

植物学报 ›› 2022, Vol. 57 ›› Issue (5): 649-660.DOI: 10.11983/CBB22049 cstr: 32102.14.CBB22049
张和臣, 王慧娟, 李艳敏, 高杰, 袁欣, 王利民, 王校晨, 赵银鸽, 符真珠*( )
)
收稿日期:2022-03-12
接受日期:2022-06-23
出版日期:2022-09-01
发布日期:2022-09-09
通讯作者:
符真珠
作者简介:*E-mail: pearlgh2005@163.com基金资助:
Zhang Hechen, Wang Huijuan, Li Yanmin, Gao Jie, Yuan Xin, Wang Limin, Wang Xiaochen, Zhao Yinge, Fu Zhenzhu*( )
)
Received:2022-03-12
Accepted:2022-06-23
Online:2022-09-01
Published:2022-09-09
Contact:
Fu Zhenzhu
About author:*E-mail: pearlgh2005@163.com摘要: 月季(Rosa hybrida)花色丰富, 是世界著名的观赏花卉。月季红双喜因其花瓣的变色特性而在市场上广受欢迎。该研究通过类黄酮和类胡萝卜素靶向代谢并结合转录组分析, 发现红双喜的黄色花瓣呈色主要源于叶绿素、类胡萝卜素以及类黄酮的积累, 红色花瓣呈色主要是花青素苷积累增加且糖苷化的结果。花青素苷合成关键基因CHI、ANS和UFGT, 以及R2R3-MYB家族的AN2-like成员在红色花瓣中的强烈表达是花青素苷积累的分子基础; 类胡萝卜素成分改变及相关基因的表达变化在红双喜花瓣变色过程中也起重要作用, 并且miRNA156可能参与其调控过程。该研究揭示了月季红双喜花瓣变色的分子和化学基础, 研究结果为观赏植物花色分子设计育种提供了重要理论依据。
张和臣, 王慧娟, 李艳敏, 高杰, 袁欣, 王利民, 王校晨, 赵银鸽, 符真珠. 月季红双喜花瓣变色的化学基础及比较转录组分析. 植物学报, 2022, 57(5): 649-660.
Zhang Hechen, Wang Huijuan, Li Yanmin, Gao Jie, Yuan Xin, Wang Limin, Wang Xiaochen, Zhao Yinge, Fu Zhenzhu. The Chemical Composition and Transcriptome Analysis Reveal the Mechanism of Color Formation in Rosa hybrida cv. ‘Double delight’. Chinese Bulletin of Botany, 2022, 57(5): 649-660.
| Gene name | Pri- mers | Sequences (5′-3′) |
|---|---|---|
| CHI | F | GCAATACTCGGAGAAGGTTTCA |
| (LOC112182551) | R | CAATCACCGCATTTCCAAC |
| ANS | F | TAGAAGAAGGGAGGCTGGAG |
| (LOC112179310) | R | TGTGGAGGATGAAGGTGAGT |
| UFGT | F | TTGTAACACACTGCGGGTG |
| (LOC112172868) | R | GAACATCTCTGAGCATTCGTG |
| PSY | F | GCTGTTGCTCACCCATCAAG |
| (LOC112190337) | R | CAAACCTCACCACACCTATCG |
| LYCB | F | ACACAGACCCTTCCCTCCAA |
| (LOC112188432) | R | TGGTTCTTCCACAACGGTTT |
| ZDE | F | CCTGCCTGTCAATCTTGTAGAC |
| (LOC112189356) | R | TCCCACTATCACCACATCCTC |
| AN2-like1 | F | GCTGTAGACTGAGGTGGCTAAA |
| (LOC112185634) | R | GTGAAAGGACGATGGGCTA |
| AN2-like2 | F | GGACGAACTGGAAACGATG |
| (LOC112193894) | R | GTGATGCTTGTGTTGAGCG |
| AN2-like3 | F | GGAAGATGGCACAAGGTTC |
| (LOC112186121) | R | GCCGAGCACTCCAATAGTTT |
| UVR8 | F | GGCAGAGTTCTTTCTTGACAGAC |
| (LOC112182836) | R | GGCAATGCTGAGAGAGTTTCA |
| PIF3 | F | TGATGAGAAGATTGACCGAGG |
| (LOC112192990) | R | AGAAGACGGCGAAAGGCTA |
| HY5 | F | GGCATACTTGAGTGACTTGGAA |
| (LOC112172411) | R | CGGCTTGCTGTTGTGTTCT |
| GAPDH | F | TATGACCAGATCAAGGCTGCT |
| (JN399220) | R | ACCAATGAAGTCGGTTGACAC |
表1 qRT-PCR所用引物
Table 1 The primers used for qRT-PCR
| Gene name | Pri- mers | Sequences (5′-3′) |
|---|---|---|
| CHI | F | GCAATACTCGGAGAAGGTTTCA |
| (LOC112182551) | R | CAATCACCGCATTTCCAAC |
| ANS | F | TAGAAGAAGGGAGGCTGGAG |
| (LOC112179310) | R | TGTGGAGGATGAAGGTGAGT |
| UFGT | F | TTGTAACACACTGCGGGTG |
| (LOC112172868) | R | GAACATCTCTGAGCATTCGTG |
| PSY | F | GCTGTTGCTCACCCATCAAG |
| (LOC112190337) | R | CAAACCTCACCACACCTATCG |
| LYCB | F | ACACAGACCCTTCCCTCCAA |
| (LOC112188432) | R | TGGTTCTTCCACAACGGTTT |
| ZDE | F | CCTGCCTGTCAATCTTGTAGAC |
| (LOC112189356) | R | TCCCACTATCACCACATCCTC |
| AN2-like1 | F | GCTGTAGACTGAGGTGGCTAAA |
| (LOC112185634) | R | GTGAAAGGACGATGGGCTA |
| AN2-like2 | F | GGACGAACTGGAAACGATG |
| (LOC112193894) | R | GTGATGCTTGTGTTGAGCG |
| AN2-like3 | F | GGAAGATGGCACAAGGTTC |
| (LOC112186121) | R | GCCGAGCACTCCAATAGTTT |
| UVR8 | F | GGCAGAGTTCTTTCTTGACAGAC |
| (LOC112182836) | R | GGCAATGCTGAGAGAGTTTCA |
| PIF3 | F | TGATGAGAAGATTGACCGAGG |
| (LOC112192990) | R | AGAAGACGGCGAAAGGCTA |
| HY5 | F | GGCATACTTGAGTGACTTGGAA |
| (LOC112172411) | R | CGGCTTGCTGTTGTGTTCT |
| GAPDH | F | TATGACCAGATCAAGGCTGCT |
| (JN399220) | R | ACCAATGAAGTCGGTTGACAC |
| No. | Type of carotenoid | Y1 (μg·g-1) | Y2 (μg·g-1) | R1 (μg·g-1) | R2 (μg·g-1) | Log2FC | Regulated |
|---|---|---|---|---|---|---|---|
| Carotenoid_22 | Neochrome palmitate | 2.2016998 | 2.3834704 | 1.0197834 | 1.0475690 | -1.1491910 | Down |
| Carotenoid_24 | Rubixanthin laurate | 0.1569250 | 0.1481073 | 0.0527210 | 0.0507537 | -1.5596845 | Down |
| Carotenoid_32 | Violaxanthin dilaurate | 3.9240426 | 4.7154605 | 2.9313100 | 2.8637581 | -0.5761227 | Unchanged |
| Carotenoid_33 | Violaxanthin-myristate-caprate | 21.7732951 | 22.4958882 | 12.8019824 | 12.7492289 | -0.7929111 | Unchanged |
| Carotenoid_34 | Violaxanthin-myristate-laurate | 5.6980954 | 6.7643503 | 4.7010832 | 5.0801542 | -0.3494983 | Unchanged |
| Carotenoid_39 | Violaxanthin dioleate | 0.7922281 | 0.8738322 | 0.4032495 | 0.3662500 | -1.11444831 | Down |
| Carotenoid_41 | Zeaxanthin palmitate | 0.1189156 | 0.1116267 | 0.3534273 | 0.3530580 | 1.61562841 | Up |
| Carotenoid_51 | β-cryptoxanthin laurate | 0.7270018 | 0.7903577 | 0.3395545 | 0.3316843 | -1.1766652 | Down |
| Carotenoid_54 | β-cryptoxanthin oleate | 0.1793999 | 0.1468413 | 0.0389599 | 0.0467119 | -1.9290470 | Down |
| Carotenoid_56 | Zeaxanthin | 5.9183084 | 6.1692434 | 17.8138771 | 17.0472808 | 1.5280984 | Up |
| Carotenoid_57 | Violaxanthin | 4.9150727 | 4.8171053 | 3.8840384 | 3.8675731 | -0.3282664 | Unchanged |
| Carotenoid_58 | Neoxanthin | 2.0484948 | 2.2917558 | 1.9106499 | 1.9435004 | -0.1713655 | Unchanged |
| Carotenoid_59 | Lutein | 7.8872005 | 8.3871299 | 18.9305539 | 18.6268263 | 1.2064982 | Up |
| Carotenoid_66 | Canthaxanthin | 0.0005338 | 0.0004385 | 0 | 0 | -Inf | Down |
表2 月季红双喜不同呈色花瓣中主要类胡萝卜素的成分含量差异
Table 2 Differential carotenoid components in different colored petals of Rosa hybrida cv. ‘Double delight’
| No. | Type of carotenoid | Y1 (μg·g-1) | Y2 (μg·g-1) | R1 (μg·g-1) | R2 (μg·g-1) | Log2FC | Regulated |
|---|---|---|---|---|---|---|---|
| Carotenoid_22 | Neochrome palmitate | 2.2016998 | 2.3834704 | 1.0197834 | 1.0475690 | -1.1491910 | Down |
| Carotenoid_24 | Rubixanthin laurate | 0.1569250 | 0.1481073 | 0.0527210 | 0.0507537 | -1.5596845 | Down |
| Carotenoid_32 | Violaxanthin dilaurate | 3.9240426 | 4.7154605 | 2.9313100 | 2.8637581 | -0.5761227 | Unchanged |
| Carotenoid_33 | Violaxanthin-myristate-caprate | 21.7732951 | 22.4958882 | 12.8019824 | 12.7492289 | -0.7929111 | Unchanged |
| Carotenoid_34 | Violaxanthin-myristate-laurate | 5.6980954 | 6.7643503 | 4.7010832 | 5.0801542 | -0.3494983 | Unchanged |
| Carotenoid_39 | Violaxanthin dioleate | 0.7922281 | 0.8738322 | 0.4032495 | 0.3662500 | -1.11444831 | Down |
| Carotenoid_41 | Zeaxanthin palmitate | 0.1189156 | 0.1116267 | 0.3534273 | 0.3530580 | 1.61562841 | Up |
| Carotenoid_51 | β-cryptoxanthin laurate | 0.7270018 | 0.7903577 | 0.3395545 | 0.3316843 | -1.1766652 | Down |
| Carotenoid_54 | β-cryptoxanthin oleate | 0.1793999 | 0.1468413 | 0.0389599 | 0.0467119 | -1.9290470 | Down |
| Carotenoid_56 | Zeaxanthin | 5.9183084 | 6.1692434 | 17.8138771 | 17.0472808 | 1.5280984 | Up |
| Carotenoid_57 | Violaxanthin | 4.9150727 | 4.8171053 | 3.8840384 | 3.8675731 | -0.3282664 | Unchanged |
| Carotenoid_58 | Neoxanthin | 2.0484948 | 2.2917558 | 1.9106499 | 1.9435004 | -0.1713655 | Unchanged |
| Carotenoid_59 | Lutein | 7.8872005 | 8.3871299 | 18.9305539 | 18.6268263 | 1.2064982 | Up |
| Carotenoid_66 | Canthaxanthin | 0.0005338 | 0.0004385 | 0 | 0 | -Inf | Down |
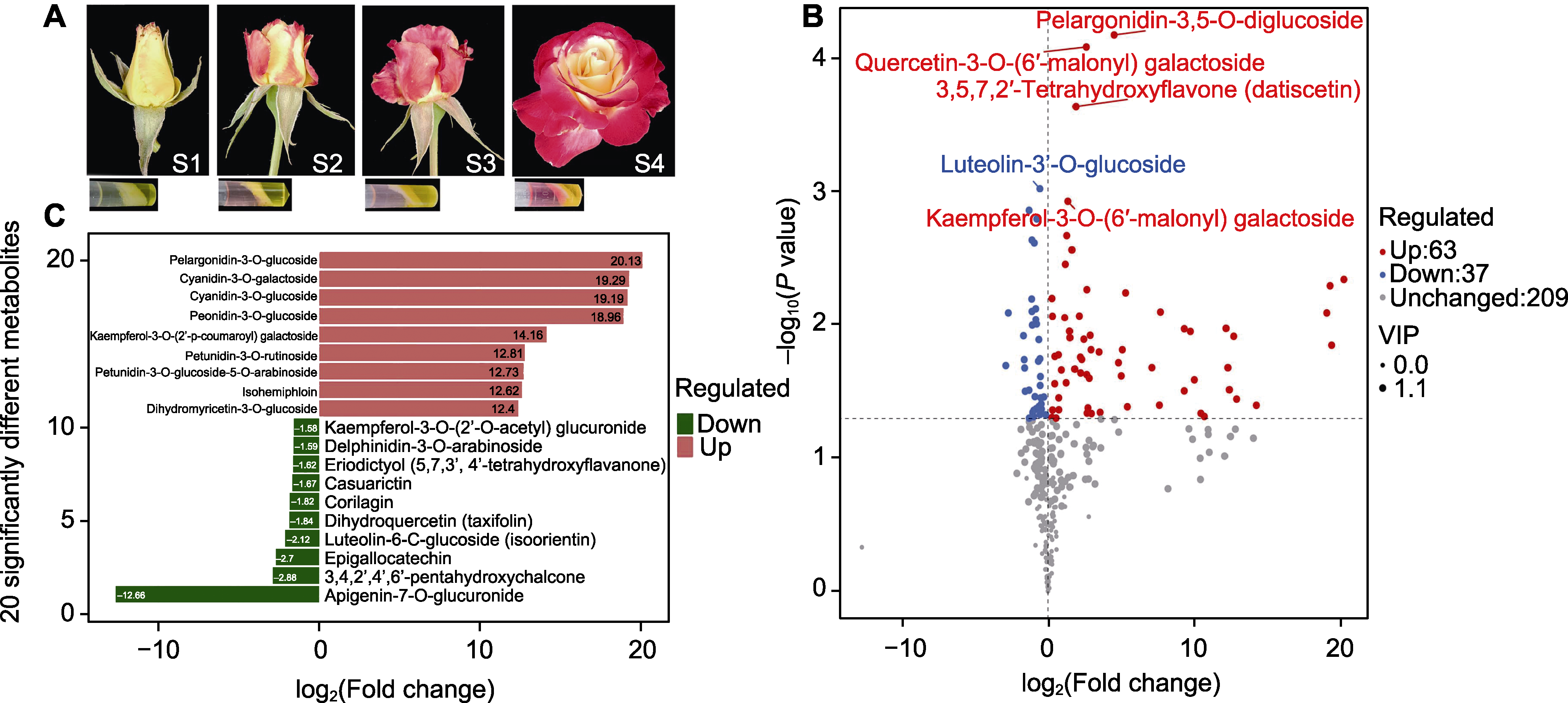
图1 月季红双喜不同发育时期花瓣呈色表型(A)、不同呈色花瓣类型中的类黄酮差异(B)及主要差异成分(C) S1: 萼片初展期; S2: 花瓣透色期; S3: 花瓣初开期; S4: 花瓣盛开期
Figure 1 The phenotype of the petal coloration (A), the difference of flavonoids (B), and the main different components (C) in petals of Rosa hybrida cv. ‘Double delight’ S1: The development stage when sepals are initially unfolded; S2: The development stage when petals are being colored; S3: The development stage when petals are just opened; S4: The development stage when petals are in full bloom
| Type | Total transcripts | Differentially expressed transcripts | Up regu- lated | Down regu- lated |
|---|---|---|---|---|
| mRNA | 36193 | 2250 | 1371 | 879 |
| miRNA | 7845 | 22 | 21 | 1 |
| LncRNA | 146605 | 51 | 24 | 27 |
表3 月季红双喜不同呈色花瓣中mRNA、miRNA和LncRNA的数量
Table 3 Transcripts of mRNA, miRNA and LncRNA in different colored petals of Rosa hybrida cv. ‘Double delight’
| Type | Total transcripts | Differentially expressed transcripts | Up regu- lated | Down regu- lated |
|---|---|---|---|---|
| mRNA | 36193 | 2250 | 1371 | 879 |
| miRNA | 7845 | 22 | 21 | 1 |
| LncRNA | 146605 | 51 | 24 | 27 |
| Comparison set | Total DEG | COG | GO | KEGG | KOG | NR | Pfam | Swiss-Prot | eggNOG |
|---|---|---|---|---|---|---|---|---|---|
| Y_vs_R | 2177 | 965 | 1904 | 1596 | 1034 | 2177 | 1923 | 1733 | 1858 |
表4 月季红双喜不同呈色花瓣中差异表达基因的功能注释
Table 4 Functional annotation of differentially expressed genes in different colored petals of Rosa hybrida cv. ‘Double delight’
| Comparison set | Total DEG | COG | GO | KEGG | KOG | NR | Pfam | Swiss-Prot | eggNOG |
|---|---|---|---|---|---|---|---|---|---|
| Y_vs_R | 2177 | 965 | 1904 | 1596 | 1034 | 2177 | 1923 | 1733 | 1858 |
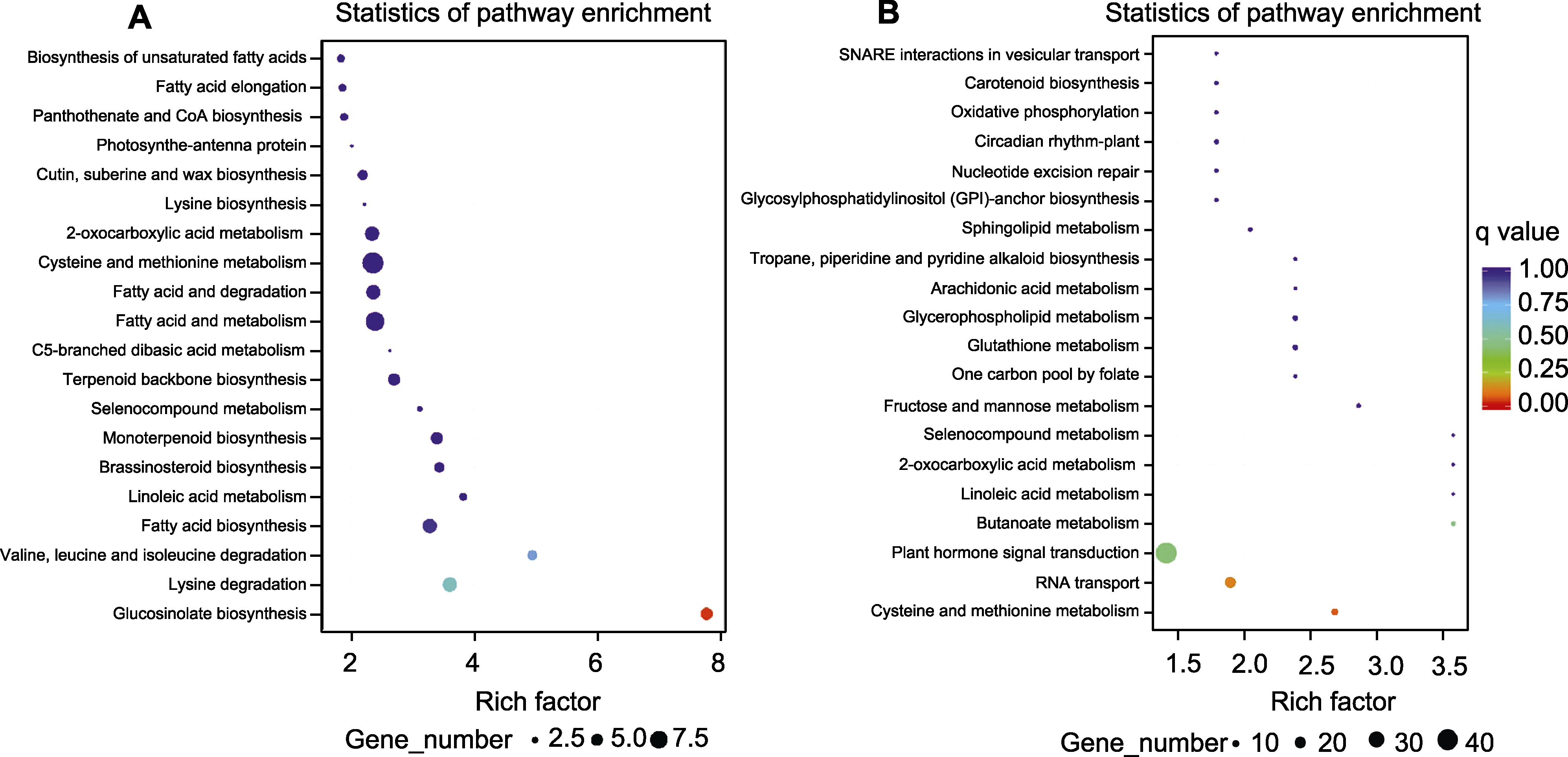
图2 月季红双喜不同呈色花瓣中差异LncRNA靶基因聚类(A)及差异siRNA靶基因聚类(B)
Figure 2 Analysis of differential LncRNA target genes (A) and siRNA target genes (B) clustering in different colored petals of Rosa hybrida cv. ‘Double delight’
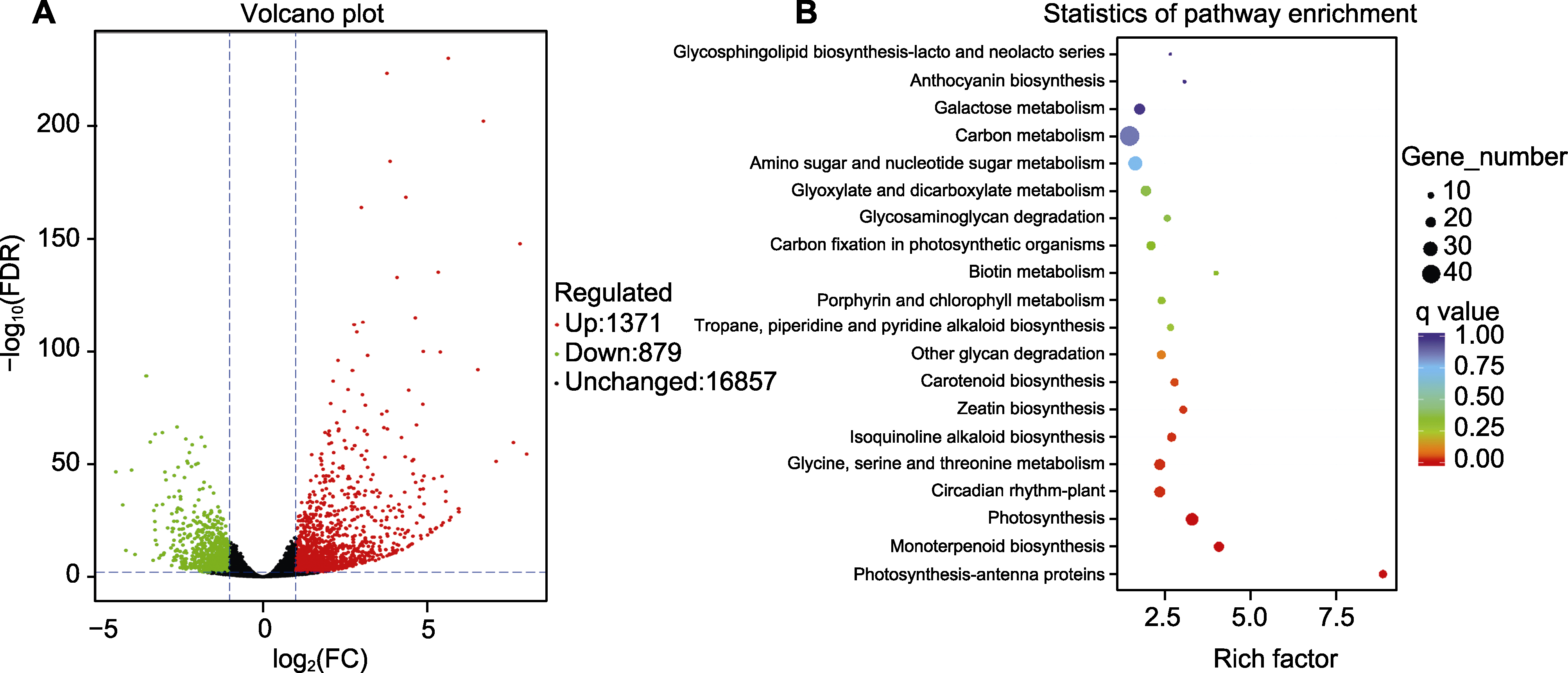
图3 月季红双喜不同呈色花瓣中的基因转录特性(A)及差异表达基因KEGG聚类(B) FDR: 错误发现率
Figure 3 Characteristics of gene expression (A) and KEGG clustering of DEGs (B) in different colored petals of Rosa hybrida cv. ‘Double delight’ FDR: False discovery rate
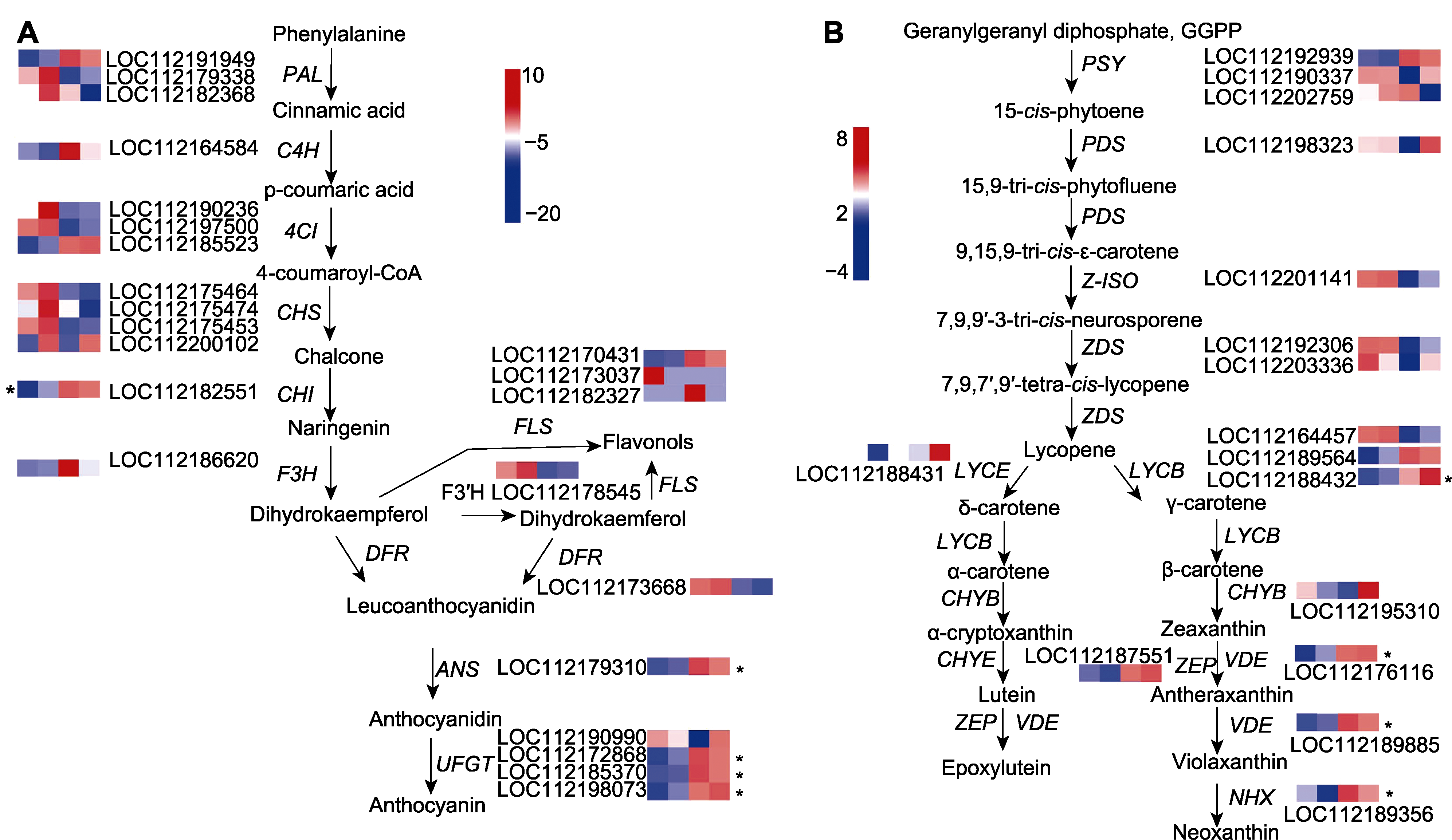
图4 月季红双喜花瓣中花青素苷及类胡萝卜素合成通路相关基因的表达特性分析 蓝色至红色代表基因表达由弱至强(彩图见网站版本)。*代表差异显著基因。
Figure 4 Expression pattern analysis of anthocyanin and carotenoid synthesis-related genes in the petals of Rosa hybrida cv. ‘Double delight’ The blue to red color represents gene expression from weak to strong (the color version is shown in online). *represent genes with significant difference.
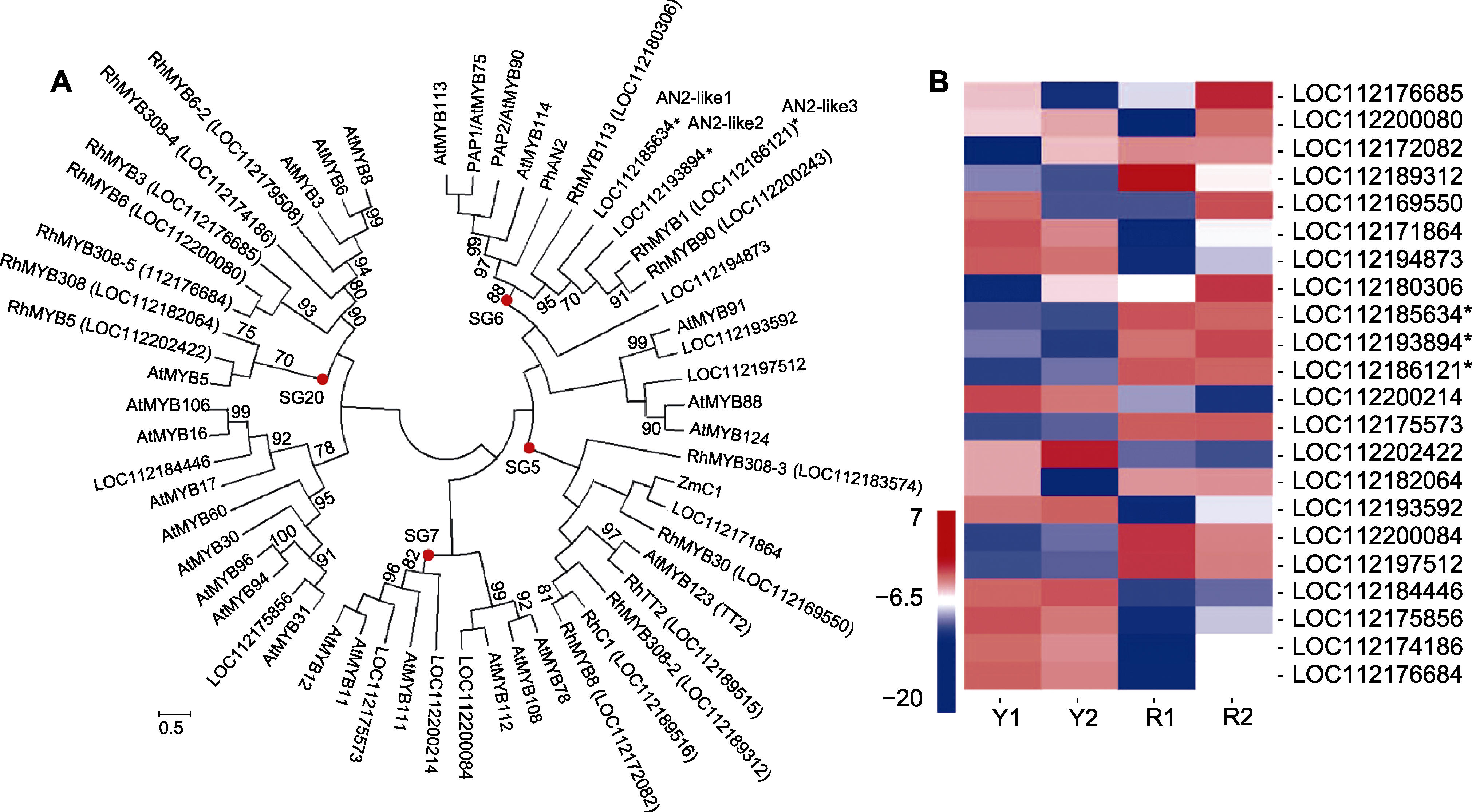
图5 月季红双喜不同呈色花瓣中差异表达R2R3-MYB基因和类黄酮调控相关R2R3-MYB基因进化分析(A)及R2R3-MYB相关基因的表达热图(B) Y和R同表2。*代表与花青素苷合成调控相关的R2R3-MYB基因。
Figure 5 Phylogenic analysis of differentially expressed R2R3-MYB and flavonoid regulation related genes (A), and heat map of the corresponding R2R3-MYB members in different colored petals of Rosa hybrida cv. ‘Double delight’ (B) Y and R see Table 2. * represent R2R3-MYB genes related to the regulation of anthocyanin biosynthesis.
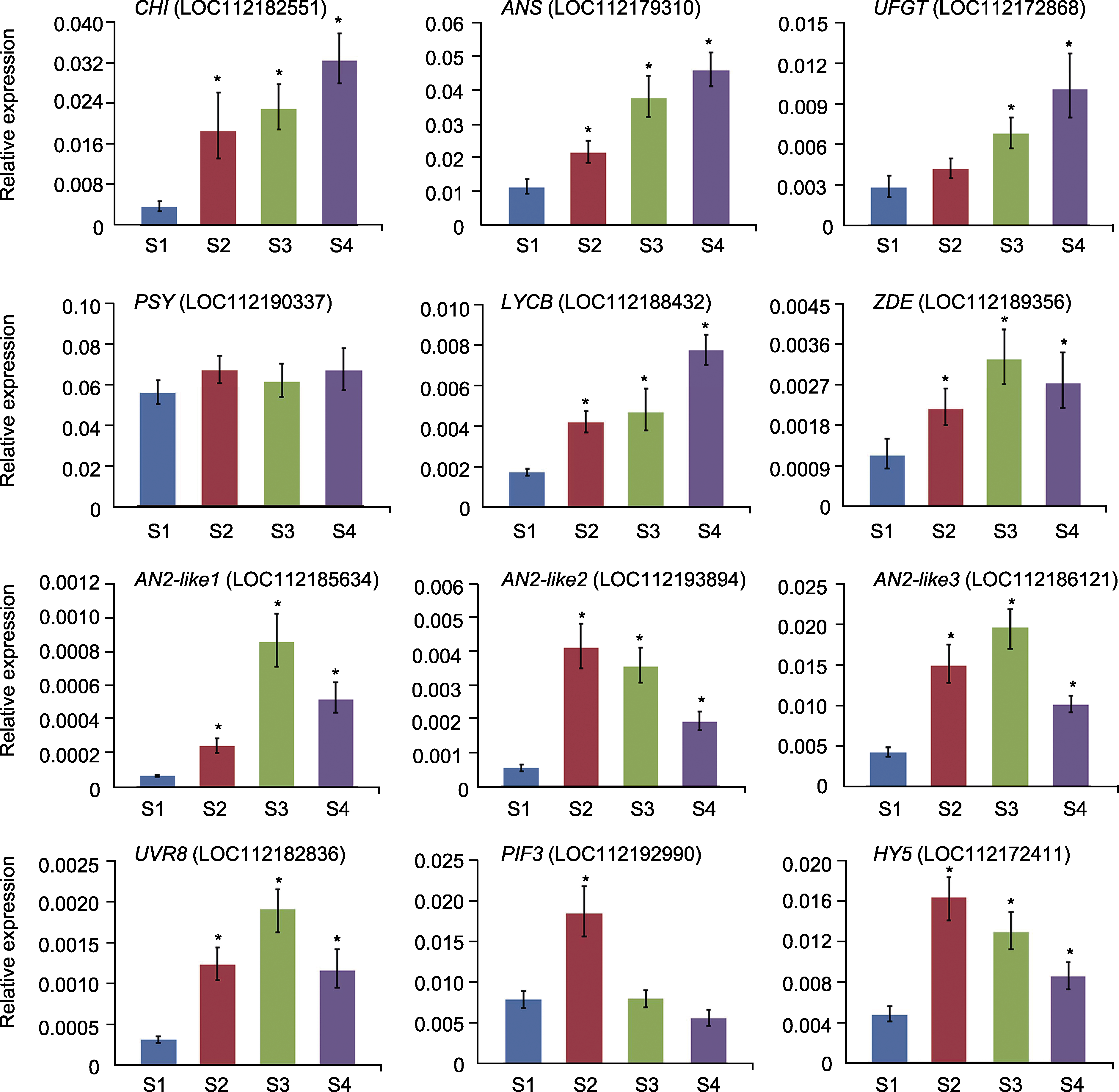
图6 qRT-PCR验证相关基因在月季红双喜花瓣不同发育时期的表达特性 S1-S4同图1。*表示与S1时期相比差异显著。
Figure 6 qRT-PCR analysis of the expression pattern of the corresponding genes in the Rosa hybrida cv. ‘Double delight’ petals at different developmental stages S1-S4 see Figure 1. * mean significant differences compared with S1 stage.
| [1] | 李茂福, 杨媛, 王华, 范又维, 孙佩, 金万梅 (2022). 月季中 R2R3-MYB基因RhMYB113c调控花青素苷合成. 园艺学报 49, 1957-1966. |
| [2] | 王峰, 杨树华, 刘新艳, 崔娇鹏, 常智慧, 葛红 (2017). 月季种质资源花色多样性及其与花青苷的关系. 园艺学报 44, 1125-1134. |
| [3] | 温佳辛, 王超林, 冯慧, 李珊珊, 王亮生, 武荣花, 赵世伟 (2021). 月季花色研究进展. 园艺学报 48, 2044-2056. |
| [4] | 张泰然, 张和臣, 武荣花 (2020). 蓝色花形成分子机理研究进展. 植物学报 55, 216-227. |
| [5] | 朱满兰, 王亮生, 张会金, 徐彦军, 郑绪辰, 王丽金 (2012). 耐寒睡莲花瓣中花青素苷组成及其与花色的关系. 植物学报 47, 437-453. |
| [6] | 邹红竹, 周琳, 韩璐璐, 吕纪杭, 王雁 (2021). 滇牡丹花瓣着色过程中类胡萝卜素成分变化和相关基因表达分析. 园艺学报 48, 1934-1944. |
| [7] | Bradley D, Xu P, Mohorianu II, Whibley A, Field D, Tavares H, Couchman M, Copsey L, Carpenter R, Li MM, Li Q, Xue YB, Dalmay T, Coen E (2017). Evolution of flower color pattern through selection on regulatory small RNAs. Science 358, 925-928. |
| [8] | Fu ZZ, Jiang H, Chao YC, Dong XY, Yuan X, Wang LM, Zhang J, Xu ML, Wang HJ, Li YM, Gao J, Zhang HC (2021). Three paralogous R2R3-MYB genes contribute to delphinidin-related anthocyanins synthesis in Petunia hybrida. J Plant Growth Regul 40, 1687-1700. |
| [9] | Fu ZZ, Shang HQ, Jiang H, Gao J, Dong XY, Wang HJ, Li YM, Wang LM, Zhang J, Shu QY, Chao YC, Xu ML, Wang R, Wang LS, Zhang HC (2020). Systematic identification of the light-quality responding anthocyanin synthesis-related transcripts in Petunia petals. Hortic Plant J 6, 428-438. |
| [10] | González-Villagra J, Kurepin LV, Reyes-Díaz MM (2017). Evaluating the involvement and interaction of abscisic acid and miRNA156 in the induction of anthocyanin biosynthesis in drought-stressed plants. Planta 246, 299-312. |
| [11] | Grotewold E (2006). The genetics and biochemistry of floral pigments. Annu Rev Plant Biol 57, 761-780. |
| [12] | Katsumoto Y, Fukuchi-Mizutani M, Fukui Y, Brugliera F, Holton TA, Karan M, Nakamura N, Yonekura-Sakakibara K, Togami J, Pigeaire A, Tao GQ, Nehra NS, Lu CY, Dyson BK, Tsuda S, Ashikari T, Kusumi T, Mason JG, Tanaka Y (2007). Engineering of the rose flavonoid biosynthetic pathway successfully generated blue-hued flowers accumulating delphinidin. Plant Cell Physiol 48, 1589-1600. |
| [13] | Klie M, Debener T (2011). Identification of superior reference genes for data normalisation of expression studies via quantitative PCR in hybrid roses (Rosa hybrida). BMC Res Notes 4, 518. |
| [14] | Koes R, Verweij W, Quattrocchio F (2005). Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci 10, 236-242. |
| [15] | Li ZJ, Zhao MY, Jin JF, Zhao LY, Xu ZD (2018). Anthocyanins and their biosynthetic genes in three novel-colored Rosa rugosa cultivars and their parents. Plant Physiol Biochem 129, 421-428. |
| [16] | Liu CC, Chi C, Jin LJ, Zhu JH, Yu JQ, Zhou YH (2018). The bZip transcription factor HY5 mediates CRY1a-induced anthocyanin biosynthesis in tomato. Plant Cell Environ 41, 1762-1775. |
| [17] | Liu ZJ, Zhang YQ, Wang JF, Li P, Zhao CZ, Chen YD, Bi YR (2015). Phytochrome-interacting factors PIF4 and PIF5 negatively regulate anthocyanin biosynthesis under red light in Arabidopsis seedlings. Plant Sci 238, 64-72. |
| [18] | Llorente B, Martinez-Garcia JF, Stange C, Rodriguez- Concepcion M (2017). Illuminating colors: regulation of carotenoid biosynthesis and accumulation by light. Curr Opin Plant Biol 37, 49-55. |
| [19] | Moehs CP, Tian L, Osteryoung KW, DellaPenna D (2001). Analysis of carotenoid biosynthetic gene expression during marigold petal development. Plant Mol Biol 45, 281-293. |
| [20] | Podolec R, Ulm R (2018). Photoreceptor-mediated regulation of the COP1/SPA E3 ubiquitin ligase. Curr Opin Plant Biol 45, 18-25. |
| [21] | Provenzano S, Spelt C, Hosokawa S, Nakamura N, Brugliera F, Demelis L, Geerke DP, Schubert A, Tanaka Y, Quattrocchio F, Koes R (2014). Genetic control and evolution of anthocyanin methylation. Plant Physiol 165, 962-977. |
| [22] | Sasaki N, Nakayama T (2015). Achievements and perspectives in biochemistry concerning anthocyanin modification for blue flower coloration. Plant Cell Physiol 56, 28-40. |
| [23] | Sui X, Zhao MY, Han X, Zhao LY, Xu ZD (2019). RrGT1, a key gene associated with anthocyanin biosynthesis, was isolated from Rosa rugosa and identified via overexpression and VIGS. Plant Physiol Biochem 135, 19-29. |
| [24] | Tanaka Y, Ohmiya A (2008). Seeing is believing: engineering anthocyanin and carotenoid biosynthetic pathways. Curr Opin Biotechnol 19, 190-197. |
| [25] | Tu ZH, Xia H, Yang LC, Zhai XY, Shen YF, Li HG (2022). The roles of microRNA-long non-coding RNA-mRNA networks in the regulation of leaf and flower development in Liriodendron chinense. Front Plant Sci 13, 816875. |
| [26] | Watkins JL, Pogson BJ (2020). Prospects for carotenoid biofortification targeting retention and catabolism. Trends Plant Sci 25, 501-512. |
| [27] | Xu WJ, Dubos C, Lepiniec L (2015). Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci 20, 176-185. |
| [28] | Yamagishi M (2011). Oriental hybrid lily Sorbonne homo-logue of LhMYB12regulates anthocyanin biosyntheses in flower tepals and tepal spots. Mol Breed 28, 381-389. |
| [29] | Zhang HC, Koes R, Shang HQ, Fu ZZ, Wang LM, Dong XY, Zhang J, Passeri V, Li YB, Jiang H, Gao J, Li YM, Wang HJ, Quattrocchio FM (2019). Identification and functional analysis of three new anthocyanin R2R3-MYB genes in Petunia. Plant Direct 3, e00114. |
| [30] | Zimmermann IM, Heim MA, Weisshaar B, Uhrig JF (2004). Comprehensive identification of Arabidopsis thaliana MYB transcription factors interacting with R/B-like BHLH proteins. Plant J 40, 22-34. |
| [1] | 王顺雨, 李杨, 吕晓琴, 李欣, 范权秀, 王晓月. 熊蜂盗蜜的花色偏好及对长距忍冬繁殖适合度的影响[J]. 生物多样性, 2025, 33(4): 24554-. |
| [2] | 热依拉穆·麦麦提吐尔逊, 艾沙江·阿不都沙拉木. 石榴花瓣和雄蕊对其传粉过程与繁殖成功的影响[J]. 生物多样性, 2023, 31(7): 22633-. |
| [3] | 薛泊宁, 张雁云, 董路. 基于类胡萝卜素着色的鸟类羽色多样性形成机制[J]. 生物多样性, 2021, 29(6): 843-854. |
| [4] | 马朝峰,戴思兰. 光受体介导信号转导调控植物开花研究进展[J]. 植物学报, 2019, 54(1): 9-22. |
| [5] | 何卿, 孙国峰, 林秦文, 李晓东, 张金政. 植物类胡萝卜素提取与分析技术研究进展[J]. 植物学报, 2018, 53(5): 700-709. |
| [6] | 胡海涛, 程珍霞, 李玲艳, 陈建华, 杨玲. 胡颓子属5种植物果实主要类胡萝卜素成分及含量[J]. 植物学报, 2016, 51(3): 306-310. |
| [7] | 蒋裕良, 白坤栋, 郭屹立, 王斌, 李冬兴, 李先琨, 刘志尚. 北热带喀斯特森林木本植物花性状及其生境分异[J]. 生物多样性, 2016, 24(2): 148-156. |
| [8] | 林魁, 徐永. LED照明对植物体内功能性化学物质积累的影响[J]. 植物学报, 2015, 50(2): 263-271. |
| [9] | 赵翔, 赵青平, 杨煦, 慕世超, 张骁. 向光素调节植物向光性及其与光敏色素/隐花色素的相互关系[J]. 植物学报, 2015, 50(1): 122-132. |
| [10] | 冯欢, 易姝利, 谢佳恒, 雷梦琦, 黄萱. 微型月季愈伤组织诱导及植株再生[J]. 植物学报, 2014, 49(5): 595-602. |
| [11] | 王小菁, 杨玉萍. 我国观赏花卉品质形成的功能基因研究进展[J]. 植物学报, 2013, 48(5): 471-480. |
| [12] | 朱满兰, 王亮生, 张会金, 徐彦军, 郑绪辰, 王丽金. 耐寒睡莲花瓣中花青素苷组成及其与花色的关系[J]. 植物学报, 2012, 47(5): 437-453. |
| [13] | 陈雪, 张金柱, 潘兵兵, 桑成瑾, 马雪, 杨涛, 车代弟. 月季愈伤组织的诱导及植株再生[J]. 植物学报, 2011, 46(5): 569-574. |
| [14] | 刘安成, 李慧, 王亮生, 庞长民, 卫伟光. 石榴类黄酮代谢产物的研究进展[J]. 植物学报, 2011, 46(2): 129-137. |
| [15] | 孙翊, 李慧, 王亮生, 戴思兰. 一种快速有效分析烟草花冠中花青素苷的方法[J]. 植物学报, 2011, 46(2): 189-196. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||