

植物学报 ›› 2025, Vol. 60 ›› Issue (1): 17-32.DOI: 10.11983/CBB24038 cstr: 32102.14.CBB24038
田建红1,†, 刘燕1,†, 尹梦琪1, 王静1, 陈婷1, 汪燕1, 姜孝成1,2,*( )
)
收稿日期:2024-03-09
接受日期:2024-07-14
出版日期:2025-01-10
发布日期:2024-07-23
通讯作者:
* 姜孝成, 博士, 湖南师范大学二级教授, 博士生导师。主要从事水稻种子活力调控及其分子机制研究, 主持和承担国家自然科学基金、国家科技部基础条件平台建设、湖南省自然科学基金等科研项目。以第一作者或通讯作者身份在Journal of Integrative Plant Biology、BMC Plant Biology等权威期刊上发表论文60余篇。兼任中国植物学会种子科学与技术专业委员会委员, 湖南省植物学会常务理事, 湖南省植物生理与分子生物学学会常务理事, 《生命科学研究》编辑委员会委员。担任Plant Biotechnology Journal、BMC Plant Biology、Molecular Breeding等国际期刊审稿人。E-mail: 作者简介:†共同第一作者
基金资助:
Jianhong Tian1,†, Yan Liu1,†, Mengqi Yin1, Jing Wang1, Ting Chen1, Yan Wang1, Xiaocheng Jiang1,2,*( )
)
Received:2024-03-09
Accepted:2024-07-14
Online:2025-01-10
Published:2024-07-23
Contact:
* E-mail: About author:†These authors contributed equally to this paper
摘要: 细胞壁相关蛋白激酶(WAK)家族成员在水稻(Oryza sativa)基因组中已注释了大约130个WAK基因, 它们在水稻生长发育和应激响应中发挥重要作用。该文探讨了水稻WAK16-RLK的编码基因OsWAK16对水稻种子活力和抗老化能力的调控作用及其生理机制。结果表明, OsWAK16敲除突变体的种子活力在种子未老化和人工老化12天时均显著低于野生型种子, 过表达种子的活力则显著高于野生型种子, 说明OsWAK16正调控种子活力和抗老化能力。同时, 相比野生型种子, 未老化和人工老化12天的OsWAK16敲除突变体种子中丙二醛含量以及种子浸泡液电导率显著增加, 抗氧化酶活性则显著下降; 过表达种子中的变化则相反。此外, 野生型、OsWAK16突变和过表达种子在人工老化处理后, OsWAK16的差异表达也引起OsPER1A、OsbZIP23、OsPIMT1、OsSdr4、OsMSRB5和OsHSP18.2等种子活力相关基因表达的协同变化。因此, 推测OsWAK16可能与其它种子活力相关基因协同作用, 行使清除细胞中活性氧的功能, 从而调控种子活力和抗老化能力。
田建红, 刘燕, 尹梦琪, 王静, 陈婷, 汪燕, 姜孝成. 水稻OsWAK16通过调节抗氧化酶活性调控种子抗老化能力(长英文摘要). 植物学报, 2025, 60(1): 17-32.
Jianhong Tian, Yan Liu, Mengqi Yin, Jing Wang, Ting Chen, Yan Wang, Xiaocheng Jiang. OsWAK16 Regulates Seed Anti-aging Ability by Modulating Antioxidant Enzyme Activity in Rice. Chinese Bulletin of Botany, 2025, 60(1): 17-32.
| Primer name | Sequence (5′-3′) | Purpose |
|---|---|---|
| gRT1 | CCGTTACAAGGCCCTTCTGT | CRISPR/Cas9 vector construction |
| OsU6aT1 | ACAGAAGGGCCTTGTAACGGC | |
| gRT2 | ATTACAACTGGTATTAGTTG | |
| OsU6bT2 | CAACTAATACCAGTTGTAATC | |
| PB-L | GCGCGCGGTCTCGCTCGACTAGTATGG | |
| PB-R | GCGCGCGGTCTCTACCGACGCGTATCC | |
| OsWAK16-cas9-F1 | GCCAGGTACTGATACCTAC | |
| OsWAK16-cas9-R1 | CTCAAGAAAAGATCAGTCGC | |
| OsWAK16-cas9-F2 | TTTACATGCACTAGTTGCCC | |
| OsWAK16-cas9-R2 | GCGCTTTCATCTGAGATTAG | |
| OsWAK16-pHB-F | ATGAGGTCGAGCTTTGTGGC | Overexpression vector construction |
| OsWAK16-pHB-R | CTAGCGTGGCAAACTGACTGAG | |
| OsWAK16-1301-F | CCAATGAGTACATTTCTGCTATAGACAG | GUS vector construction |
| OsWAK16-1301-R | CTTTGCGCCAGCCGAGAC | |
| OsActin1-F | CAATGTGCCAGCTATGTATGTCGCC | Quantitative internal control |
| OsActin1-R | TTCCCGTTCAGCAGTGGTAGTGAAG | |
| qRT-OsWAK16-F | GATGGCAACTTTACTACAA | OsWAK16 expression analysis |
| qRT-OsWAK16-R | TGTGATAATACTCTGGGTC | |
| OsPER1A-F | GACCCGGACGAGAAGGATTC | Gene expression analysis |
| OsPER1A-R | ACCACCTCATCCATGTTCCG | |
| OsbZIP23-F | CTGGGAAATGGGCTGGTCT | |
| OsbZIP23-R | CCATCTTGCCGAAGCCATT | |
| OsPIMT1-F | CACCGACTGTGGTCAAGC | |
| OsPIMT1-R | AGCACCAGGAGGCACAAA | |
| OsSdr4-F | AAGACGGCGGAGGAGGTGGA | |
| OsSdr4-R | CATGGACGGATGACCACTTGC | |
| OsMSRB5-F | GCCATAAACCGAACACCG | |
| OsMSRB5-R | GCTCATCAGTAGGCGTCTTG | |
| OsHSP18.2-F | GCTCAAGTCCTCCGACATCAAG | |
| OsHSP18.2-R | TCCGCAGGTACTTGCACGAC |
表1 实验所用引物
Table 1 The primers used in the experiments
| Primer name | Sequence (5′-3′) | Purpose |
|---|---|---|
| gRT1 | CCGTTACAAGGCCCTTCTGT | CRISPR/Cas9 vector construction |
| OsU6aT1 | ACAGAAGGGCCTTGTAACGGC | |
| gRT2 | ATTACAACTGGTATTAGTTG | |
| OsU6bT2 | CAACTAATACCAGTTGTAATC | |
| PB-L | GCGCGCGGTCTCGCTCGACTAGTATGG | |
| PB-R | GCGCGCGGTCTCTACCGACGCGTATCC | |
| OsWAK16-cas9-F1 | GCCAGGTACTGATACCTAC | |
| OsWAK16-cas9-R1 | CTCAAGAAAAGATCAGTCGC | |
| OsWAK16-cas9-F2 | TTTACATGCACTAGTTGCCC | |
| OsWAK16-cas9-R2 | GCGCTTTCATCTGAGATTAG | |
| OsWAK16-pHB-F | ATGAGGTCGAGCTTTGTGGC | Overexpression vector construction |
| OsWAK16-pHB-R | CTAGCGTGGCAAACTGACTGAG | |
| OsWAK16-1301-F | CCAATGAGTACATTTCTGCTATAGACAG | GUS vector construction |
| OsWAK16-1301-R | CTTTGCGCCAGCCGAGAC | |
| OsActin1-F | CAATGTGCCAGCTATGTATGTCGCC | Quantitative internal control |
| OsActin1-R | TTCCCGTTCAGCAGTGGTAGTGAAG | |
| qRT-OsWAK16-F | GATGGCAACTTTACTACAA | OsWAK16 expression analysis |
| qRT-OsWAK16-R | TGTGATAATACTCTGGGTC | |
| OsPER1A-F | GACCCGGACGAGAAGGATTC | Gene expression analysis |
| OsPER1A-R | ACCACCTCATCCATGTTCCG | |
| OsbZIP23-F | CTGGGAAATGGGCTGGTCT | |
| OsbZIP23-R | CCATCTTGCCGAAGCCATT | |
| OsPIMT1-F | CACCGACTGTGGTCAAGC | |
| OsPIMT1-R | AGCACCAGGAGGCACAAA | |
| OsSdr4-F | AAGACGGCGGAGGAGGTGGA | |
| OsSdr4-R | CATGGACGGATGACCACTTGC | |
| OsMSRB5-F | GCCATAAACCGAACACCG | |
| OsMSRB5-R | GCTCATCAGTAGGCGTCTTG | |
| OsHSP18.2-F | GCTCAAGTCCTCCGACATCAAG | |
| OsHSP18.2-R | TCCGCAGGTACTTGCACGAC |
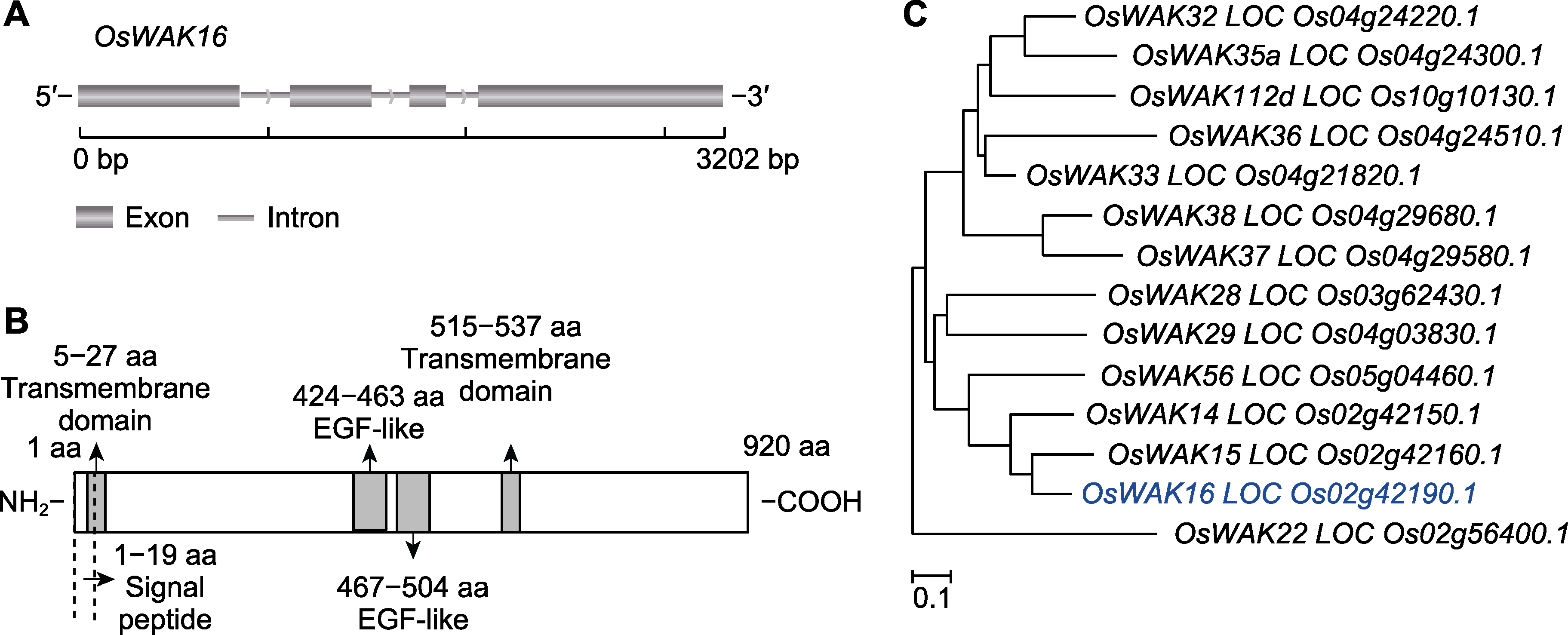
图1 OsWAK16的生物信息学分析 (A) OsWAK16基因结构; (B) OsWAK16蛋白结构; (C) OsWAK16与水稻其它WAK同源基因序列构建的系统进化树(蓝色字体为OsWAK16)。
Figure 1 Bioinformatics analysis of OsWAK16 (A) OsWAK16 gene structure; (B) OsWAK16 protein structure; (C) Phylogenetic tree of OsWAK16 and its homologous genes from Oryza sativa (OsWAK16 is highlighted in blue).
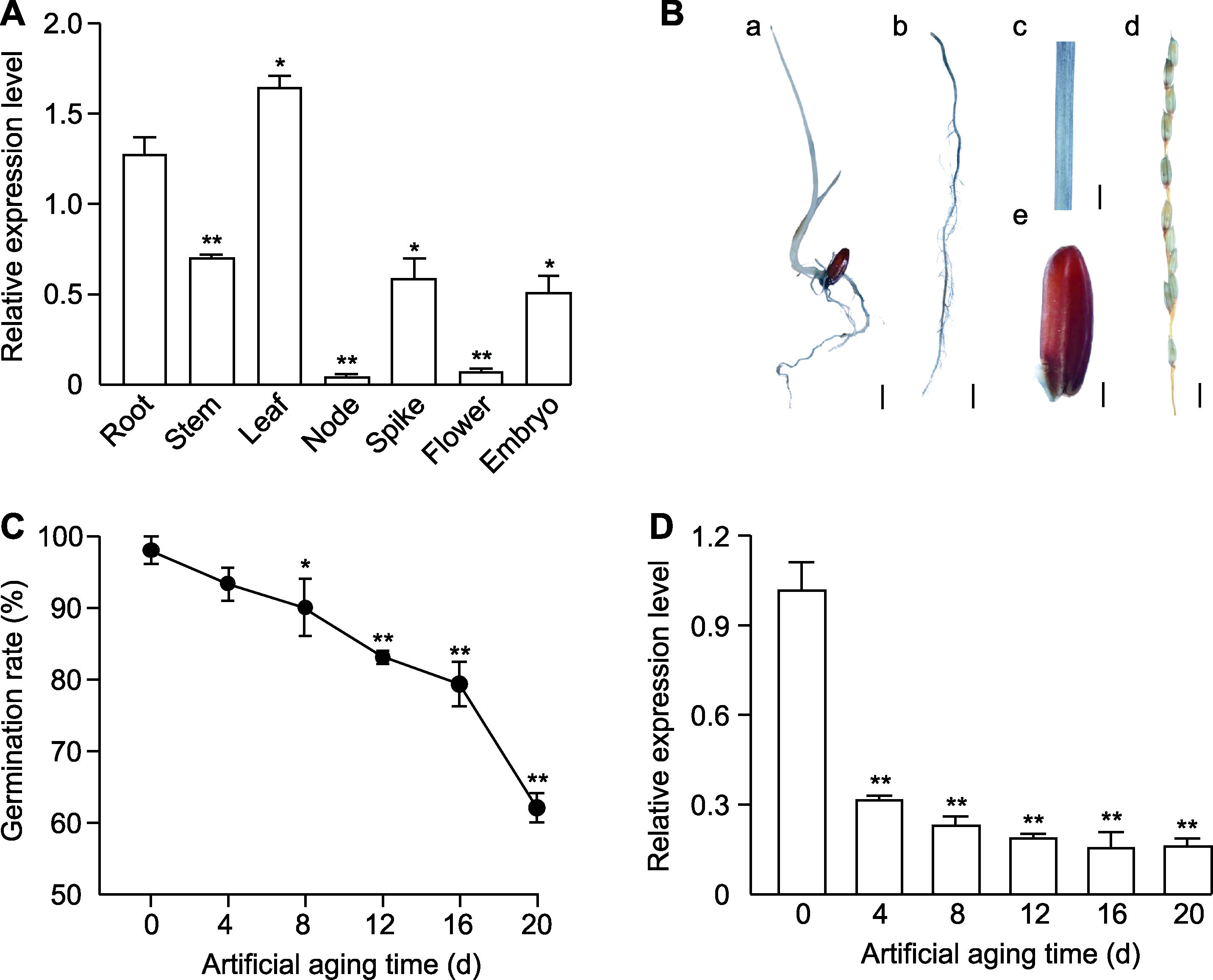
图2 OsWAK16在水稻不同组织及不同活力种子中的表达模式 (A) OsWAK16在Kasalath (WT)不同组织中的表达水平; (B) OsWAK16启动子驱动的GUS表达分析(a: 幼苗; b: 根; c: 叶; d: 穗; e: 种子); (C) 老化处理不同时间WT种子的萌发率; (D) 老化处理不同时间WT种子中OsWAK16的表达水平。(B) Bars in a-d=1 cm; bar in e=0.1 cm。数值为平均值±标准差(n=3)。根与其它组织之间的差异显著性(A)以及未老化种子与老化不同天数种子之间的差异显著性(C, D)用Student’s t-test计算(* P<0.05, ** P<0.01)。
Figure 2 Expression patterns of OsWAK16 in different tissues and seeds of rice with different vigor (A) Expression level of OsWAK16 in different tissues of Kasalath (WT); (B) Analysis of OsWAK16 promoter-driven GUS expression (a: Seedling; b: Root; c: Leaf; d: Spikelet; e: Seed); (C) Germination rate of WT seeds at different time of artificial aging; (D) Expression of OsWAK16 in WT seeds at different time of artificial aging. (B) Bars in a-d=1 cm; bar in e=0.1 cm. Data represent means±SD (n=3). Significant differences between root and other tissues (A), and significant differences between unaged seeds and seeds artificially aged for different days (C, D) were determined using Student’s t-test (* P<0.05, ** P<0.01).
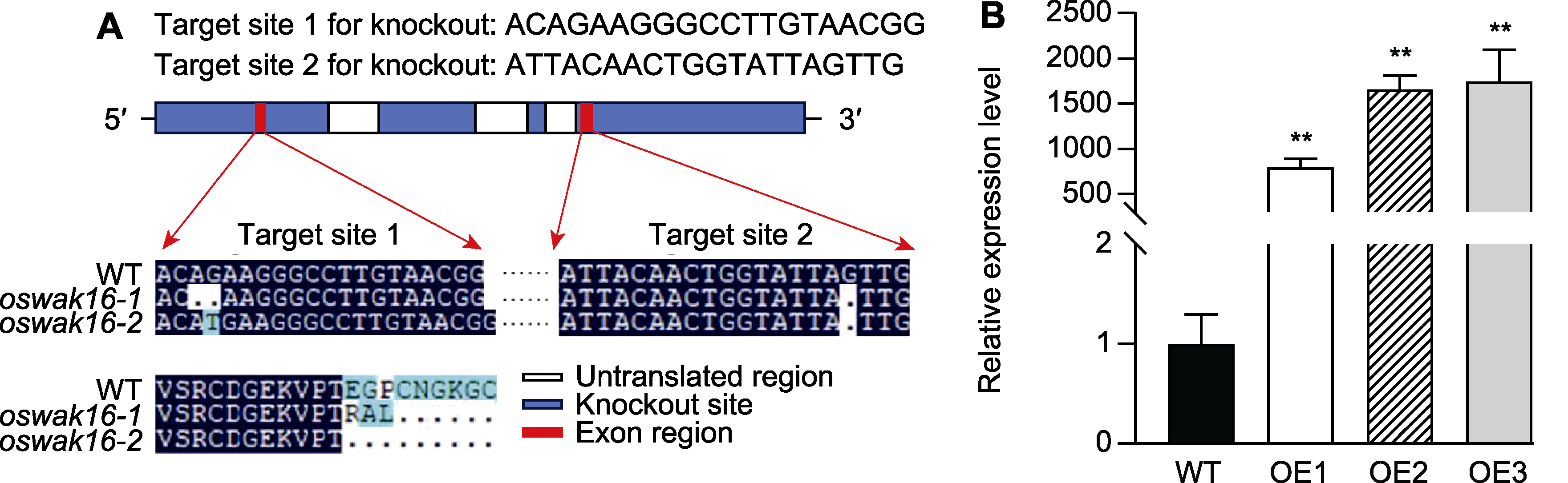
图3 OsWAK16转基因水稻株系的构建 (A) CRISPR/Cas9的OsWAK16靶位点(靶位点1和靶位点2)及突变体oswak16-1和oswak16-2的碱基序列和对应的氨基酸序列变化; (B) 野生型(WT)和OsWAK16过表达株系OE-1、OE-2和OE-3叶片中OsWAK16的相对表达量, 数值为平均值±标准差(n=3), WT与过表达株系间的差异显著性用Student’s t-test计算(** P<0.01)。
Figure 3 Construction of transgenic rice lines of OsWAK16 (A) Target sites (target site 1 and target site 2) selected by CRISPR/Cas9 and changes of the nucleotide sequences as well as the corresponding amino acid sequences of mutants oswak16-1 and oswak16-2; (B) Relative expression levels of OsWAK16 in leaves of wild type (WT) and overexpression lines OE-1, OE-2, and OE-3, data represent means±SD (n=3), significant differences between WT and overexpression lines were determined using Student’s t-test (** P<0.01).
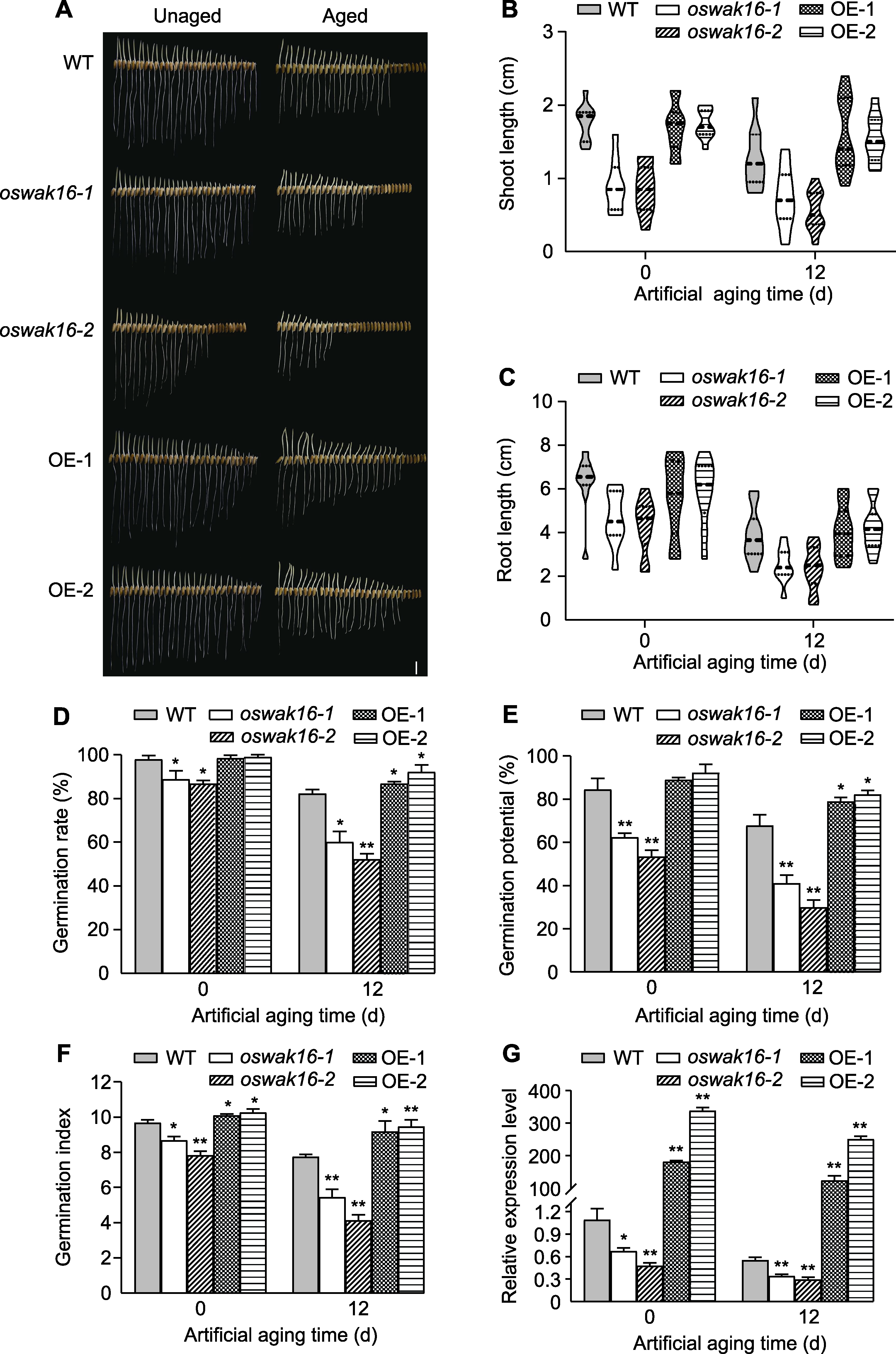
图4 Kasalath (WT)、oswak16突变体和OsWAK16过表达株系种子的萌发表型及其相关活力指标 (A) 未老化和人工老化12天种子的萌发表型(bar=1 cm); (B) 幼苗芽长; (C) 幼苗根长; (D) 萌发率; (E) 萌发势; (F) 萌发指数; (G) OsWAK16相对表达量。数值为平均值±标准差(n=3), WT与其它基因型之间的差异显著性用Student’s t-test计算(* P<0.05, ** P<0.01)。
Figure 4 Seed germination phenotypes and the related indexes of rice Kasalath (WT), oswak16 mutants and OsWAK16 overexpression lines (A) Germination phenotypes of unaged seeds and seeds with 12 days of artificial aging (bar=1 cm); (B) Seedling shoot length; (C) Seedling root length; (D) Germination rate; (E) Germination potential; (F) Germination index; (G) The relative expression level of OsWAK16. Data represent means±SD (n=3), significant differences between WT and other genotypes were determined using Student’s t-test (* P<0.05, ** P<0.01).
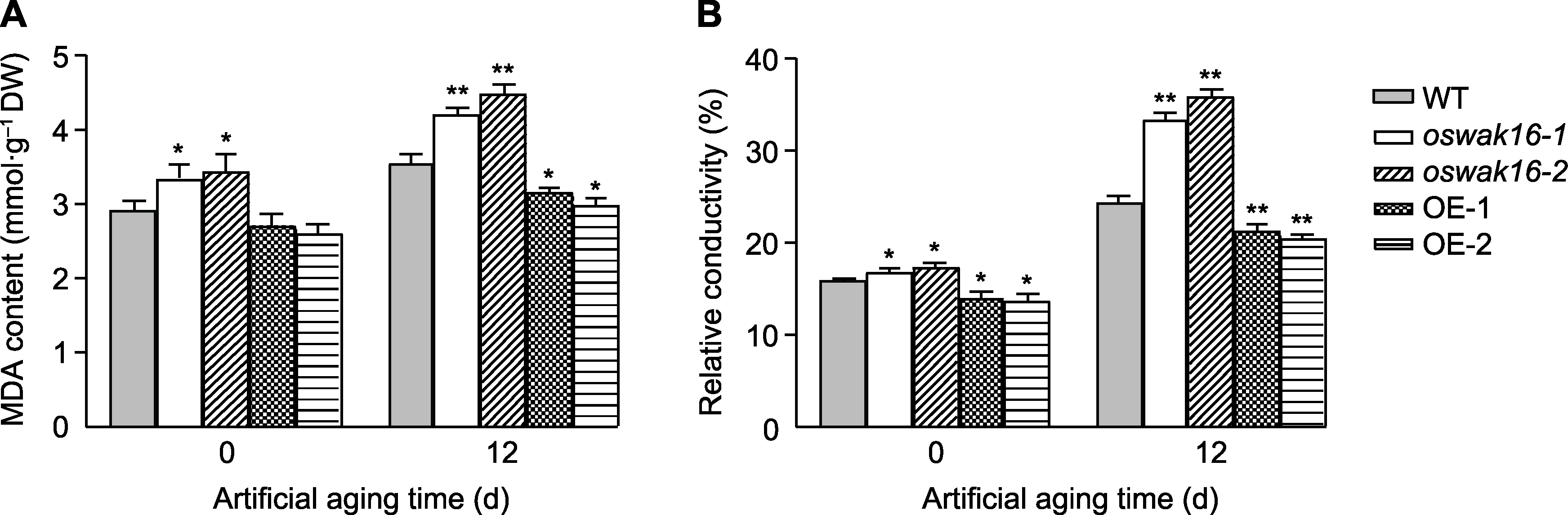
图5 Kasalath (WT)、oswak16突变体和OsWAK16过表达株系种子老化处理前后丙二醛(MDA)含量(A)和种子浸泡液的相对电导率(EC) (B)比较 数值为平均值±标准差(n=3), WT与其它基因型之间的差异显著性用Student’s t-test计算(* P<0.05, ** P<0.01)。
Figure 5 Comparison of malondialdehyde (MDA) content (A) and relative conductivity (EC) (B) of seeds of Kasalath (WT), oswak16 mutants and OsWAK16 overexpression lines before and after artificial aging Data represent means±SD (n=3); significant differences between WT and other genotypes were determined using Student’s t-test (* P<0.05, ** P<0.01).
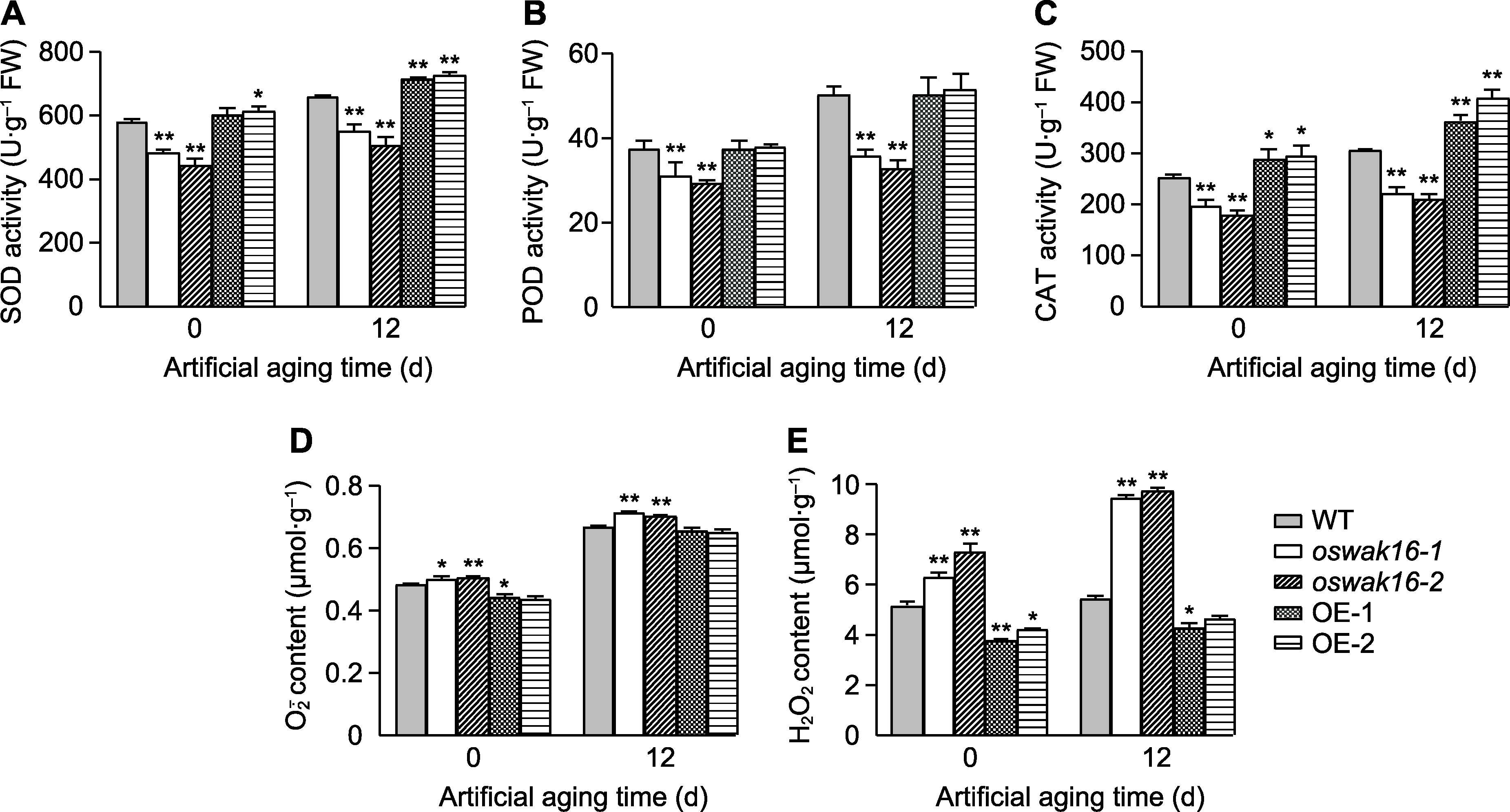
图6 Kasalath (WT)、oswak16突变体和OsWAK16过表达株系种子人工老化前后抗氧化酶活性以及H2O2和O2-.含量比较 (A) 超氧化物歧化酶(SOD); (B) 过氧化物酶(POD); (C) 过氧化氢酶(CAT); (D) O2-. ; (E) H2O2。数值为平均值±标准差(n=3), WT与其它基因型之间的差异显著性用Student’s t-test计算(* P<0.05, ** P<0.01)。
Figure 6 Comparison of antioxidase activities and H2O2 and O2-. contents in seeds before and after artificial aging of Kasalath (WT), oswak16 mutants and OsWAK16 overexpression lines (A) Superoxide dismutase (SOD); (B) Peroxidase (POD); (C) Catalase (CAT); (D) O2-. ; (E) H2O2. Data represent means±SD (n=3); significant differences between WT and other genotypes were determined using Student’s t-test (* P<0.05, ** P<0.01).
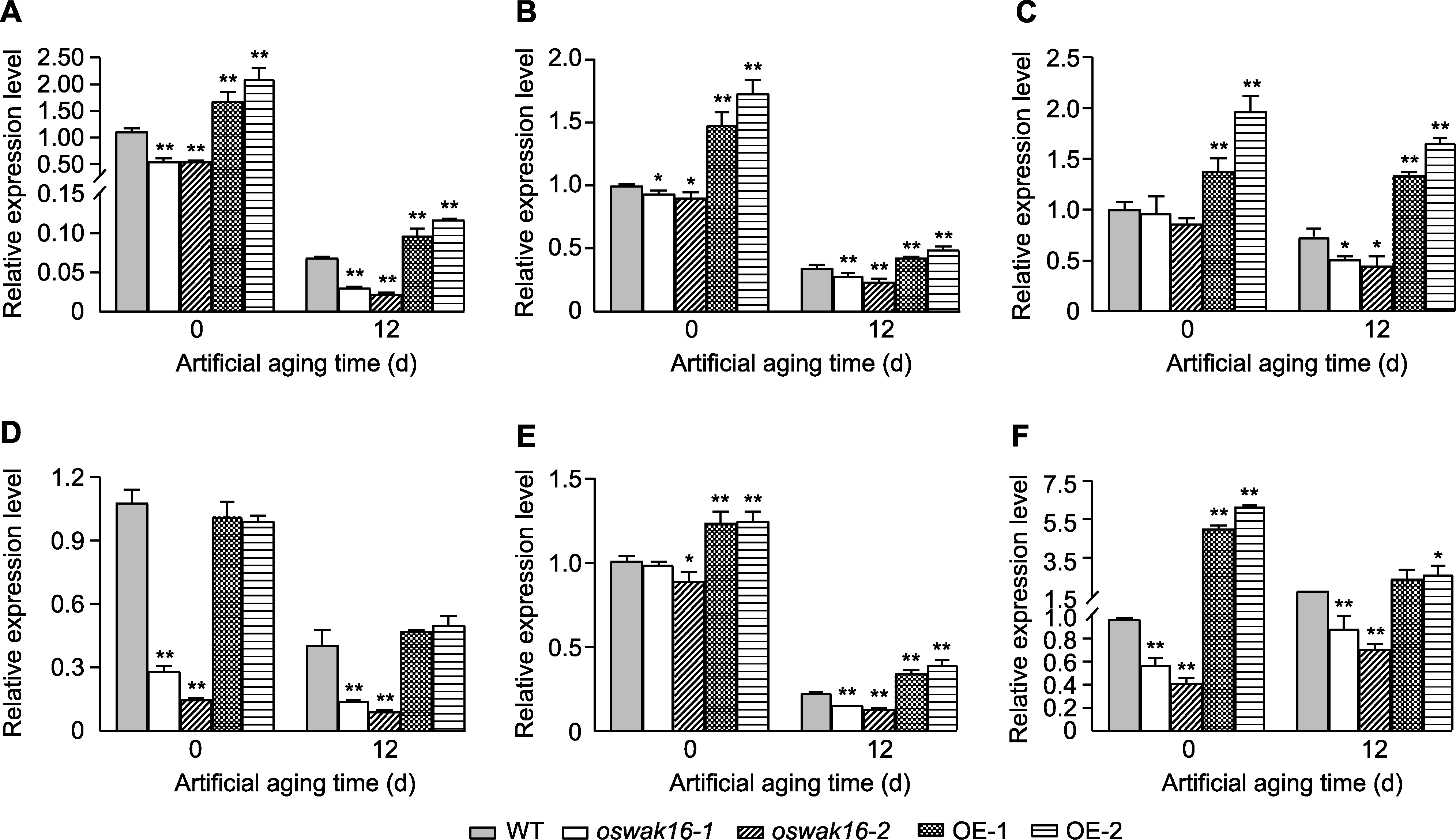
图7 Kasalath (WT)、oswak16突变体和OsWAK16过表达株系种子中种子活力相关基因表达量分析 (A) OsPER1A; (B) OsbZIP23; (C) OsPIMT1; (D) OsSdr4; (E) OsMSRB5; (F) OsHSP18.2。数值为平均值±标准差(n=3), WT与其它基因型之间的差异显著性用Student’s t-test计算(* P<0.05, ** P<0.01)。
Figure 7 Analysis of seed vigor-related genes expression in seeds before and after artificial aging of Kasalath (WT), oswak16 mutants and OsWAK16 overexpression lines (A) OsPER1A; (B) OsbZIP23; (C) OsPIMT1; (D) OsSdr4; (E) OsMSRB5; (F) OsHSP18.2. Data represent means±SD (n=3), significant differences between WT and other genotypes were determined using Student’s t-test (* P<0.05, ** P<0.01).
| [1] | An JY, Liu YH, Han JJ, He C, Chen M, Zhu XB, Hu WM, Song WJ, Hu J, Guan YJ (2022). Transcriptional multiomics reveals the mechanism of seed deterioration in Nicotiana tabacum L. and Oryza sativa L. J Adv Res 42, 163-176. |
| [2] |
Brutus A, Sicilia F, Macone A, Cervone F, De Lorenzo G (2010). A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc Natl Acad Sci USA 107, 9452-9457.
DOI PMID |
| [3] | Chang HW, Zhang FL, Yang ZR, Kong DJ, Zheng QL, Hao LZ (2015). Physiological and biochemical responses of allium mongolicum seeds to storage aging. Plant Physiol J 51, 1075-1081. (in Chinese) |
| 常海文, 张凤兰, 杨忠仁, 孔德娟, 郑清岭, 郝丽珍 (2015). 沙葱种子贮藏陈化过程中的生理生化应答反应. 植物生理学报 51, 1075-1081. | |
| [4] | Chen BX, Fu H, Gao JD, Zhang YX, Huang WJ, Chen ZJ, Zhang Q, Yan SJ, Liu J (2022). Identification of metabolomic biomarkers of seed vigor and aging in hybrid rice. Rice 15, 7. |
| [5] | Chu Z, Mao GF, Wu M, Wu HK (2023). Relationship between electrical conductivity of seed soaking solution and seed vigor in rice (Oryza sativa L.). J Agric Sci Technol 25, 35-41. (in Chinese) |
|
曲宗普尺, 毛光锋, 吴敏, 吴洪恺 (2023). 水稻种子浸泡液电导率与种子活力的关系. 中国农业科技导报 25, 35-41.
DOI |
|
| [6] | Christoff AP, de Lima JC, de Ross BCF, Sachetto-Martins G, Margis-Pinheiro M, Margis R (2014). The wall-associated kinase gene family in rice ge- nomes. Plant Sci 229, 181-192. |
| [7] |
Delteil A, Gobbato E, Cayrol B, Estevan J, Michel-Romiti C, Dievart A, Kroj T, Morel JB (2016). Several wall-associated kinases participate positively and negatively in basal defense against rice blast fungus. BMC Plant Biol 16, 17.
DOI PMID |
| [8] | Gao JD, Fu H, Zhou XQ, Chen ZJ, Luo Y, Cui BY, Chen GH, Liu J (2016). Comparative proteomic analysis of seed embryo proteins associated with seed storability in rice (Oryza sativa L) during natural aging. Plant Physiol Biochem 103, 31-44. |
| [9] | Gao QM, Lu XX, Zhu LY, Xin X, Jiang XC (2019). Correlation studies on MDA and 4-HNE contents in soybean seed aging. Seed 38(4), 1-9. (in Chinese) |
| 高琴梅, 卢新雄, 朱凌燕, 辛霞, 姜孝成 (2019). 大豆种子老化MDA和4-HNE的含量变化相关性研究. 种子 38(4), 1-9. | |
| [10] | Ghassemi-Golezani K, Khomari S, Valizadeh M (2009). Effects of seed and seedling vigor on antioxidative isozyme activity and cold acclimation capability of winter oilseed rape. J Food Agric Environ 7, 452-456. |
| [11] | Goel A, Sheoran IS (2003). Lipid peroxidation and peroxide-scavenging enzymes in cotton seeds under natural ageing. Biol Plant 46, 429-434. |
| [12] | Han GH, Huang RN, Hong LH, Xu JX, Hong YG, Wu YH, Chen WW (2023). The transcription factor NAC102 confers cadmium tolerance by regulating WAKL11 expression and cell wall pectin metabolism in Arabidopsis. J Integr Plant Biol 65, 2262-2278. |
| [13] | Harkenrider M, Sharma R, De Vleesschauwer D, Tsao L, Zhang XT, Chern M, Canlas P, Zuo SM, Ronald PC (2016). Overexpression of rice wall-associated kinase 25 (OsWAK25) alters resistance to bacterial and fungal pathogens. PLoS One 11, e0147310. |
| [14] | Hazra A, Varshney V, Verma P, Kamble NU, Ghosh S, Achary RK, Gautam S, Majee M (2022). Methionine sulfoxide reductase B5 plays a key role in preserving seed vigor and longevity in rice (Oryza sativa). New Phytol 236, 1042-1060. |
| [15] | He YQ, Cheng JP, He Y, Yang B, Cheng YH, Yang C, Wang ZS (2019). Influence of isopropylmalate synthase OsIPMS1 on seed vigour associated with amino acid and energy metabolism in rice. Plant Biotechnol J 17, 322-337. |
| [16] |
He ZH, Fujiki M, Kohorn BD (1996). A cell wall-associated receptor-like protein kinase. J Biol Chem 271, 19789-19793.
DOI PMID |
| [17] |
He ZH, He DZ, Kohorn BD (1998). Requirement for the induced expression of a cell wall associated receptor kinase for survival during the pathogen response. Plant J 14, 55-63.
DOI PMID |
| [18] | Hu W, Lv YY, Lei WR, Li X, Chen YH, Zheng LQ, Xia Y, Shen ZG (2014). Cloning and characterization of the Oryza sativa wall-associated kinase gene OsWAK11 and its transcriptional response to abiotic stresses. Plant Soil 384, 335-346. |
| [19] |
Huang JX, Cai MH, Long QZ, Liu LL, Lin QY, Jiang L, Chen SH, Wan JM (2014). OsLOX2, a rice type I lipoxygenase, confers opposite effects on seed germination and longevity. Transgenic Res 23, 643-655.
DOI PMID |
| [20] | Kanneganti V, Gupta AK (2011). RNAi mediated silencing of a wall associated kinase, OsiWAK1 in Oryza sativa results in impaired root development and sterility due to anther indehiscence: wall associated kinases from Oryza sativa. Physiol Mol Biol Plants 17, 65-77. |
| [21] |
Kaur H, Petla B, Kamble NU, Singh A, Rao V, Salvi P, Ghosh S, Majee M (2015). Differentially expressed seed aging responsive heat shock protein OsHSP18.2 implicates in seed vigor, longevity and improves germination and seedling establishment under abiotic stress. Front Plant Sci 6, 713.
DOI PMID |
| [22] | Kim DH, Han SH (2018). Seed coat and aging conditions affect germination and physiological changes of aging Korean pine seeds. J For Res 23, 372-379. |
| [23] |
Kohorn BD, Kobayashi M, Johansen S, Friedman HP, Fischer A, Byers N (2006). Wall-associated kinase 1 (WAK1) is crosslinked in endomembranes, and transport to the cell surface requires correct cell-wall synthesis. J Cell Sci 119, 2282-2290.
PMID |
| [24] |
Kohorn BD, Kohorn SL (2012). The cell wall-associated kinases, WAKs, as pectin receptors. Front Plant Sci 3, 88.
DOI PMID |
| [25] |
Koornneef M, Bentsink L, Hilhorst H (2002). Seed dormancy and germination. Curr Opin Plant Biol 5, 33-36.
DOI PMID |
| [26] | Kumari S, Joshi R, Singh K, Roy S, Tripathi AK, Singh P, Singla-Pareek SL, Pareek A (2015). Expression of a cyclophilin OsCyp2-P isolated from a salt-tolerant landrace of rice in tobacco alleviates stress via ion homeostasis and limiting ROS accumulation. Funct Integr Genomics 15, 395-412. |
| [27] |
Lally D, Ingmire P, Tong HY, He ZH (2001). Antisense expression of a cell wall-associated protein kinase, WAK4, inhibits cell elongation and alters morphology. Plant Cell 13, 1317-1331.
DOI PMID |
| [28] | Li H, Zhou SY, Zhao WS, Su SC, Peng YL (2009). A novel wall-associated receptor-like protein kinase gene, OsWAK1, plays important roles in rice blast disease resistance. Plant Mol Biol 69, 337-346. |
| [29] | Li SM, Dong LP, Sun JY, Ma J (2012). Effect of artificial accelerated aging of 2 wheat cultivars on seed germination and physiological and biochemical characteristics. J Jilin Agricul Sci 37(5), 18-20. (in Chinese) |
| 李淑梅, 董丽平, 孙君艳, 马俊 (2012). 人工加速老化对2个小麦品种发芽和种子生理生化特性的影响. 吉林农业科学 37(5), 18-20. | |
| [30] | Li XF, Zhou XX, Liu ZM (2005). On physiological and biochemical changes of artificially aged pepper seeds. J Hunan Agricul Univ Nat Sci 31, 265-268. (in Chinese) |
| 李雪峰, 邹学校, 刘志敏 (2005). 辣椒种子人工老化及劣变的生理生化变化. 湖南农业大学学报(自然科学版) 31, 265-268. | |
| [31] | Lim S, Park J, Lee N, Jeong J, Toh S, Watanabe A, Kim J, Kang H, Kim DH, Kawakami N, Choi G (2013). ABA-INSENSITIVE3, ABA-INSENSITIVE5, and DELLAs interact to activate the expression of SOMNUS and other high-temperature-inducible genes in imbibed seeds in Arabidopsis. Plant Cell 25, 4863-4878. |
| [32] | Liu J, Gui J, Gao W, Ma JF, Wang QZ (2016). Review of the physiological and biochemical reactions and molecular mechanisms of seed aging. Acta Ecol Sin 36, 4997-5006. (in Chinese) |
| 刘娟, 归静, 高伟, 马俊峰, 王佺珍 (2016). 种子老化的生理生化与分子机理研究进展. 生态学报 36, 4997-5006. | |
| [33] |
Livak KJ, Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402-408.
DOI PMID |
| [34] |
Ma XL, Zhang QY, Zhu QL, Liu W, Chen Y, Qiu R, Wang B, Yang ZF, Li HY, Lin YR, Xie YY, Shen RX, Chen SF, Wang Z, Chen YL, Guo JX, Chen LT, Zhao XC, Dong ZC, Liu YG (2015). A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol Plant 8, 1274-1284.
DOI PMID |
| [35] | Ma YX, Wang ZH, Humphries J, Ratcliffe J, Bacic A, Johnson KL, Qu GQ (2024). WALL-ASSOCIATED KINASE Like 14 regulates vascular tissue development in Arabidopsis and tomato. Plant Sci 341, 112013. |
| [36] | McDonald MB (1999). Seed deterioration: physiology, repair and assessment. Seed Sci Technol 27, 177-237. |
| [37] |
Osakabe Y, Maruyama K, Seki M, Satou M, Shinozaki K, Yamaguchi-Shinozaki K (2005). Leucine-rich repeat receptor-like kinase1 is a key membrane-bound regulator of abscisic acid early signaling in Arabidopsis. Plant Cell 17 1105-1119.
PMID |
| [38] | Pérez-Rodríguez JL, Ramos Aquino RG, Lorente González GY, González-Olmedo JL, Martínez Montero ME (2023). ROS production and antioxidant enzyme activity in relation to germination and vigor during tobacco seed development. Vegetos 36, 506-515. |
| [39] | Petla BP, Kamble NU, Kumar M, Verma P, Ghosh S, Singh A, Rao V, Salvi P, Kaur H, Saxena SC, Majee M (2016). Rice PROTEIN L-ISOASPARTYL METHYLTRANS- FERASE isoforms differentially accumulate during seed maturation to restrict deleterious isoAsp and reactive oxygen species accumulation and are implicated in seed vigor and longevity. New Phytol 211, 627-645. |
| [40] | Qun S, Wang JH, Sun BQ (2007). Advances on seed vigor physiological and genetic mechanisms. Agric Sci China 6, 1060-1066. |
| [41] |
Sattler SE, Gilliland LU, Magallanes-Lundback M, Pollard M, DellaPenna D (2004). Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell 16, 1419-1432.
DOI PMID |
| [42] |
Shiu SH, Bleecker AB (2001). Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc Natl Acad Sci USA 98, 10763-10768.
DOI PMID |
| [43] | Sun Q, Wang JH, Sun BQ (2007). Advances on seed vigor physiological and genetic mechanisms. Agric Sci China 6, 1060-1066. |
| [44] | Tamzil MS, Alfiko Y, Mubarok AF, Purwantomo S, Suwanto A, Budiarti S (2021). Development of auxotrophic Agrobacterium tumefaciens AGL1 by Tn5 transposon for rice (Oryza sativa L.) transformation. Biotechnol Bioproc Eng 26, 641-649. |
| [45] |
Tripathi RK, Aguirre JA, Singh J (2021). Genome-wide analysis of wall associated kinase (WAK) gene family in barley. Genomics 113, 523-530.
DOI PMID |
| [46] |
Wagner TA, Kohorn BD (2001). Wall-associated kinases are expressed throughout plant development and are required for cell expansion. Plant Cell 13, 303-318.
DOI PMID |
| [47] | Wang WQ, Xu DY, Sui YP, Ding XH, Song XJ (2022). A multiomic study uncovers a bZIP23-PER1A-mediated deto- xification pathway to enhance seed vigor in rice. Proc Natl Acad Sci USA 119, e2026355119. |
| [48] | Wang YC, Wang Y, Lu M, Wu HK, Cao DD (2018). Effects of artificial aging on physiological characteristics of rice seeds in different dormancy properties. Seed 37(6), 15-19. (in Chinese) |
| 王仪春, 王洋, 陆敏, 吴洪恺, 曹栋栋 (2018). 人工老化处理对不同休眠特性水稻种子生理特性的影响. 种子 37(6), 15-19. | |
| [49] | Wang YH, Xie HG, Chen FH, Lin Q, Cui LL, Wu FX, Wei YD, Luo X, Chen LP, Cai QH, Xie HA, Zhang JF (2023). Analysis of the high seed storability trait in indica rice “Fuxiangzhan”. Chin Sci Bull 68, 3857-3868. (in Chinese) |
| 王颖姮, 谢鸿光, 陈飞鹤, 林强, 崔丽丽, 吴方喜, 魏毅东, 罗曦, 陈丽萍, 蔡秋华, 谢华安, 张建福 (2023). 籼稻福香占耐储藏性的蛋白质组学分析. 科学通报 68, 3857-3868. | |
| [50] | Xia Y, Yin SJ, Zhang KL, Shi XT, Lian CL, Zhang HS, Hu ZB, Shen ZG (2018). OsWAK11, a rice wall-associated kinase, regulates Cu detoxification by alteration the immobilization of Cu in cell walls. Environ Exp Bot 150, 99-105. |
| [51] |
Xu J, Yang J, Duan XG, Jiang YM, Zhang P (2014). Increased expression of native cytosolic Cu/Zn superoxide dismutase and ascorbate peroxidase improves tolerance to oxidative and chilling stresses in cassava (Manihot esculenta Crantz). BMC Plant Biol 14, 208.
DOI PMID |
| [52] | Xu M, Li JL, Zhu H, Jin LL, Wang ZS (2018). Comparative study on physiological and biochemical indexes changes in the aging process of cotton seeds. Seed 37(2), 14-18. (in Chinese) |
| 徐敏, 李憬霖, 朱鹤, 金路路, 王子胜 (2018). 棉花种子老化过程中生理生化指标变化比较研究. 种子 37(2), 14-18. | |
| [53] | Yadav S, Parihar S (2013). Seed germination and viability testing—principles and techniques. In: Basu S, Pariha SS, Lal SK, Arun Kumar MB, eds. Emerging Paradigms in Hybrid Seed Production, Plant Variety Protection, Value Addition and Quality Assurance for Enhancing Productivity and Sustainable Crop Production. New Delhi: Indian Agricultural Research Institute. pp. 205-217. |
| [54] | Yan WQ, Hu PL, Ni YX, Zhao H, Liu XT, Cao HC, Jia M, Tian BM, Miao HM, Liu HY (2023). Genome-wide characterization of the wall-associated kinase-like (WAKL) family in sesame (Sesamum indicum) identifies a SiWAKL6 gene involved in resistance to Macrophomina Phaseolina. BMC Plant Biol 23, 624. |
| [55] | Yang YQ, Wang XF (2004). Advances on relation-ship between biomembrane and seed vigor. Chin Bull Bot 21(6), 641-648. (in Chinese) |
| 杨永青, 汪晓峰 (2004). 种子活力与生物膜的研究现状. 植物学通报 21(6), 641-648. | |
| [56] | Yin XY, Hou XW (2017). Role of OsWAK124, a rice wall-associated kinase, in response to environmental heavy metal stresses. Pak J Bot 49, 1255-1261. |
| [57] | Zhang CQ, Xu Y, Lu Y, Yu HX, Gu MH, Liu QQ (2011). regulates stem elongation and seed development in rice. Planta 234, 541-554. |
| [58] | Zhang HB, Yang GJ, Gao WD, Zhu Y, Huang F, Pei HF, Li QM (2019). Study on the seed vigor of Toona sinensis under specific storage conditions. For Res 32(2), 152-159. |
| 张海波, 杨桂娟, 高卫东, 祝燕, 黄放, 裴昊斐, 李庆梅 (2019). 香椿种子特定贮藏条件下活力变化的研究. 林业科学研究 32(2), 152-159. | |
| [59] |
Zhang RG, Guo XC, Zhang YL, Tian CR (2020). Influence of modified atmosphere treatment on post-harvest reactive oxygen metabolism of pomegranate peels. Nat Prod Res 34, 740-744.
DOI PMID |
| [60] | Zhang YX, Fan F, Zhang QJ, Luo YJ, Liu QJ, Gao JD, Liu J, Chen GH, Zhang HQ (2022). Identification and functional analysis of long non-coding RNA (lncRNA) in response to seed aging in rice. Plants 11, 3223. |
| [61] | Zhao B, Zhang H, Chen TX, Ding L, Zhang LY, Ding XL, Zhang J, Qian Q, Xiang Y (2022). Sdr4dominates pre- harvest sprouting and facilitates adaptation to local climatic condition in Asian cultivated rice. J Integr Plant Biol 64, 1246-1263. |
| [62] | Zhao J, He YQ, Huang SL, Wang ZF (2021). Advances in the identification of quantitative trait loci and genes involved in seed vigor in rice. Front Plant Sci 12, 659307. |
| [63] | Zhao S, Zhao YL, Pan XQ, Zhang JJ, Huang DF (2019). Artificial aging of cabbage seeds and biological effects. North Hortic (24), 7-13. (in Chinese) |
| 赵硕, 赵颖雷, 潘学勤, 章竞瑾, 黄丹枫 (2019). 甘蓝种子的人工老化及其生物学效应. 北方园艺 (24), 7-13. | |
| [64] | Zhou YL, Chu P, Chen HH, Li Y, Liu J, Ding Y, Tsang EWT, Jiang LW, Wu KQ, Huang SZ (2012). Overexpression of Nelumbo nucifera metallothioneins 2a and 3 enhances seed germination vigor in Arabidopsis. Planta 235, 523-537. |
| [1] | 樊蓓, 任敏, 王延峰, 党峰峰, 陈国梁, 程国亭, 杨金雨, 孙会茹. 番茄SlWRKY45转录因子在响应低温和干旱胁迫中的功能(长英文摘要)[J]. 植物学报, 2025, 60(2): 186-203. |
| [2] | 余玉蓉, 吴浩, 高娅菲, 赵媛博, 李小玲, 卜贵军, 薛丹, 刘正祥, 武海雯, 吴林. 模拟氮沉降对鄂西南湿地泥炭藓生理及形态特征的影响[J]. 植物生态学报, 2023, 47(11): 1493-1506. |
| [3] | 王琦, 许艳丽, 闫鹏, 董好胜, 张薇, 卢霖, 董志强. PAC对谷子花后土壤氮素供应和叶片抗氧化特性的影响[J]. 植物学报, 2023, 58(1): 90-107. |
| [4] | 都业勤, 张迪, 王赛, 王磊, 闫兴富, 唐占辉. 湿地植物大花百合种群的性系统特征[J]. 生物多样性, 2021, 29(10): 1321-1335. |
| [5] | 李晶, 周天阳, 鲁雪丽, 李新涛, 孙斌, 孟红杰. 珍稀植物连香树在其中国分布区北缘的种子性状及幼苗更新限制[J]. 生物多样性, 2020, 28(10): 1161-1173. |
| [6] | 许馨露, 李丹丹, 马元丹, 翟建云, 孙建飞, 高岩, 张汝民. 四季桂抗氧化防御系统对干旱、高温及协同胁迫的响应[J]. 植物学报, 2018, 53(1): 72-81. |
| [7] | 许红梅, 李进, 张元明. 水分条件对人工培养齿肋赤藓光化学效率及生理特性的影响[J]. 植物生态学报, 2017, 41(8): 882-893. |
| [8] | 刘盟盟, 贾丽, 程路芸, 张洪芹, 臧晓琳, 宝音陶格涛, 张汝民, 高岩. 冷蒿酚酸及其抗氧化防御酶活性对机械损伤的响应[J]. 植物生态学报, 2017, 41(2): 219-230. |
| [9] | 陈思羽, 刘鹏, 朱末, 夏冬冬, 李亮, 徐克章, 陈展宇, 张治安. 大豆植株不同冠层种子活力及其萌发中抗氧化酶活性[J]. 植物学报, 2016, 51(1): 24-30. |
| [10] | 尹本丰, 张元明. 冻融过程对荒漠区不同微生境下齿肋赤藓渗透调节物含量和抗氧化酶活力的影响[J]. 植物生态学报, 2015, 39(5): 517-529. |
| [11] | 郭慧媛, 马元丹, 王丹, 左照江, 高岩, 张汝民, 王玉魁. 模拟酸雨对毛竹叶片抗氧化酶活性及释放绿叶挥发物的影响[J]. 植物生态学报, 2014, 38(8): 896-903. |
| [12] | 耿东梅, 单立山, 李毅, Жигунов Анатолий Васильевич. 土壤水分胁迫对红砂幼苗叶绿素荧光和抗氧化酶活性的影响[J]. 植物学报, 2014, 49(3): 282-291. |
| [13] | 陈坚,李妮亚,刘强,钟才荣,黄敏,曾佳. NaCl处理下两种引进红树的光合及抗氧化防御能力[J]. 植物生态学报, 2013, 37(5): 443-453. |
| [14] | 刘柿良, 马明东, 潘远智, 魏刘利, 何成相, 杨开茂. 不同光强对两种桤木幼苗光合特性和抗氧化系统的影响[J]. 植物生态学报, 2012, 36(10): 1062-1074. |
| [15] | 刘长成, 刘玉国, 郭柯. 四种不同生活型植物幼苗对喀斯特生境干旱的生理生态适应性[J]. 植物生态学报, 2011, 35(10): 1070-1082. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||