

植物学报 ›› 2023, Vol. 58 ›› Issue (5): 760-769.DOI: 10.11983/CBB22141 cstr: 32102.14.CBB22141
余晓敏1, 王亚琴1, 刘雨菡1, 易庆平2, 程文翰2, 朱钰1, 段枫1, 张莉雪1, 何燕红1,2,*( )
)
收稿日期:2022-07-02
接受日期:2022-12-02
出版日期:2023-09-01
发布日期:2023-09-21
通讯作者:
*E-mail: hyh2010@mail.hzau.edu.cn
基金资助:
Yu Xiaomin1, Wang Yaqin1, Liu Yuhan1, Yi Qingping2, Cheng Wenhan2, Zhu Yu1, Duan Feng1, Zhang Lixue1, He Yanhong1,2,*( )
)
Received:2022-07-02
Accepted:2022-12-02
Online:2023-09-01
Published:2023-09-21
Contact:
*E-mail: hyh2010@mail.hzau.edu.cn
摘要: 以万寿菊(Tagetes erecta)里程碑·黄色的小叶为外植体, 采用农杆菌介导法探究抗生素浓度、菌株类型、菌液浓度、侵染时间、共培养时间、乙酰丁香酮浓度和抗褐化剂种类对万寿菊遗传转化效率的影响。结果表明, 头孢霉素(Cef)和硫酸卡那霉素(Kan)的适宜浓度分别为100 mg·L-1和10 mg·L-1; EHA105菌株的稳定转化效率最高; 菌液浓度OD600=0.1、侵染5分钟及共培养1天为最佳侵染条件。此外, 在侵染过程中添加100 µmol·L-1乙酰丁香酮(AS)和筛选培养基中添加0.2 g·L-1聚乙烯吡咯烷酮(PVP)均能提高出芽率。经GUS染色、PCR检测以及Southern blot检测证明转化成功, 转化效率达到4%左右。研究结果为万寿菊基因功能研究和转基因育种奠定了基础。
余晓敏, 王亚琴, 刘雨菡, 易庆平, 程文翰, 朱钰, 段枫, 张莉雪, 何燕红. 根癌农杆菌介导万寿菊遗传转化体系的建立. 植物学报, 2023, 58(5): 760-769.
Yu Xiaomin, Wang Yaqin, Liu Yuhan, Yi Qingping, Cheng Wenhan, Zhu Yu, Duan Feng, Zhang Lixue, He Yanhong. Establishment of Agrobacterium tumefaciens-mediated Genetic Transformation System of Marigold (Tagetes erecta). Chinese Bulletin of Botany, 2023, 58(5): 760-769.
| Culture medium types | Formula |
|---|---|
| MS | 4.405 g·L-1 MS+30 g·L-1 sucrose+8 g·L-1 agar |
| Regeneration medium | 4.405 g·L-1 MS+40 g·L-1 sucrose+8 g·L-1 agar+0.2 mg·L-1 TDZ+0.5 mg·L-1 IBA |
| Co-culture medium | 4.405 g·L-1 MS+40 g·L-1 sucrose+8 g·L-1 agar+0.2 mg·L-1 TDZ+0.5 mg·L-1 IBA+0.5 g·L-1 MES+ 100 μmol·L-1 AS |
| Screening medium | 4.405 g·L-1 MS+40 g·L-1 sucrose+8 g·L-1 agar+0.2 mg·L-1 TDZ+0.5 mg·L-1 IBA+0.5 g·L-1 MES+10 mg·L-1 Kan+100 mg·L-1 Cef |
| Elongation medium/ rooting medium | 4.405 g·L-1 MS+30 g·L-1 sucrose+8 g·L-1 agar+0.5 g·L-1 MES+10 mg·L-1 Kan+100 mg·L-1 Cef |
表1 万寿菊里程碑·黄色遗传转化培养基
Table 1 Medium used for genetic transformation of marigold Milestone Yellow
| Culture medium types | Formula |
|---|---|
| MS | 4.405 g·L-1 MS+30 g·L-1 sucrose+8 g·L-1 agar |
| Regeneration medium | 4.405 g·L-1 MS+40 g·L-1 sucrose+8 g·L-1 agar+0.2 mg·L-1 TDZ+0.5 mg·L-1 IBA |
| Co-culture medium | 4.405 g·L-1 MS+40 g·L-1 sucrose+8 g·L-1 agar+0.2 mg·L-1 TDZ+0.5 mg·L-1 IBA+0.5 g·L-1 MES+ 100 μmol·L-1 AS |
| Screening medium | 4.405 g·L-1 MS+40 g·L-1 sucrose+8 g·L-1 agar+0.2 mg·L-1 TDZ+0.5 mg·L-1 IBA+0.5 g·L-1 MES+10 mg·L-1 Kan+100 mg·L-1 Cef |
| Elongation medium/ rooting medium | 4.405 g·L-1 MS+30 g·L-1 sucrose+8 g·L-1 agar+0.5 g·L-1 MES+10 mg·L-1 Kan+100 mg·L-1 Cef |
| Kan concentration (mg·L-1) | Callus induction rate (%) | Regeneration rate (%) |
|---|---|---|
| 0 | 100.00±0.00 a | 72.50±5.00 a |
| 2 | 100.00±0.00 a | 67.50±12.58 a |
| 4 | 100.00±0.00 a | 50.00±14.14 b |
| 6 | 97.50±5.00 a | 15.00±10.00 c |
| 8 | 95.00±5.77 ab | 7.50±5.00 d |
| 10 | 92.50±5.00 b | 0.00±0.00 e |
| 15 | 65.00±5.77 c | 0.00±0.00 e |
| 20 | 37.50±12.58 d | 0.00±0.00 e |
| 30 | 27.50±5.00 d | 0.00±0.00 e |
| 40 | 15.00±5.77 e | 0.00±0.00 e |
| 50 | 0.00±0.00 f | 0.00±0.00 e |
表2 不同浓度Kan对万寿菊小叶再生的影响
Table 2 Effect of Kan concentration on leaflet regeneration of marigold
| Kan concentration (mg·L-1) | Callus induction rate (%) | Regeneration rate (%) |
|---|---|---|
| 0 | 100.00±0.00 a | 72.50±5.00 a |
| 2 | 100.00±0.00 a | 67.50±12.58 a |
| 4 | 100.00±0.00 a | 50.00±14.14 b |
| 6 | 97.50±5.00 a | 15.00±10.00 c |
| 8 | 95.00±5.77 ab | 7.50±5.00 d |
| 10 | 92.50±5.00 b | 0.00±0.00 e |
| 15 | 65.00±5.77 c | 0.00±0.00 e |
| 20 | 37.50±12.58 d | 0.00±0.00 e |
| 30 | 27.50±5.00 d | 0.00±0.00 e |
| 40 | 15.00±5.77 e | 0.00±0.00 e |
| 50 | 0.00±0.00 f | 0.00±0.00 e |
| Cef concentration (mg·L-1) | Callus induction rate (%) | Regeneration rate (%) | Growth of bacteria |
|---|---|---|---|
| 0 | 100.00±0.00 a | 60.00±8.16 a | Grow well |
| 100 | 97.50±5.00 a | 47.50±12.58 ab | Completely inhibited |
| 200 | 97.50±5.00 a | 40.00±8.16 abc | Completely inhibited |
| 300 | 65.00±5.77 b | 35.00±12.91 bc | Completely inhibited |
| 400 | 37.50±12.58 c | 37.50±17.08 bc | Completely inhibited |
| 500 | 27.50±5.00 c | 27.50±12.58 c | Completely inhibited |
表3 不同浓度Cef对万寿菊小叶再生和菌落生长的影响
Table 3 Effect of Cef concentration on leaflet regeneration of marigold and bacteria growth
| Cef concentration (mg·L-1) | Callus induction rate (%) | Regeneration rate (%) | Growth of bacteria |
|---|---|---|---|
| 0 | 100.00±0.00 a | 60.00±8.16 a | Grow well |
| 100 | 97.50±5.00 a | 47.50±12.58 ab | Completely inhibited |
| 200 | 97.50±5.00 a | 40.00±8.16 abc | Completely inhibited |
| 300 | 65.00±5.77 b | 35.00±12.91 bc | Completely inhibited |
| 400 | 37.50±12.58 c | 37.50±17.08 bc | Completely inhibited |
| 500 | 27.50±5.00 c | 27.50±12.58 c | Completely inhibited |
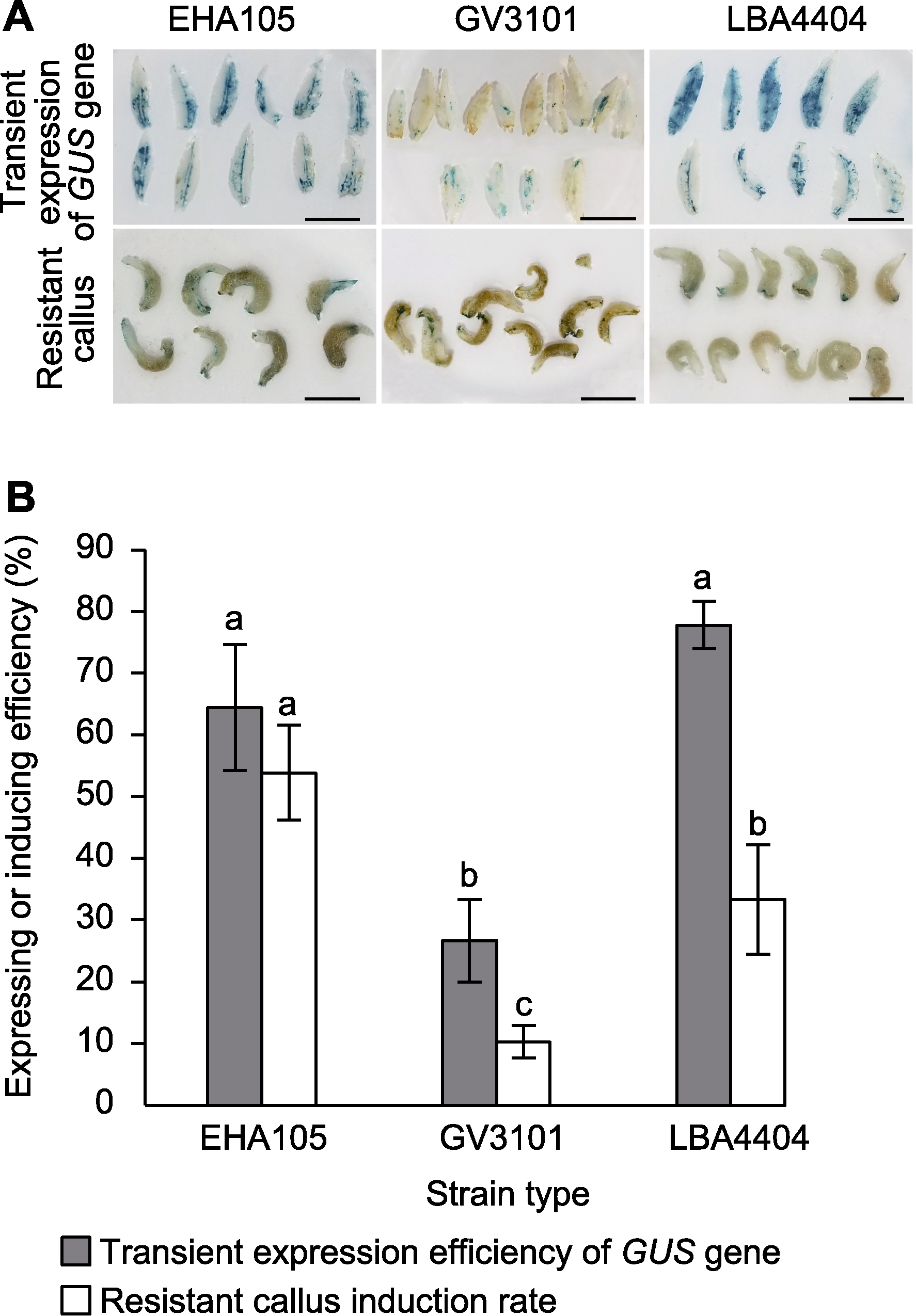
图1 菌株类型对万寿菊遗传转化效率的影响 (A) 3种菌株的GUS基因瞬时表达和抗性愈伤组织示意图(bars=1 cm); (B) 菌株类型对GUS基因瞬时表达效率及抗性愈伤组织诱导效率的影响(相同颜色柱形图上不同小写字母表示在0.05水平差异显著)
Figure 1 Effects of strain types on genetic transformation efficiency of marigold (A) Schematic diagram of transient expression of GUS gene and resistant callus in three strains (bars=1 cm); (B) Effect of strain types on transient expression efficiency of GUS gene and induction rate of resistant calli (different lowercase letters on the same color histogram indicate significant differences at 0.05 level)
| No. | OD600 | Infection time (d) | Co-culture time (d) | Transient expression rate of GUS gene (%) | Callus induction rate (%) | Resistance bud rate (%) | PCR positive rate (%) |
|---|---|---|---|---|---|---|---|
| 1 | 0.1 | 5 | 1 | 100 | 90.67±2.31 abcd | 45.33 | 4.00 |
| 2 | 0.1 | 10 | 2 | 100 | 91.85±7.83 abcd | 27.55 | 0.00 |
| 3 | 0.1 | 20 | 3 | 100 | 77.11±6.99 e | 20.25 | 0.00 |
| 4 | 0.1 | 30 | 4 | 100 | 86.20±11.95 cde | 0.00 | 0.00 |
| 5 | 0.5 | 5 | 2 | 100 | 96.67±2.88 abc | 28.33 | 0.00 |
| 6 | 0.5 | 10 | 1 | 100 | 86.67±0.00 bcde | 22.22 | 3.33 |
| 7 | 0.5 | 20 | 4 | 100 | 93.62±6.45 abc | 3.16 | 0.00 |
| 8 | 0.5 | 30 | 3 | 100 | 96.30±3.21 abc | 0.93 | 0.00 |
| 9 | 1 | 5 | 3 | 100 | 97.10±2.51 ab | 23.53 | 0.00 |
| 10 | 1 | 10 | 4 | 100 | 91.61±4.93 abcd | 13.51 | 0.00 |
| 11 | 1 | 20 | 1 | 100 | 81.25±6.25 de | 28.57 | 0.00 |
| 12 | 1 | 30 | 2 | 100 | 86.03±1.27 cde | 9.00 | 0.00 |
| 13 | 2 | 5 | 4 | 100 | 95.45±7.87 abc | 17.65 | 1.96 |
| 14 | 2 | 10 | 3 | 100 | 100.00±0.00 a | 5.71 | 0.00 |
| 15 | 2 | 20 | 2 | 100 | 86.18±3.66 cde | 12.64 | 0.00 |
| 16 | 2 | 30 | 1 | 100 | 86.11±4.34 cde | 0.00 | 0.00 |
表4 不同侵染条件对万寿菊转化效率的影响
Table 4 Effects of different infection conditions on transformation efficiency of marigold
| No. | OD600 | Infection time (d) | Co-culture time (d) | Transient expression rate of GUS gene (%) | Callus induction rate (%) | Resistance bud rate (%) | PCR positive rate (%) |
|---|---|---|---|---|---|---|---|
| 1 | 0.1 | 5 | 1 | 100 | 90.67±2.31 abcd | 45.33 | 4.00 |
| 2 | 0.1 | 10 | 2 | 100 | 91.85±7.83 abcd | 27.55 | 0.00 |
| 3 | 0.1 | 20 | 3 | 100 | 77.11±6.99 e | 20.25 | 0.00 |
| 4 | 0.1 | 30 | 4 | 100 | 86.20±11.95 cde | 0.00 | 0.00 |
| 5 | 0.5 | 5 | 2 | 100 | 96.67±2.88 abc | 28.33 | 0.00 |
| 6 | 0.5 | 10 | 1 | 100 | 86.67±0.00 bcde | 22.22 | 3.33 |
| 7 | 0.5 | 20 | 4 | 100 | 93.62±6.45 abc | 3.16 | 0.00 |
| 8 | 0.5 | 30 | 3 | 100 | 96.30±3.21 abc | 0.93 | 0.00 |
| 9 | 1 | 5 | 3 | 100 | 97.10±2.51 ab | 23.53 | 0.00 |
| 10 | 1 | 10 | 4 | 100 | 91.61±4.93 abcd | 13.51 | 0.00 |
| 11 | 1 | 20 | 1 | 100 | 81.25±6.25 de | 28.57 | 0.00 |
| 12 | 1 | 30 | 2 | 100 | 86.03±1.27 cde | 9.00 | 0.00 |
| 13 | 2 | 5 | 4 | 100 | 95.45±7.87 abc | 17.65 | 1.96 |
| 14 | 2 | 10 | 3 | 100 | 100.00±0.00 a | 5.71 | 0.00 |
| 15 | 2 | 20 | 2 | 100 | 86.18±3.66 cde | 12.64 | 0.00 |
| 16 | 2 | 30 | 1 | 100 | 86.11±4.34 cde | 0.00 | 0.00 |
| Factor | OD600 | Infection time (d) | Co-culture time (d) |
|---|---|---|---|
| K1 | 4.00 | 5.96 | 7.33 |
| K2 | 3.33 | 3.33 | 0.00 |
| K3 | 0.00 | 0.00 | 0.00 |
| K4 | 1.96 | 0.00 | 1.96 |
| K1 | 1.00 | 1.49 | 1.83 |
| K2 | 0.83 | 0.83 | 0.00 |
| K3 | 0.00 | 0.00 | 0.00 |
| K4 | 0.49 | 0.00 | 0.49 |
| R | 1.00 | 1.49 | 1.83 |
表5 不同侵染条件下PCR阳性率的极差分析
Table 5 Range analysis of PCR positive rate under different infection conditions
| Factor | OD600 | Infection time (d) | Co-culture time (d) |
|---|---|---|---|
| K1 | 4.00 | 5.96 | 7.33 |
| K2 | 3.33 | 3.33 | 0.00 |
| K3 | 0.00 | 0.00 | 0.00 |
| K4 | 1.96 | 0.00 | 1.96 |
| K1 | 1.00 | 1.49 | 1.83 |
| K2 | 0.83 | 0.83 | 0.00 |
| K3 | 0.00 | 0.00 | 0.00 |
| K4 | 0.49 | 0.00 | 0.49 |
| R | 1.00 | 1.49 | 1.83 |
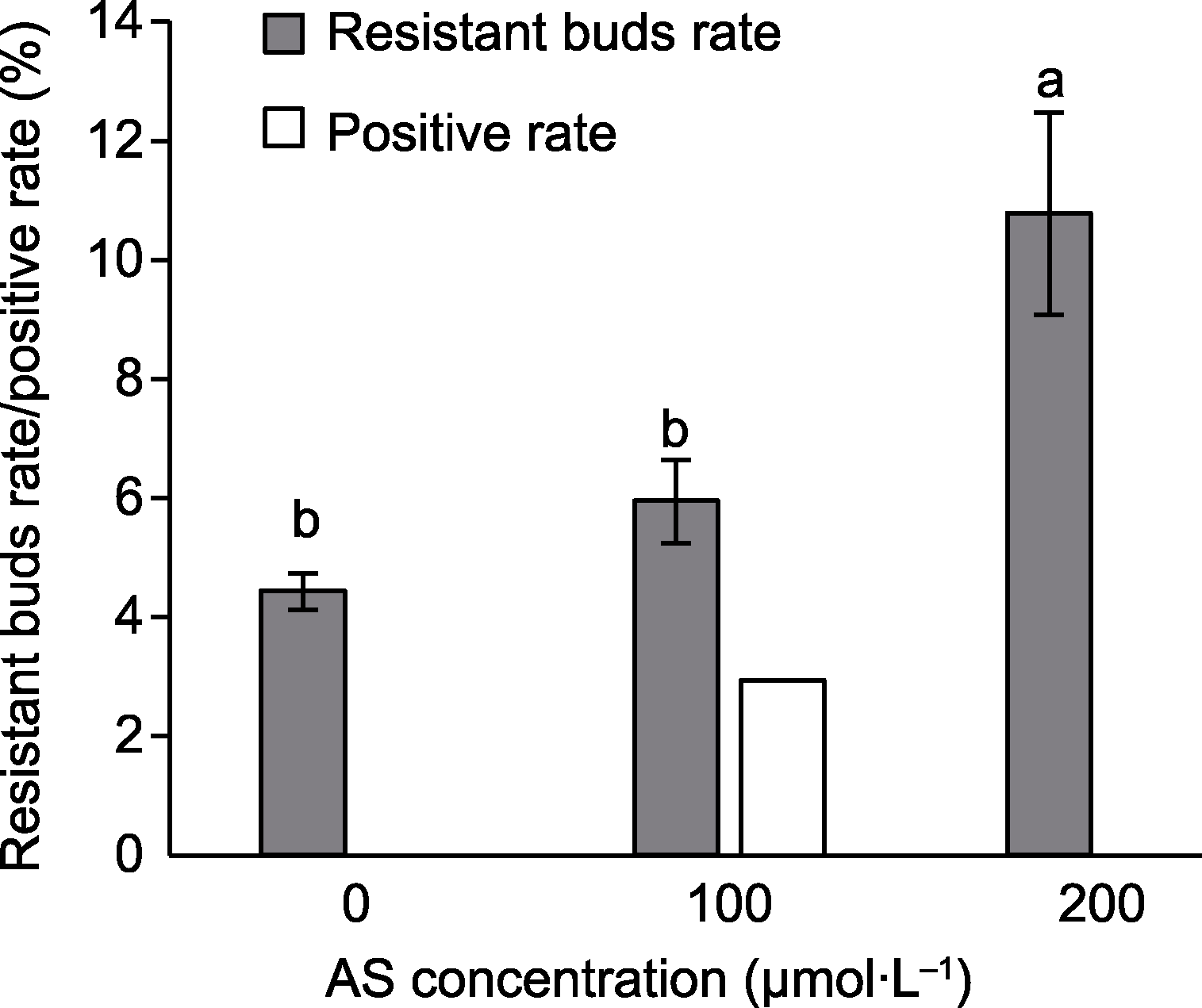
图2 乙酰丁香酮(AS)对万寿菊转化效率的影响 相同颜色柱形图上不同小写字母表示在0.05水平差异显著。
Figure 2 Effect of acetosyringone (AS) on transformation efficiency of marigold Different lowercase letters on the same color histogram indicate significant differences at 0.05 level.
| Anti-browning agent and concentration | No. of resistant buds | Resistance buds rate (%) | No. of positive seedlings | PCR positive rate (%) | Description of budding state |
|---|---|---|---|---|---|
| CK | 4 | 4.00 | 0 | 0.00 | Grow well |
| 0.2 g·L-1 CA | 6 | 6.45 | 0 | 0.00 | Hard texture, more clustered buds, basically no main stem |
| 0.4 g·L-1 CA | 9 | 9.00 | 0 | 0.00 | Hard texture, more clustered buds, basically no main stem |
| 0.6 g·L-1 CA | 12 | 12.00 | 0 | 0.00 | Hard texture, more clustered buds, basically no main stem |
| 0.2 g·L-1 PVP | 12 | 12.00 | 4 | 4.00 | Grow well |
| 0.4 g·L-1 PVP | 7 | 7.00 | 0 | 0.00 | Grow well |
| 0.6 g·L-1 PVP | 7 | 7.37 | 1 | 1.11 | Grow well |
表6 柠檬酸(CA)和聚乙烯吡咯烷酮(PVP)对万寿菊转化效率的影响
Table 6 Effects of citric acid (CA) and polyvinyl pyrrolidone (PVP) on transformation efficiency of marigold
| Anti-browning agent and concentration | No. of resistant buds | Resistance buds rate (%) | No. of positive seedlings | PCR positive rate (%) | Description of budding state |
|---|---|---|---|---|---|
| CK | 4 | 4.00 | 0 | 0.00 | Grow well |
| 0.2 g·L-1 CA | 6 | 6.45 | 0 | 0.00 | Hard texture, more clustered buds, basically no main stem |
| 0.4 g·L-1 CA | 9 | 9.00 | 0 | 0.00 | Hard texture, more clustered buds, basically no main stem |
| 0.6 g·L-1 CA | 12 | 12.00 | 0 | 0.00 | Hard texture, more clustered buds, basically no main stem |
| 0.2 g·L-1 PVP | 12 | 12.00 | 4 | 4.00 | Grow well |
| 0.4 g·L-1 PVP | 7 | 7.00 | 0 | 0.00 | Grow well |
| 0.6 g·L-1 PVP | 7 | 7.37 | 1 | 1.11 | Grow well |

图3 万寿菊遗传转化流程图 (A) 万寿菊复叶; (B) 小叶培养; (C) 小叶诱导形成愈伤组织; (D) 瞬时转化叶片GUS染色; (E) 抗性芽; (F) 抗性苗; (G) 抗性苗生根培养; (H) 阳性苗; (I) 阳性苗移栽。(A)-(D), (G)-(I) Bars=1 cm; (E), (F) Bars=0.5 cm
Figure 3 Genetic transformation process diagram of marigold (A) Marigold compound leaves; (B) Leaflet culture; (C) Leaflet-induced calli; (D) GUS staining of transiently transformed leaves; (E) Resistance buds; (F) Resistant seedlings; (G) Rooting culture of resistant seedlings; (H) Positive seedlings; (I) Transplantation of positive seedlings. (A)-(D), (G)-(I) Bars=1 cm; (E), (F) Bars=0.5 cm
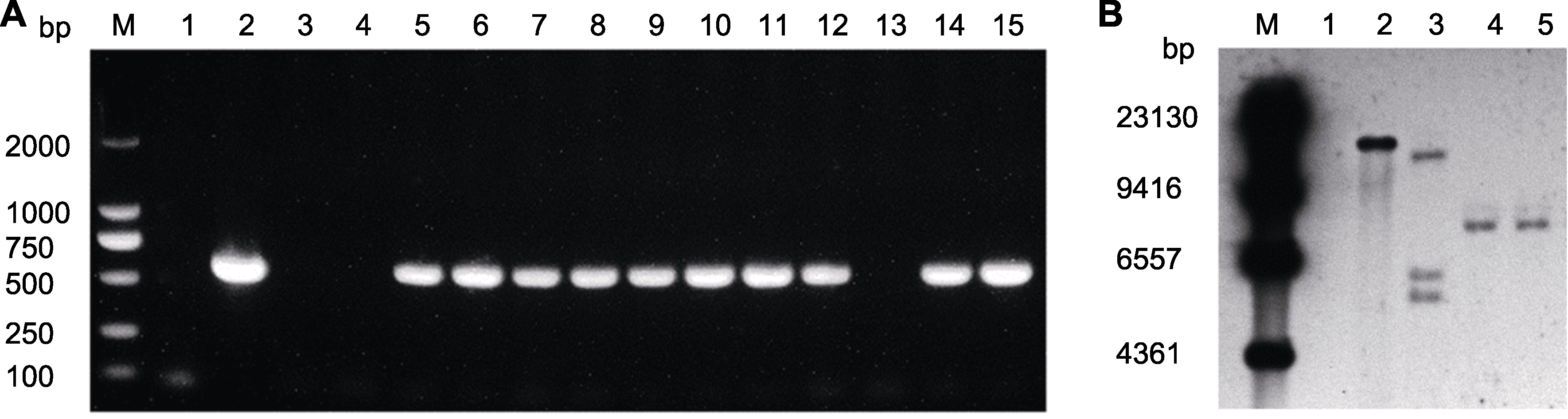
图5 万寿菊抗性苗PCR检测及Southern blot验证 (A) 抗性苗PCR扩增检测(M: 2000 DNA分子量标准; 1: ddH2O; 2: 质粒; 3, 4: 阴性对照, 即未侵染的叶片DNA; 5-15: 抗性苗DNA); (B) 阳性苗Southern blot检测(用HindIII酶切, 以NPT-II为探针。M: DNA分子量标准; 1: 阴性对照, 即未侵染的叶片DNA; 2: 阳性对照, 即质粒; 3-5: 阳性苗DNA)。
Figure 5 PCR amplification detection and Southern blot results of marigold resistant seedlings (A) PCR amplification detection of resistant seedlings (M: 2000 DNA marker; 1: ddH2O; 2: Plasmid; 3, 4: Negative control, DNA of uninfected leaf; 5-15: DNA of resistant seedlings); (B) Southern blot results of positive seedlings (the NPT-II probe was digested with HindIII. M: DNA marker; 1: Negative control, DNA of uninfected leaf; 2: Positive control, plasmid; 3-5: DNA of positive seedlings).
| [1] | 陈利文, 唐楠 (2021). 不同品种色素万寿菊主要农艺性状评价. 安徽农业科学 49(4), 53-55. |
| [2] |
付洪冰, 崔崇士, 赵曦, 刘琦 (2010). 农杆菌介导南瓜遗传转化体系的建立. 植物学报 45, 472-478.
DOI |
| [3] | 符勇耀, 杨利平, 郑开敏, 徐文姬 (2021). 药用万寿菊多倍体的诱导与特征分析. 热带作物学报 42, 1318-1325. |
| [4] | 郭彩珍 (2021). 响应面法优化紫丁香愈伤组织诱导条件及防褐化研究. 种子 40(8), 141-145, 148. |
| [5] | 梁艳, 赵雪莹, 白雪, 刘德强, 张妍, 潘朋 (2021). PVP处理对黑皮油松外植体酚类物质形成及酶活性的影响. 林业科学 57(10), 166-174. |
| [6] | 刘翰升, 赵春莉, 刘玥, 国伟强 (2019). 镉胁迫对万寿菊属植物幼苗生理及富集的影响. 福建农业学报 34, 1221-1227. |
| [7] | 刘香利, 赵惠贤, 郭蔼光 (2011). 农杆菌介导的小麦遗传转化研究进展. 安徽农业科学 39, 19065-19066. |
| [8] | 王关林, 方宏筠 (2002). 植物基因工程(第2版). 北京: 科学出版社. pp. 393. |
| [9] | 王亚琴, 韦陆丹, 王文静, 刘宝骏, 张春玲, 张俊卫, 何燕红 (2020). 万寿菊再生体系的建立及优化. 植物学报 55, 749-759. |
| [10] | 杨帆 (2011). 色素万寿菊psy双边界载体及遗传转化体系建立和SSR-PCR体系的优化. 硕士论文. 上海: 上海交通大学. pp. 14-23. |
| [11] | 曾益, 李婧怡, 周明康, 曹栋才, 娄琳琳, 何恒, 何梦雪, 邹俊杰, 殷中琼 (2021). 万寿菊茎叶醇提物的镇痛抗炎活性研究. 四川农业大学学报 39, 451-458. |
| [12] | 张海霞, 张少英, 付增娟 (2013). 乙酰丁香酮对甜菜遗传转化的影响. 广东农业科学 40(22), 22-24. |
| [13] | 张嫔 (2012). 万寿菊属植物染色体核型分析及万寿菊psy基因遗传转化体系影响因素的研究. 硕士论文. 上海: 上海交通大学. pp. 36-47. |
| [14] |
Amoah BK, Wu H, Sparks C, Jones HD (2001). Factors influencing Agrobacterium-mediated transient expression of uidA in wheat inflorescence tissue. J Exp Bot 52, 1135-1142.
DOI PMID |
| [15] |
Cervera M, López MM, Navarro L, Peña L (1998). Virulence and supervirulence of Agrobacterium tumefaciensin woody fruit plants. Physiol Mol Plant Pathol 52, 67-78.
DOI URL |
| [16] |
Chintakovid W, Visoottiviseth P, Khokiattiwong S, Lauengsuchonkul S (2008). Potential of the hybrid marigolds for arsenic phytoremediation and income generation of remediators in Ron Phibun District, Thailand. Chemosphere 70, 1532-1537.
PMID |
| [17] |
Chitrakar B, Zhang M, Bhandari B (2019). Edible flowers with the common name “Marigold”: their therapeutic values and processing. Trends Food Sci Technol 89, 76-87.
DOI URL |
| [18] |
Godoy-Hernández G, Berzunza EA, Concha LC, Miranda- Ham MDL (2006). Agrobacterium-mediated transient transformation of marigold (Tagetes erecta). Plant Cell Tissue Organ Cult 84, 365-368.
DOI URL |
| [19] |
Gupta V, Rahman LU (2015). An efficient plant regeneration and Agrobacterium-mediated genetic transformation of Tagetes erecta. Protoplasma 252, 1061-1070.
DOI URL |
| [20] |
Jefferson RA, Kavanagh TA, Bevan MW (1987). GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6, 3901-3907.
DOI PMID |
| [21] |
Lacatusu I, Badea G, Popescu M, Bordei N, Istrati D, Moldovan L, Seciu AM, Panteli MI, Rasit I, Badea N (2017). Marigold extract, azelaic acid and black caraway oil into lipid nanocarriers provides a strong anti-inflammatory effect in vivo. Ind Crops Prod 109, 141-150.
DOI URL |
| [22] |
Maleki SS, Mohammadi K, Ji KS (2018). Study on factors influencing transformation efficiency in Pinus massoniana using Agrobacterium tumefaciens. Plant Cell Tissue Organ Cult 133, 437-445.
DOI |
| [23] |
Manivannan A, Narasegowda S, Prakash T (2021). Comparative study on color coordinates, phenolics, flavonoids, carotenoids, and antioxidant potential of marigold (Tagetes sp.) with diverse colored petals. J Food Meas Charact 15, 4343-4353.
DOI |
| [24] |
Mir RA, Argal S, Ahanger MA, Tomar NS, Agarwal RM (2022). Variation in phenolic compounds, antioxidant activity and osmotica of different cultivars of Tagetes erecta L. at different growth stages and effect of its leachates on germination and growth of wheat (Triticum aestivum L.). J Plant Growth Regul 41, 907-921.
DOI |
| [25] |
Nuoendagula, Narushima M, Uesugi M, Murai Y, Katayama Y, Iimura Y, Kajita S (2017). In vitro regeneration and Agrobacterium-mediated transformation of male- sterile marigold (Tagetes erecta L.). Plant Biotechnol (Tokyo) 34, 125-129.
DOI PMID |
| [26] |
Sessitsch A, Hardoim P, Döring J, Weilharter A, Krause A, Woyke T, Mitter B, Hauberg-Lotte L, Friedrich F, Rahalkar M, Hurek T, Sarkar A, Bodrossy L, Van Overbeek L, Brar D, Van Elsas JD, Reinhold-Hurek B (2012). Functional characteristics of an endophyte community colonizing rice roots as revealed by metagenomic analysis. Mol Plant Microbe Interact 25, 28-36.
DOI URL |
| [27] |
Shabbir M, Rather LJ, Mohammad F (2018). Economically viable UV-protective and antioxidant finishing of wool fabric dyed with Tagetes erecta flower extract: valorization of marigold. Ind Crops Prod 119, 277-282.
DOI URL |
| [28] |
Stachel SE, Messens E, Van Montagu M, Zambryski P (1985). Identification of the signal molecules produced by wounded plant cells that activate T-DNA transfer in Agrobacterium tumefaciens. Nature 318, 624-629.
DOI |
| [29] |
Vanegas PE, Valdez-Morales M, Valverde ME, Cruz-Hernández A, Paredes-López O (2006). Particle bombardment, a method for gene transfer in marigold. Plant Cell Tissue Organ Cult 84, 359-363.
DOI URL |
| [1] | 李晶晶, 李艳飞, 王安琪, 王佳颖, 邓成燕, 卢敏, 马剑英, 戴思兰. 菊花品种‘万代风光’再生及遗传转化体系的建立[J]. 植物学报, 2025, 60(4): 1-0. |
| [2] | 曾文丹, 严华兵, 吴正丹, 尚小红, 曹升, 陆柳英, 肖亮, 施平丽, 程冬, 龙紫媛, 李婕宇. 发根农杆菌介导的野葛毛状根遗传转化体系[J]. 植物学报, 2025, 60(3): 425-434. |
| [3] | 张小雨, 贾国栋, 余新晓, 孙立博, 蒋涛. 不同退化程度小叶杨人工林冠层气孔导度特征及其环境响应[J]. 植物生态学报, 2024, 48(9): 1143-1156. |
| [4] | 李宇琛, 赵海霞, 姜希萍, 黄馨田, 刘亚玲, 吴振映, 赵彦, 付春祥. 根癌农杆菌介导的蒙古冰草稳定遗传转化体系建立[J]. 植物学报, 2024, 59(4): 600-612. |
| [5] | 王文静, 朱钰, 张洪铭, 韦陆丹, 易庆平, 余晓敏, 刘雨菡, 张莉雪, 程文翰, 何燕红. 万寿菊舌状花花冠裂片突变体的形态鉴定及连锁标记开发[J]. 植物学报, 2023, 58(6): 893-904. |
| [6] | 杨澜, 刘雅, 项阳, 孙秀娟, 颜景畏, 张阿英. 谷子茎尖体外遗传转化体系的建立与优化[J]. 植物学报, 2021, 56(1): 71-79. |
| [7] | 王亚琴, 韦陆丹, 王文静, 刘宝骏, 张春玲, 张俊卫, 何燕红. 万寿菊再生体系的建立及优化[J]. 植物学报, 2020, 55(6): 749-759. |
| [8] | 李俊华,刘世宇,李成龙,韩林林,董亚辉,张晓丽,赵喜亭,李明军. 铁棍山药微型块茎遗传转化体系的建立[J]. 植物学报, 2019, 54(1): 72-80. |
| [9] | 吴国栋, 修宇, 王华芳. 优化子叶节转化法培育大豆MtDREB2A转基因植株[J]. 植物学报, 2018, 53(1): 59-71. |
| [10] | 陈定帅, 董正武, 高磊, 陈效民, 彭新华, 司炳成, 赵英. 不同降水条件下科尔沁沙地小叶锦鸡儿和盐蒿的水分利用动态[J]. 植物生态学报, 2017, 41(12): 1262-1272. |
| [11] | 赵喜亭, 蒋丽微, 王苗, 朱玉婷, 张文芳, 李明军. 怀黄菊间接体胚受体再生体系的建立及CmTGA1的遗传转化[J]. 植物学报, 2016, 51(4): 525-532. |
| [12] | 赵玮, 胡中民, 杨浩, 张雷明, 郭群, 乌志颜, 刘德义, 李胜功. 浑善达克沙地榆树疏林和小叶杨人工林碳密度特征及其与林龄的关系[J]. 植物生态学报, 2016, 40(4): 318-326. |
| [13] | 李垚, 张兴旺, 方炎明. 小叶栎分布格局对末次盛冰期以来气候变化的响应[J]. 植物生态学报, 2016, 40(11): 1164-1178. |
| [14] | 郑肖然, 赵国琴, 李小雁, 李柳, 吴华武, 张思毅, 张志华. 氢同位素在内蒙古小叶锦鸡儿灌丛水分来源研究中的应用[J]. 植物生态学报, 2015, 39(2): 184-196. |
| [15] | 王丽, 王芹芹, 王幼群. 蚕豆叶片小叶脉不同发育时期ATP酶和酸性磷酸酶的细胞化学超微结构定位[J]. 植物学报, 2014, 49(1): 78-86. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||