

植物学报 ›› 2021, Vol. 56 ›› Issue (1): 62-70.DOI: 10.11983/CBB20174 cstr: 32102.14.CBB20174
杜鹏飞1,2,3, 王玉2, 曹英萍2, 杨松2, 孙志超2, 毛德才3,4, 鄢家俊3,4, 李达旭3,4, 孙美贞5, 付春祥2,*( ), 白史且3,4,*(
), 白史且3,4,*( )
)
收稿日期:2020-11-02
接受日期:2021-01-05
出版日期:2021-01-01
发布日期:2021-01-15
通讯作者:
付春祥,白史且
作者简介:610852681@qq.com基金资助:
Pengfei Du1,2,3, Yu Wang2, Yingping Cao2, Song Yang2, Zhichao Sun2, Decai Mao3,4, Jiajun Yan3,4, Daxu Li3,4, Meizhen Sun5, Chunxiang Fu2,*( ), Shiqie Bai3,4,*(
), Shiqie Bai3,4,*( )
)
Received:2020-11-02
Accepted:2021-01-05
Online:2021-01-01
Published:2021-01-15
Contact:
Chunxiang Fu,Shiqie Bai
摘要: 川草2号老芒麦(Elymus sibiricus)是青藏高原地区治理荒漠化和建设高产人工草地的主要栽培草种。用川草2号老芒麦5种外植体诱导愈伤组织, 经分化测试, 仅幼穗愈伤组织能分化再生。以当代培养25天和35天的结构致密坚硬的幼穗愈伤组织为受体, 分别进行农杆菌侵染和基因枪转化, 结果只有基因枪能转化成功。在基因枪转化过程中, 采用高渗培养和滤纸干燥2种方式预处理愈伤组织, 结果表明滤纸干燥处理比高渗处理转化效率高。当代诱导25天的幼穗愈伤组织, 滤纸干燥处理2小时转化效率最高, 达40%。该研究成功获得了基因枪转化的以川草2号老芒麦幼穗愈伤为受体的阳性愈伤组织。
杜鹏飞, 王玉, 曹英萍, 杨松, 孙志超, 毛德才, 鄢家俊, 李达旭, 孙美贞, 付春祥, 白史且. 基因枪介导的老芒麦遗传转化体系的建立. 植物学报, 2021, 56(1): 62-70.
Pengfei Du, Yu Wang, Yingping Cao, Song Yang, Zhichao Sun, Decai Mao, Jiajun Yan, Daxu Li, Meizhen Sun, Chunxiang Fu, Shiqie Bai. Establishment of Biolistic Mediated Transformation System for Elymus sibiricus. Chinese Bulletin of Botany, 2021, 56(1): 62-70.
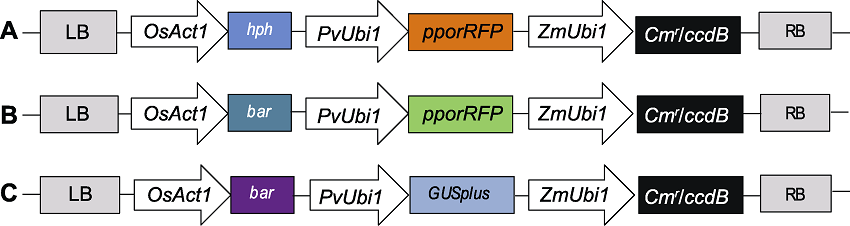
图1 PANIC6A (A)、PANIC6D (B)和PANIC6E (C) T-DNA区段 LB: T-DNA区段左边界; OsAct1: 水稻启动子; hph/bar: 筛选标记基因; PvUbi1: 柳枝稷启动子; pporRFP/GUSplus: 红色荧光/葡萄糖苷酸酶报告基因; ZmUbi1: 玉米启动子; Cmr: 氯霉素抗性基因; ccdB: 目的基因; RB: T-DNA区段右边界
Figure 1 T-DNA segment of PANIC6A (A), PANIC6D (B) and PANIC6E (C) LB: Left boundary of T-DNA segment; OsAct1: Rice promoter; hph/bar: Screening marker genes; PvUbi1: Switchgrass promoter; pporRFP/GUSplus: Red fluorescence/glucuronidase reporter gene; ZmUbi1: Maize promoter; Cmr: Chloramphenicol resistant gene; ccdB: Target gene; RB: Right boundary of T-DNA segment
| Infect schemes | Infection methods | |||
|---|---|---|---|---|
| Predrying treatment (10 min) | Vacuum treatment (10 min) | Ultrasonic treatment (5 min) | Vacuum treatment (10 min) | |
| 1 | - | + | + | + |
| 2 | - | + | + | - |
| 3 | - | - | + | + |
| 4 | + | + | + | + |
表1 4种农杆菌侵染方案
Table 1 Four Agrobacterium infection methods
| Infect schemes | Infection methods | |||
|---|---|---|---|---|
| Predrying treatment (10 min) | Vacuum treatment (10 min) | Ultrasonic treatment (5 min) | Vacuum treatment (10 min) | |
| 1 | - | + | + | + |
| 2 | - | + | + | - |
| 3 | - | - | + | + |
| 4 | + | + | + | + |
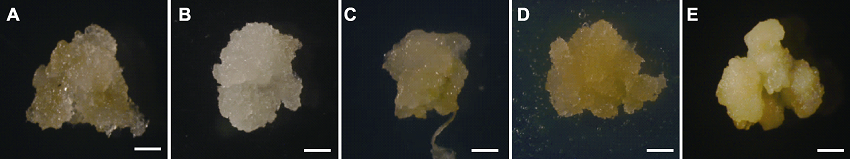
图2 不同外植体诱导出愈伤后约30天的显微图 (A)-(E) 分别表示用种子(成熟胚)、根、下胚轴、茎和幼穗诱导出的愈伤。Bars=50 μm
Figure 2 Micrographs of calli from different explants (30 d) (A)-(E) Callus induced by seeds (mature embryo), roots, hypocotyl, stem and inflorescence, respectively. Bars=50 μm
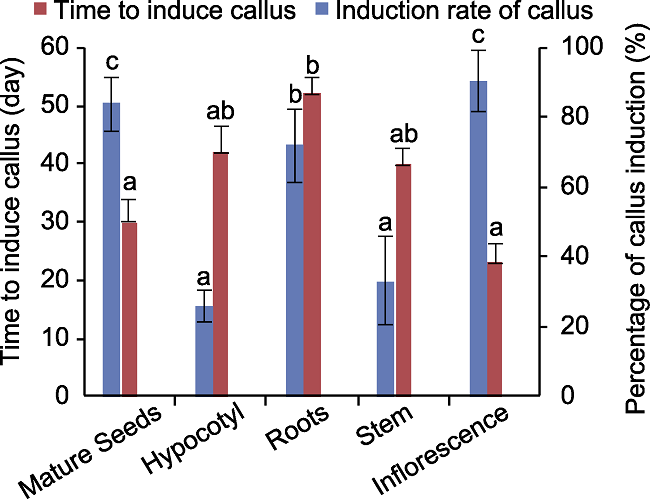
图3 川草2号老芒麦不同外植体愈伤诱导所用时间及诱导率 误差线为标准误(SE), 样本容量为60。不同小写字母表示差异显著(P<0.05)。
Figure 3 Callus induction time and induction rate of different explants in Elymus sibiricus cv. ‘Chuancao No.2’ The error bars indicate standard error (SE), the sample size was 60. Different lowercase letters indicate significant differences (P<0.05).
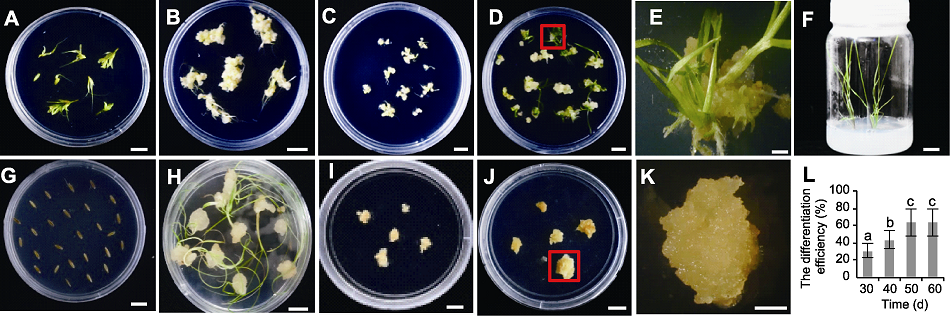
图4 川草2号老芒麦组培再生体系建立及幼穗愈伤分化效率随继代时间的变化 (A) 幼穗接种于愈伤诱导培养基; (B) 幼穗诱导35天后的愈伤; (C) 挑选图(B)中结构致密坚硬的愈伤置于继代培养基15天; (D) 图(C)愈伤置于分化培养基约35天; (E) 图(D)中红色框内愈伤分化显微图; (F) 图(D)中分化苗置于生根培养基约30天; (G) 种子接种于愈伤诱导培养基; (H) 种子诱导35天后的愈伤; (I) 挑选图(H)中结构致密坚硬的愈伤置于继代培养基15天; (J) 图(I)愈伤置于分化培养基约35天; (K) 图(J)中红色框内愈伤分化显微图; (L) 不同再生时间下的幼穗愈伤分化效率(误差线为标准误, 样本容量为30, 不同小写字母表示差异显著(P<0.05))。(D), (F), (G), (J) Bars=2 cm; (A)-(C), (E), (H), (I), (K) Bars=1 cm
Figure 4 The process diagram of tissue culture and regeneration system and the plot of callus differentiation efficiency of Elymus sibiricus cv. ‘Chuancao No.2’ with subculture time (A) Inflorescence of ‘Chuancao No.2’ placed in callus induction medium; (B) Callus induced by inflorescence of ‘Chuancao No.2’ for 35 d; (C) The callus with dense and hard structure in figure (B) was selected and placed in medium for 15 d; (D) Callus in figure (C) cultured in differentiation medium for 35 d; (E) The micrograph of callus in red frame in figure (D); (F) Differentiated seedlings in figure (D) placed in rooting medium for 30 d; (G) Seeds placed in callus induction medium; (H) Callus induced by seeds for 35 d; (I) The callus with dense and hard structure in figure (H) was selected and placed in subculture medium for 15 d; (J) Callus in figure (I) cultured in differentiation medium for 35 d; (K) The micrograph of callus in red frame in figure (J); (L) The differentiation efficiency of callus induced by inflorescence at different time periods (The error line is the standard error, and the sample size is 30; Different lowercase letters indicate significant differences at P<0.05). Bars in (D), (F), (G), (J)=2 cm; Bars in (A)-(C), (E), (H), (I), (K)=1 cm
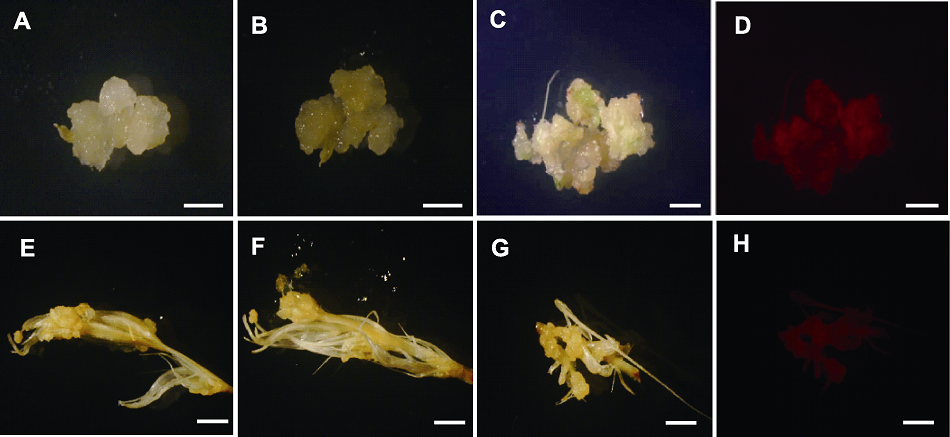
图5 根癌农杆菌侵染川草2号老芒麦幼穗和幼穗愈伤 (A), (B) 农杆菌侵染幼穗愈伤GUS染色前后图片; (C), (D) 农杆菌侵染幼穗愈伤RFP荧光下明场和暗场图片; (E), (F) 农杆菌直接侵染幼穗后GUS染色前后图片; (G), (H) 农杆菌直接侵染幼穗后RFP下明场和暗场图片。Bars=50 μm
Figure 5 Callus of inflorescence and inflorescence infected by Agrobacterium tumefaciens in Elymus sibiricus cv. ‘Chuancao No.2’ (A), (B) Callus induced by inflorescence before and after GUS staining after Agrobacterium infection; (C), (D) The bright and dark field images of RFP fluorescence after Agrobacterium infection of callus induced by inflorescence; (E), (F) The images before and after GUS staining directly after Agrobacterium infection with inflorescence; (G), (H) The bright and dark field images of RFP fluorescence after Agrobacterium infection of inflorescence, respectively. Bars=50 μm
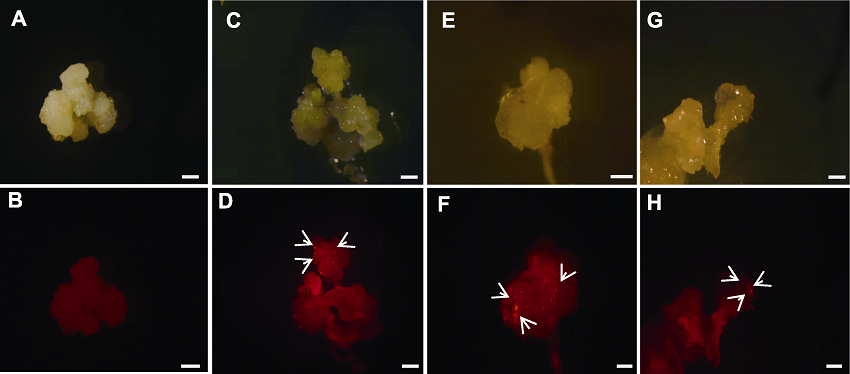
图6 不同诱导时间和预处理方式下的幼穗愈伤基因枪轰击后的荧光显微图 (A), (B) 25天幼穗愈伤(未进行轰击); (C), (D) 25天幼穗愈伤常规高渗处理; (E), (F) 25天幼穗愈伤滤纸干燥处理2小时; (G), (H) 35天的幼穗愈伤滤纸干燥处理2小时。箭头所示为阳性转化愈伤。Bars=20 μm
Figure 6 Fluorescence micrographs of inflorescence callus after bombardment with different induction time and pretreatment methods (A), (B) Callus of inflorescence on 25 d (no bombardment); (C), (D) Conventional hyperosmotic treatment of inflorescence callus at 25 d; (E), (F) Inflorescence callus filter paper dried for 2 h on 25 d; (G), (H) Inflorescence callus filter paper dried for 2 h on 35 d. Arrows indicate the positive transgenic calli. Bars =20 μm
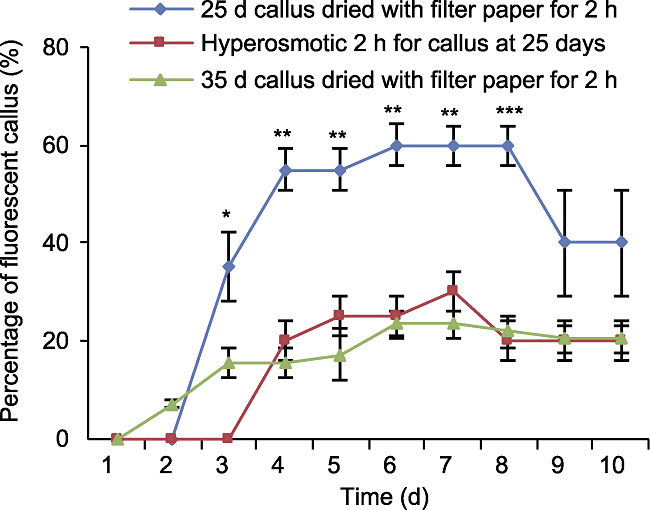
图7 基因枪轰击后荧光愈伤百分比随持续观测时间的变化趋势 误差线为标准误差, 样本容量为30。25天幼穗愈伤滤纸干燥2小时荧光愈伤比率均显著高于其它2种处理方式(* P<0.05, ** P<0.01, *** P<0.001)。
Figure 7 The trend of percentage of fluorescent callus after gene gun bombardment with continuous observation time The error line is the standard error, and the sample size is 30. The fluorescence callus ratio of the 25-day inflorescence callus after 2 h drying on filter paper was significantly higher than that of the other two treatments (* P<0.05, ** P<0.01, *** P<0.001).
| [1] | 白史且, 鄢家俊 (2020). 老芒麦种质资源研究与利用. 北京: 科学出版社. pp. 21-26. |
| [2] | 陈默君, 贾慎修 (2002). 中国饲用植物. 北京: 中国农业出版社. pp. 52-68. |
| [3] | 郭夏宇, 李合松, 彭克勤, 黄志刚, 萧浪涛 (2011). 南荻的组织培养与快速繁殖技术. 植物生理学报 47, 987-990. |
| [4] |
霍秀文, 魏建华, 张辉, 米福贵, 云锦凤 (2004). 冰草属植物组织培养再生体系的建立. 华北农学报 19, 17-20.
DOI URL |
| [5] | 李才旺, 柏正强, 曹毅, 汤茂林 (1999). 提高多年生人工草地建植当年产草量的研究. 四川草原 ( 1), 5-7. |
| [6] | 李达旭 (2006). 几种禾本科牧草遗传转化体系的建立和抗虫转基因研究. 博士论文. 成都: 四川大学. pp. 85-123. |
| [7] | 李达旭, 张杰, 赵建, 张艺, 李力, 刘素君, 陈飞, 杨志荣 (2006). 根癌农杆菌介导转化川草二号老芒麦胚性愈伤组织. 植物生理与分子生物学学报 32, 45-51. |
| [8] | 王关林, 方宏筠 (2002). 植物基因工程(第2版). 北京: 科学出版社. pp. 321-327. |
| [9] | 王宇 (2014). 几种牧草再生体系和遗传转化体系的优化. 硕士论文. 兰州: 兰州大学. pp. 46-51. |
| [10] | 王元富, 杨智永, 盘朝邦 (1995). 川草2号老芒麦选育报告. 四川草原 ( 1), 19-24. |
| [11] | 吴召林, 祁娟, 刘文辉, 金鑫, 杨航, 宿敬龙, 李明 (2020). 氮素形态及其配比对老芒麦生长及生理特性的影响. 草业科学 37, 942-951. |
| [12] | 鄢家俊, 白史且, 马啸, 干友民, 张建波 (2007). 老芒麦遗传多样性及育种研究进展. 植物学通报 24, 226-231. |
| [13] | 杨静 (2019). 根癌农杆菌介导单子叶植物遗传转化研究进展. 种子科技 37(18), 10-12. |
| [14] | 喻修道, 徐兆师, 陈明, 李连城, 马有志 (2010). 小麦转基因技术研究及其应用. 中国农业科学 43, 1539-1553. |
| [15] | 张童 (2009). 禾本科植物离体再生体系研究进展. 中国新技术新产品 ( 1), 169. |
| [16] | 周国栋, 李志勇, 李鸿雁, 师文贵, 李兴酉, 刘磊, 韩海波 (2011). 老芒麦种质资源的研究进展. 草业科学 28, 2026-2031. |
| [17] | 周妍彤, 张琳, 郭强, 田小霞, 孟林, 崔国文 (2018). 长穗偃麦草幼穗离体培养高频再生体系的建立. 植物生理学报 54, 1475-1480. |
| [18] | Chen KL, Gao CX (2015). Developing CRISPR technology in major crop plants. In: Zhang F, Puchta H, Thomson J, eds. Advances in New Technology for Targeted Modification of Plant Genomes. New York: Springer. pp. 145-159. |
| [19] | Cheng M, Lowe BA, Spencer TM, Ye XD, Armstrong CL (2004). Factors influencing Agrobacterium-mediated transformation of monocotyledonous species. In Vitro Cell Dev Biol Plant 40, 31-45. |
| [20] | De Cleene M, de Ley J (1976). The host range of crown gall. Bot Rev 42, 389-466. |
| [21] | Feng ZY, Zhang BT, Ding WN, Liu XD, Yang DL, Wei PL, Cao FQ, Zhu SH, Zhang F, Mao YF, Zhu JK (2013). Efficient genome editing in plants using a CRISPR/Cas system. Cell Res 23, 1229-1232. |
| [22] | Lee KW, Chinzorig O, Choi GJ, Kim KY, Ji HC, Park HS, Lee SH (2012). Callus induction and plant regeneration from mature seeds of Siberian wildrye grass (Elymus sibiricus L.). J Anim Plant Sci 22, 518-521. |
| [23] | Li DX, Zhang J, Zhao J, Zhang Y, Chen F, Zhu JQ, Liu SJ, Yang ZR (2006). Plant regeneration via somatic embryogenesis of Elymus sibiricus cv. ‘Chuancao No.2’. Plant Cell Tissue Organ Cult 84, 285-292. |
| [24] | Li JF, Norville JE, Aach J, McCormack M, Zhang DD, Bush J, Church GM, Sheen J (2013). Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat Biotechnol 31, 688-691. |
| [25] |
Liang Z, Zhang K, Chen KL, Gao CX (2014). Targeted mutagenesis in Zea mays using TALENs and the CRISPR/ Cas system. J Genet Genomics 41, 63-68.
URL PMID |
| [26] |
Mann DGJ, Lafayette PR, Abercrombie LL, King ZR, Mazarei M, Halter MC, Poovaiah CR, Baxter H, Shen H, Dixon RA, Parrott WA, Stewart CN Jr (2012). Gateway-compatible vectors for high-throughput gene functional analysis in switchgrass (Panicum virgatum L.) and other monocot species. Plant Biotechnol J 10, 226-236.
DOI URL PMID |
| [27] | Mao YF, Zhang H, Xu NF, Zhang BT, Gao F, Zhu JK (2013). Application of the CRISPR-Cas system for efficient genome engineering in plants. Mol Plant 6, 2008-2011. |
| [28] | McGuire PE, Dvôrák J (1981). High salt tolerance potential in wheatgrasses. Crop Sci 21, 702-705. |
| [29] |
Shan QW, Wang YP, Li J, Zhang Y, Chen KL, Liang Z, Zhang K, Liu JX, Xi JJ, Qiu JL, Gao CX (2013). Targeted genome modification of crop plants using a CRISPR- Cas system. Nat Biotechnol 31, 686-688.
URL PMID |
| [30] | Vasil IK (1994). Molecular improvement of cereals. Plant Mol Biol 25, 925-937. |
| [31] | Wang ZY, Ge YX (2005). Agrobacterium-mediated high efficiency transformation of tall fescue (Festuca arundinacea). J Plant Physiol 162, 103-113. |
| [32] | Wang ZY, Ge YX (2006). Recent advances in genetic transformation of forage and turf grasses. In Vitro Cell Dev Biol Plant 42, 1-18. |
| [1] | 刘叶飞, 赵海霞, 姜希萍, 邱锐, 周昕越, 赵彦, 付春祥. 野大麦高效组培快繁及农杆菌介导的愈伤侵染体系建立[J]. 植物学报, 2023, 58(3): 440-448. |
| [2] | 朱昀 王猛 贾志伟 练云 金颖 王国英. 一种从富含多糖的玉米幼穗中提取RNA的方法[J]. 植物学报, 2007, 24(05): 624-628. |
| [3] | 王亚琴 段中岗 黄江康 梁承邺. 水稻幼穗培养高效再生系统的建立[J]. 植物学报, 2004, 21(01): 52-60. |
| [4] | 张景昱 张中林 苏宁 宋艳茹. 叶绿体遗传转化:植物导入外源基因的新途径[J]. 植物学报, 2001, 18(03): 288-294. |
| [5] | 范树国 梁承邺 刘鸿先. 水稻离体育性变异研究[J]. 植物学报, 2000, 17(03): 232-241. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||