

Chinese Bulletin of Botany ›› 2021, Vol. 56 ›› Issue (1): 56-61.DOI: 10.11983/CBB20095 cstr: 32102.14.CBB20095
• TECHNIQUES AND METHODS • Previous Articles Next Articles
Zhengjun Xia1,*( ), Yuzhuo Li1,2, Jinlong Zhu1, Hongyan Wu1, Kun Xu1, Hong Zhai1
), Yuzhuo Li1,2, Jinlong Zhu1, Hongyan Wu1, Kun Xu1, Hong Zhai1
Received:2020-05-26
Accepted:2020-10-05
Online:2021-01-01
Published:2021-01-15
Contact:
Zhengjun Xia
Zhengjun Xia, Yuzhuo Li, Jinlong Zhu, Hongyan Wu, Kun Xu, Hong Zhai. A Rapid, Non-destructive and Continuous Sampling Technique and DNA Extraction for Soybean Seed[J]. Chinese Bulletin of Botany, 2021, 56(1): 56-61.
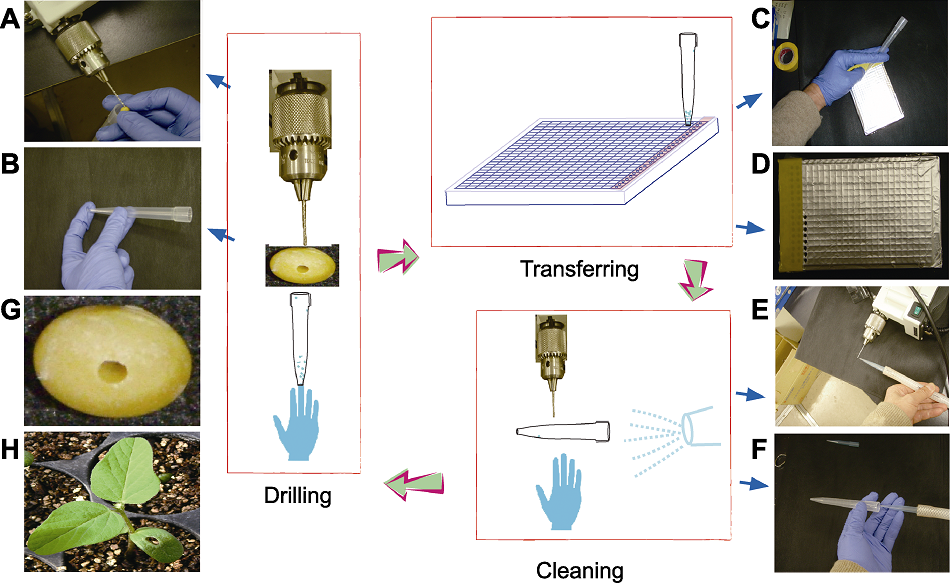
Figure 1 Procedure for seed drilling and sample collection (A) Drilling of seed tissue; (B) Dilled tissue was collected by a pipette tip; (C) Transferring into a 384 deep-well plate; (D) When all wells in a row of the 384 deep-well plate were filled, the row will be sealed with a sticky tape; (E) Cleaning of the driller; (F) Cleaning of the tip and gloves; (G) Appearance of drilled seed; (H) Seedling of drilled seed (14 days after being sown). In the middle panel, the whole procedure is shown, in which seed sampling is performed by drilling, transferring and cleaning before proceeding to the next one.
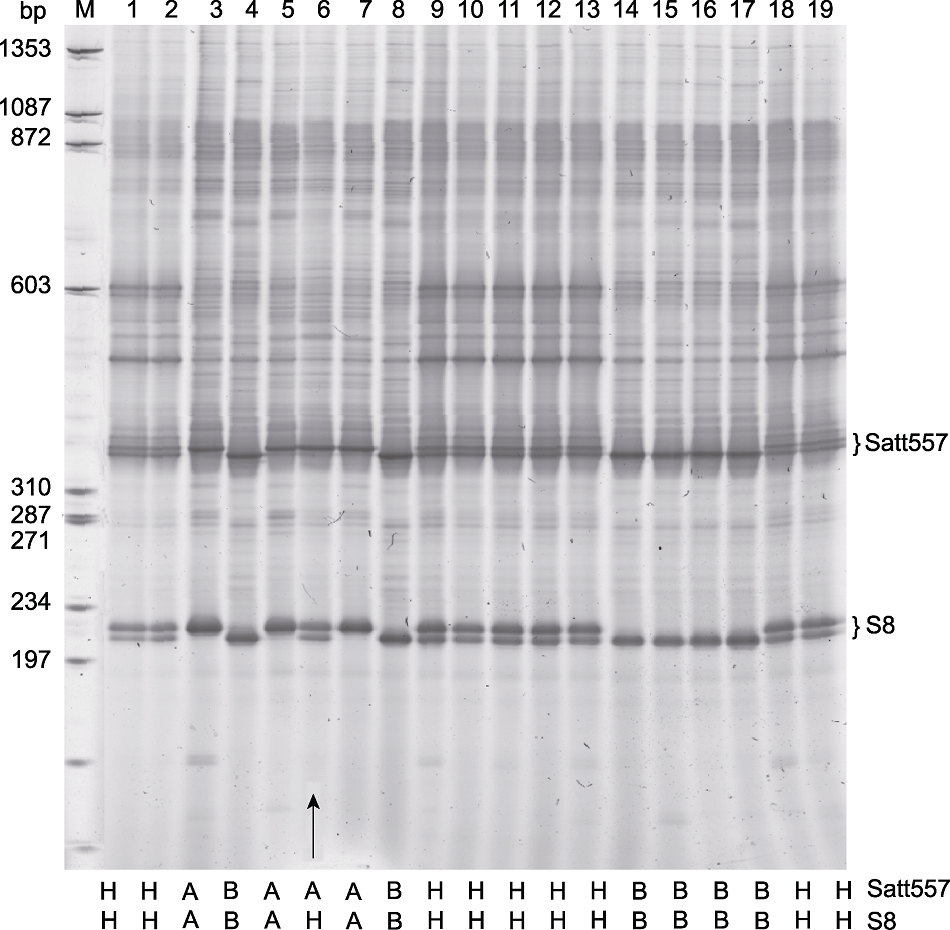
Figure 2 Genotyping of a soybean genetic population through seed-drilling and thereafter DNA extraction Accurate identification of recombinant occuring between two markers, Satt557 and S8, using one tube PCR (arrow). M: Molecular weight marker ΦX174 HaeIII; 1-19: F2 population that were derived from Harosoy-E1 × Harosoy (e1) (the genotypes of each individual were indicated at the bottom of each lane).
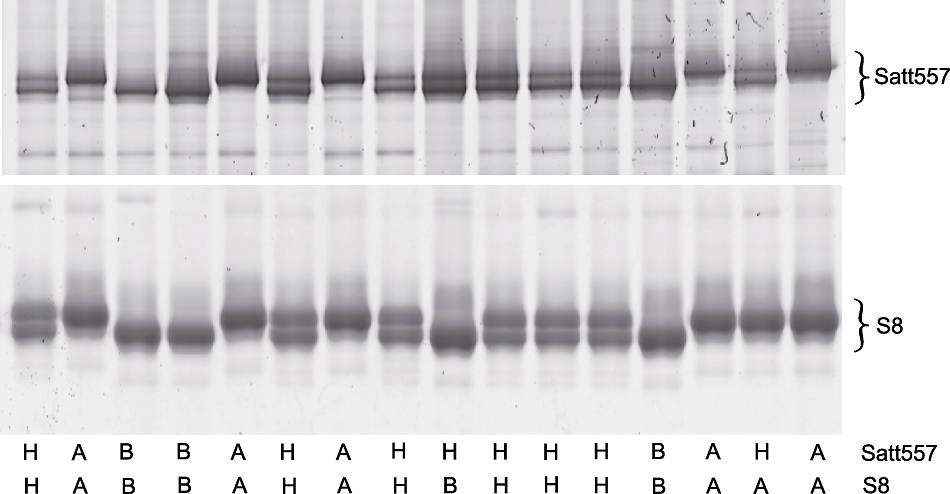
Figure 3 Verification of genotyping results obtained through seed-drilling DNA were extracted from leaves of the plants that were developed from the drilled seeds, thereafter genotyping of Satt557 and S8 were performed to verify the accuracy of genotyping data of seed. The upper and the bottom gels are shown the genotyping result of Satt557 and S8, respectively (the genotypes of each individual were indicated at the bottom of each lane).
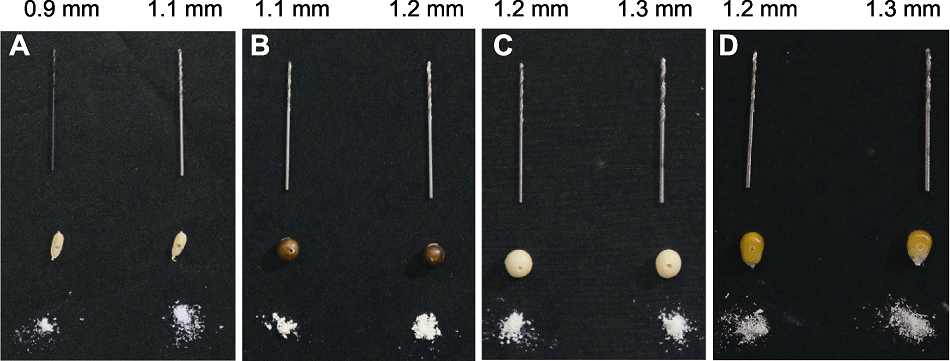
Figure 4 The seed dimensions of rice, soybean and maize and the selection of their appropriate drillers (A) Rice seed (average drilling depth is (1.52±0.12) mm; average acquired tissue weight is (1.54±0.12) mg for the 0.9 mm diameter driller; average acquired tissue weight is (2.30±0.18) mg for the 1.1 mm diameter driller). (B) Soybean seed (small size) (average drilling depth is (3.88±0.37) mm; average acquired tissue weight is (5.85±0.99) mg for the 1.1 mm diameter driller; average acquired tissue weight is (6.96±0.66) mg for the 1.2 mm diameter driller). (C) Soybean seed (large size) (average drilling depth is (4.63±0.43) mm; average acquired tissue weight is (8.31±0.79) mg for the 1.2 mm diameter driller; average acquired tissue weight is (9.75±0.92) mg for the 1.3 mm diameter driller). (D) Maize seed (average drilling depth is (3.78±0.46) mm; average acquired tissue weight is (6.79±0.83) mg for the 1.2 mm diameter driller; average acquired tissue weight is (7.97±0.98) mg for the 1.3 mm diameter driller.
| [1] | 程文, 夏正俊, 冯献忠, 杨素欣 (2016). 一种快速、无损大豆种子DNA提取方法的建立和应用. 植物学报 51, 68-73. |
| [2] | 薛勇彪, 种康, 韩斌, 桂建芳, 王台, 傅向东, 何祖华, 储成才, 田志喜, 程祝宽, 林少扬 (2015). 开启中国设计育种新篇章——“分子模块设计育种创新体系”战略性先导科技专项进展. 中国科学院院刊 30, 393-402. |
| [3] | Kamiya M, Kiguchi T (2003). Rapid DNA extraction method from soybean seeds. Breed Sci 53, 277-279. |
| [4] |
King Z, Serrano J, Boerma HR, Li ZL (2014). Non-toxic and efficient DNA extractions for soybean leaf and seed chips for high-throughput and large-scale genotyping. Biotechnol Lett 36, 1875-1879.
URL PMID |
| [5] | McCarthy PL, Hansen JL, Zemetra RS, Berger PH (2002). Rapid identification of transformed wheat using a half- seed PCR assay. Biotechniques 32, 560-564. |
| [6] | von Post R, von Post L, Dayteg C, Nilsson M, Forster BP, Tuvesson S (2003). A high-throughput DNA extraction method for barley seed. Euphytica 130, 255-260. |
| [7] |
Xia ZJ, Tsubokura Y, Hoshi M, Hanawa M, Yano C, Okamura K, Ahmed TA, Anai T, Watanabe S, Hayashi M, Kawai T, Hossain KG, Masaki H, Asai K, Yamanaka N, Kubo N, Kadowaki K, Nagamura Y, Yano M, Sasaki T, Harada K (2007). An integrated high-density linkage map of soybean with RFLP, SSR, STS, and AFLP markers using a single F2 population. DNA Res 14, 257-269.
DOI URL PMID |
| [8] |
Xia ZJ, Watanabe S, Yamada T, Tsubokura Y, Nakashima H, Zhai H, Anai T, Sato S, Yamazaki T, Lü SX, Wu HY, Tabata S, Harada K (2012). Positional cloning and characterization reveal the molecular basis for soybean maturity locus E1 that regulates photoperiodic flowering. Proc Natl Acad Sci USA 109, E2155-E2164.
DOI URL PMID |
| [1] | Zhou xin-yu, huiliang liu, GAO Bei, LU Yuting, TAO Lingqing, WEN Xiaohu, ZHANG Lan, ZHANG Yuan-Ming. Reproductive Biology of the Endangered and Endemic Species Nymphaea candida C. Presl in Xinjiang [J]. , 2025, 49(濒危植物的保护与恢复): 0-. |
| [2] | SHANGGUAN Yao-Yao, SU Shi-Ping, GU Xue-Dan, ZHANG Zheng-Zhong, ZHAO Hu, LI Yi, WEI Xing-Yu. Response of Reaumuria songorica seedlings to photoperiod and light quality ratio [J]. Chin J Plant Ecol, 2025, 49(5): 788-800. |
| [3] | LI Xin-Yi, ZHANG Li-Fang, WU You-Gui, GUO Jing, LAN Rong-Guang, LÜ Hong-Fei, YU Ming-Jian. Growth characteristics of Abies beshanzuensis seedlings at different altitudes and the influencing factors [J]. Chin J Plant Ecol, 2025, 49(4): 610-623. |
| [4] | Zhao Ling, Guan Ju, Liang Wenhua, Zhang Yong, Lu Kai, Zhao Chunfang, Li Yusheng, Zhang Yadong. Mapping of QTLs for Heat Tolerance at the Seedling Stage in Rice Based on a High-density Bin Map [J]. Chinese Bulletin of Botany, 2025, 60(3): 342-353. |
| [5] | ZHANG Hui, ZHAO Yun-Peng, LIU Xiao-Chen, GUO Zeng-Peng, HU Guo-Rui, FENG Yan-Hao, MA Miao-Jun. Dynamics of soil seed bank and its role in plant community regeneration during alpine meadow degradation [J]. Chin J Plant Ecol, 2025, 49(1): 74-82. |
| [6] | Jianhong Tian, Yan Liu, Mengqi Yin, Jing Wang, Ting Chen, Yan Wang, Xiaocheng Jiang. OsWAK16 Regulates Seed Anti-aging Ability by Modulating Antioxidant Enzyme Activity in Rice [J]. Chinese Bulletin of Botany, 2025, 60(1): 17-32. |
| [7] | MA Dong-Feng, JIA Cun-Zhi, WANG Xue-Peng, ZHAO Peng-Peng, HU Xiao-Wen. Effect of multi-species grouping on restoration of alpine degraded meadows in Gannan, China [J]. Chin J Plant Ecol, 2025, 49(1): 93-102. |
| [8] | Wenjie Qu, Lei Wang, Wenyan Kang, Xinguo Yang, Jianjun Qu, Xue Zhang. Seed supply and regeneration potential of sand-fixing vegetation with different establishment years in the southeastern edge of the Tengger Desert [J]. Biodiv Sci, 2025, 33(1): 24254-. |
| [9] | Hongju Li, Weicai Yang. A Micropeptide With a Big Role: New Molecular Mechanism in Seed Desiccation [J]. Chinese Bulletin of Botany, 2024, 59(6): 869-872. |
| [10] | SUN Long, LI Wen-Bo, LOU Hu, YU Cheng, HAN Yu, HU Tong-Xin. Effects of fire disturbance on seed germination of Larix gmelinii [J]. Chin J Plant Ecol, 2024, 48(6): 770-779. |
| [11] | XU Zi-Yi, JIN Guang-Ze. Variation and trade-offs in fine root functional traits of seedlings of different mycorrhizal types in mixed broadleaf-Korean pine forests [J]. Chin J Plant Ecol, 2024, 48(5): 612-622. |
| [12] | YUAN Han, ZHONG Ai-Wen, LIU Song-Ping, PENG Yan-Song, XU Lei. Differences in the germination characteristics of Schoenoplectiella triangulata seeds and methods for breaking seed dormancy [J]. Chin J Plant Ecol, 2024, 48(5): 638-650. |
| [13] | Yan Luo, Qiyuan Liu, Yuanbing Lü, Yue Wu, Yaoyu Tian, Tian An, Zhenhua Li. Photothermal Sensitivity of Phytochrome Mutants During Seed Germination in Arabidopsis thaliana [J]. Chinese Bulletin of Botany, 2024, 59(5): 752-762. |
| [14] | Yang Ding, Yingqun Feng, Jinyu Zhang, Bo Wang. Seed predation and dispersal by animals of an endangered endemic species Pinus dabeshanensis [J]. Biodiv Sci, 2024, 32(3): 23401-. |
| [15] | Xiaobo Zhu, Zhang Dong, Mengjin Zhu, Jin Hu, Cheng Lin, Min Chen, Yajing Guan. Indispensable Material for Germination: Long-lived mRNAs of Plant Seed [J]. Chinese Bulletin of Botany, 2024, 59(3): 355-372. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||