [1] | Yi Song, Hanghang Chen, Xin Cui, Zhifeng Lu, Shipeng Liao, Yangyang Zhang, Xiaokun Li, Rihuan Cong, Tao Ren, Jianwei Lu. Potassium Nutrient Status-mediated Leaf Growth of Oilseed Rape (Brassica napus) and Its Effect on Phyllosphere Microorganism [J]. Chinese Bulletin of Botany, 2024, 59(1): 54-65. |
| [2] | Ziwen Tang, Dongping Zhang. Research Progress on the Molecular Mechanism of Starch Accumulation in Rice Endosperm [J]. Chinese Bulletin of Botany, 2023, 58(4): 612-621. |
| [3] | Xiaoqing Yang,Xiaoqin Huang,Xiaoyang Han,Tengfei Liu,Xiaowei Yue,Ran Yi. Effect of Exogenous Substances on Cold Tolerance and Key Sucrose Metabolic Gene Expression in Camellia sinensis [J]. Chinese Bulletin of Botany, 2020, 55(1): 21-30. |
| [4] | Shuhui Zhang,Hong Wang,Wenru Wang,Xuelian Wu,Yuansong Xiao,Futian Peng. Effects of Sucrose on Seedling Growth and Development and SnRK1 Activity in Prunus persica [J]. Chinese Bulletin of Botany, 2019, 54(6): 744-752. |
| [5] | Xuewei Song,Jiebing Wei,Shaokang Di,Yongzhen Pang. Recent Advances in the Regulation Mechanism of Transcription Factors and Metabolic Engineering of Anthocyanins [J]. Chinese Bulletin of Botany, 2019, 54(1): 133-156. |
| [6] | Shang Su, Lijin Wang, Jie Wu, Bing Li, Weiwei Wang, Liangsheng Wang. Review: Chemical Compositions and Functions of Vaccinium uliginosum [J]. Chinese Bulletin of Botany, 2016, 51(5): 691-704. |
| [7] | Yuliang Jiang, Kundong Bai, Yili Guo, Bin Wang, Dongxing Li, Xiankun Li, Zhishang Liu. Floral traits of woody plants and their habitat differentiations in a northern tropical karst forest [J]. Biodiv Sci, 2016, 24(2): 148-156. |
| [8] | Zhixin Zhu, Yingqing Lu. Plant Color Mutants and the Anthocyanin Pathway [J]. Chinese Bulletin of Botany, 2016, 51(1): 107-119. |
| [9] | Kui Lin, Yong Xu. Effect of LED Illumination on the Accumulation of Functional Chemicals in Plants [J]. Chinese Bulletin of Botany, 2015, 50(2): 263-271. |
| [10] | Yi Zhang, Dabing Zhang, Man Liu. The Molecular Mechanism of Long-distance Sugar Transport in Plants [J]. Chinese Bulletin of Botany, 2015, 50(1): 107-121. |
| [11] | Manlan Zhu, Liangsheng Wang, Huijin Zhang, Yanjun Xu, Xuchen Zheng, Lijin Wang. Relationship Between the Composition of Anthocyanins and Flower Color Variation in Hardy Water Lily (Nymphaea spp.) Cultivars [J]. Chinese Bulletin of Botany, 2012, 47(5): 437-453. |
| [12] | Xue Chen, Jinzhu Zhang, Bingbing Pan, Chengjin Sang, Xue Ma, Tao Yang, Daidi Che. Callus Induction and Plant Regeneration of Rose [J]. Chinese Bulletin of Botany, 2011, 46(5): 569-574. |
| [13] | WANG Wei, CAI Yi-Xia, YANG Jian-Chang, ZHU Qing-Sen. Effects of soil water deficit on physiological causes of rice grain-filling [J]. Chin J Plant Ecol, 2011, 35(2): 195-202. |
| [14] | Qiliang Jian, Longying Wen, Tuo Chen, Manxiao Zhang, Shijian Xu. Seasonal Changes in the Contents of Pigments and Anthocyanins Synthetase Activity of Sabina przewalskii and Sabina chinensis [J]. Chinese Bulletin of Botany, 2010, 45(06): 698-704. |
| [15] | Wei Sun;Chonghui Li;Liangsheng Wang;Silan Dai*. Analysis of Anthocyanins and Flavones in Different-colored Flowers of Chrysanthemum [J]. Chinese Bulletin of Botany, 2010, 45(03): 327-336. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||