

植物学报 ›› 2021, Vol. 56 ›› Issue (5): 584-593.DOI: 10.11983/CBB21104 cstr: 32102.14.CBB21104
收稿日期:2021-07-01
接受日期:2021-08-09
出版日期:2021-09-01
发布日期:2021-08-31
通讯作者:
沈文锦,高彩吉
作者简介:gaocaiji@m.scnu.edu.cn基金资助:
Jiayi Kuang, Hongqing Li, Wenjin Shen*( ), Caiji Gao*(
), Caiji Gao*( )
)
Received:2021-07-01
Accepted:2021-08-09
Online:2021-09-01
Published:2021-08-31
Contact:
Wenjin Shen,Caiji Gao
摘要: 邻近标记作为近些年发展起来的一项检测活细胞内蛋白互作关系和亚细胞结构蛋白组的新型技术, 已成功应用于多种动植物体系的研究。该技术通过给诱饵蛋白融合一个具有特定催化连接活性的酶, 在酶的催化作用下将小分子底物(如生物素)共价连接到酶邻近的内源蛋白, 通过富集和分析被标记的蛋白可获得与诱饵互作的蛋白组。经定向进化产生的生物素连接酶TurboID具有无蛋白毒性及催化效率高的优势。利用TurboID介导的邻近标记技术分析感兴趣蛋白的邻近蛋白组, 可研究细胞内瞬时发生或微弱的蛋白互作网络, 进而解析复杂的生物学过程。该文详细描述了在拟南芥(Arabidopsis thaliana)中基于TurboID的邻近标记实验方法及注意事项, 旨在为利用这一新技术研究植物蛋白互作关系提供参考。
邝嘉怡, 李洪清, 沈文锦, 高彩吉. 基于TurboID的植物蛋白邻近标记实验方法. 植物学报, 2021, 56(5): 584-593.
Jiayi Kuang, Hongqing Li, Wenjin Shen, Caiji Gao. Methods for TurboID-based Proximal Labeling in Plants. Chinese Bulletin of Botany, 2021, 56(5): 584-593.
| Solution | Composition | Working concentration |
|---|---|---|
| Protein Extraction Buffer | Tris-HCl (pH7.5) | 50 mmol∙L-1 |
| NaCl | 150 mmol∙L-1 | |
| Sodium deoxycholate | 0.5% (w/v) | |
| SDS | 0.1% (w/v) | |
| EDTA | 1 mmol∙L-1 | |
| Triton X-100 | 1% (v/v) | |
| DTT | 1 mmol∙L-1 | |
| PMSF | 1 mmol∙L-1 | |
| Leupeptin | 10 μg∙mL-1 | |
| Protease Inhibitor Cocktail | 1× | |
| 4×SDS Sample Buffer | Tris-HCl (pH6.8) | 200 mmol∙L-1 |
| SDS | 8% (w/v) | |
| Glycerol | 40% (v/v) | |
| β-mercaptoethanol | 20% (v/v) | |
| Bromophenol blue | 0.1% (w/v) | |
| 1×PBS buffer (pH7.4) | NaH2PO4∙2H2O | 0.263 g∙L-1 |
| Na2HPO4∙12H2O | 1.856 g∙L-1 | |
| NaCl | 8 g∙L-1 | |
| KCl | 0.2 g∙L-1 | |
| 1×PBST | 1×PBS buffer containing 0.05% Tween-20 | |
| Biotin stock (stored at -20°C) | Dissolve biotin in DMSO to a final concentration of 50 mmol∙L-1 | |
表1 实验所用相关试剂配方
Table 1 Related reagent formulations used in experiment
| Solution | Composition | Working concentration |
|---|---|---|
| Protein Extraction Buffer | Tris-HCl (pH7.5) | 50 mmol∙L-1 |
| NaCl | 150 mmol∙L-1 | |
| Sodium deoxycholate | 0.5% (w/v) | |
| SDS | 0.1% (w/v) | |
| EDTA | 1 mmol∙L-1 | |
| Triton X-100 | 1% (v/v) | |
| DTT | 1 mmol∙L-1 | |
| PMSF | 1 mmol∙L-1 | |
| Leupeptin | 10 μg∙mL-1 | |
| Protease Inhibitor Cocktail | 1× | |
| 4×SDS Sample Buffer | Tris-HCl (pH6.8) | 200 mmol∙L-1 |
| SDS | 8% (w/v) | |
| Glycerol | 40% (v/v) | |
| β-mercaptoethanol | 20% (v/v) | |
| Bromophenol blue | 0.1% (w/v) | |
| 1×PBS buffer (pH7.4) | NaH2PO4∙2H2O | 0.263 g∙L-1 |
| Na2HPO4∙12H2O | 1.856 g∙L-1 | |
| NaCl | 8 g∙L-1 | |
| KCl | 0.2 g∙L-1 | |
| 1×PBST | 1×PBS buffer containing 0.05% Tween-20 | |
| Biotin stock (stored at -20°C) | Dissolve biotin in DMSO to a final concentration of 50 mmol∙L-1 | |
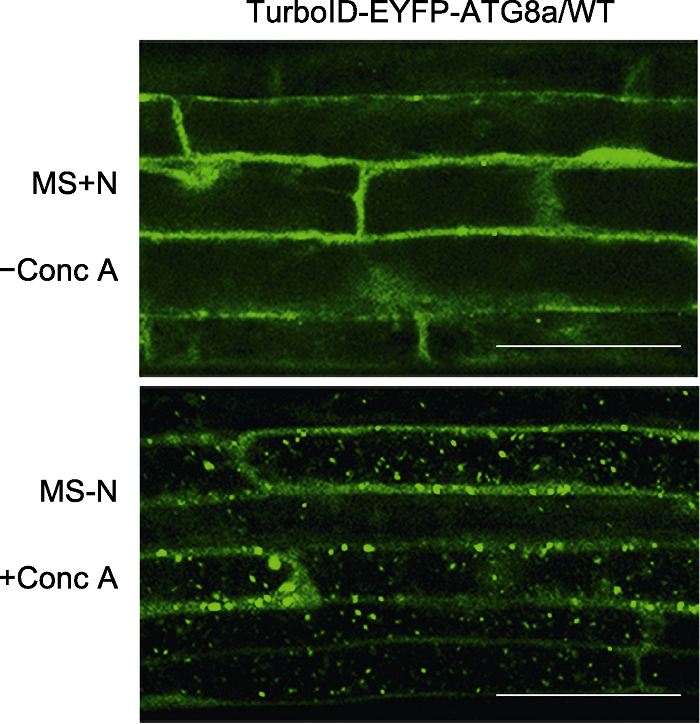
图2 稳定转化拟南芥中TurboID-EYFP-ATG8a的亚细胞定位 将6日龄转TurboID-EYFP-ATG8a拟南芥幼苗转移至含1 µmol∙L-1 Conc A的MS缺氮液体培养基(MS-N)处理8小时, 在激光共聚焦荧光显微镜下观察亚细胞定位。Bars=50 μm
Figure 2 Subcellular localization of TurboID-EYFP-ATG8a in stable Arabidopsis lines 6-day-old transgenic Arabidopsis seedlings overexpressing TurboID-EYFP-ATG8a were transferred to nitrogen (N)- deficient medium with 1 µmol∙L-1 Conc A for 8 h followed by confocal microscopic analysis. Bars=50 μm
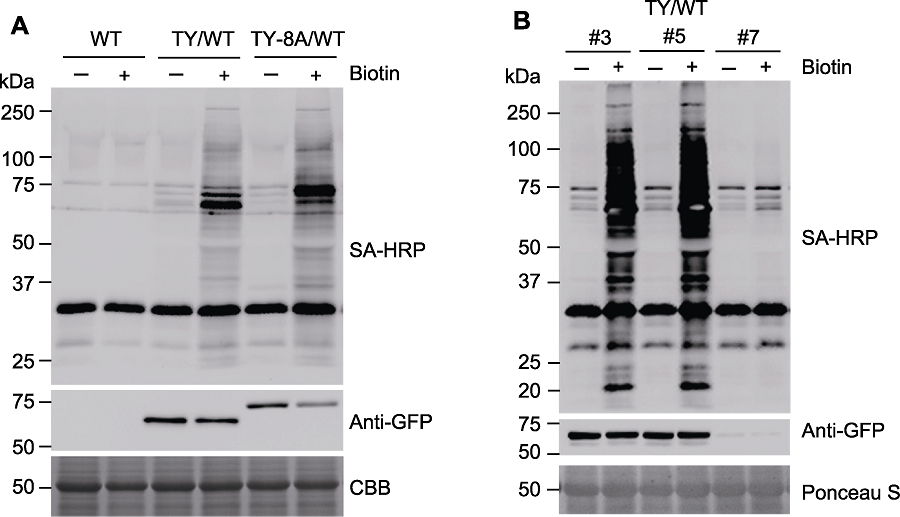
图3 生物素标记蛋白的免疫印迹检测 (A) 对野生型(WT)、转TurboID-EYFP (TY/WT)和转TurboID-EYFP-ATG8a (TY-8A/WT)拟南芥幼苗分别进行生物素处理(+, 50 µmol∙L-1, 3小时)或不进行处理(-), 使用SA-HRP与anti-GFP抗体进行免疫印迹检测, 分析TurboID-EYFP的活性与表达水平; (B) 对不同株系的转TurboID-EYFP (TY/WT)拟南芥幼苗进行生物素处理(+, 50 µmol∙L-1, 3小时)或不进行处理(-), 进行免疫印迹检测。SA-HRP: 辣根过氧化物酶标记链霉亲和素; CBB: 考马斯亮蓝
Figure 3 Immunoblot analysis of biotinylated protein (A) Wild-type (WT), transgenic Arabidopsis seedlings overexpressing TurboID-EYFP (TY/WT) or TurboID-EYFP-ATG8a (TY-8A/WT) were treated with 50 µmol∙L-1 biotin (+) for 3 h or 0 (-), activity and expression of TurboID-EYFP construct were analyzed by immunoblots with SA-HRP and anti-GFP antibodies; (B) Different lines of transgenic Arabidopsis seedlings overexpressing TurboID-EYFP (TY/WT) were treated with 50 µmol∙L-1 biotin (+) for 3 h or 0 (-), followed by immunoblots. SA-HRP: Streptavidin-HRP; CBB: Coomassie Brilliant Blue
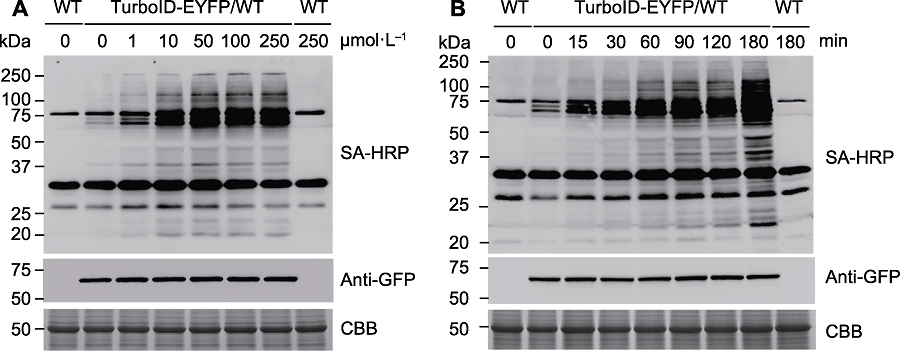
图4 邻近标记中生物素浓度与处理时间的优化 (A) 使用不同浓度的生物素溶液分别对野生型和转TurboID-EYFP拟南芥处理1小时, 使用SA-HRP和anti-GFP抗体进行免疫印迹检测, 分析TurboID-EYFP的活性与表达水平; (B) 用50 µmol∙L-1生物素溶液处理野生型和转TurboID-EYFP拟南芥不同时间, 然后抽提蛋白并进行免疫印迹分析。SA-HRP: 辣根过氧化物酶标记链霉亲和素; CBB: 考马斯亮蓝
Figure 4 The optimization of biotin concentration and labeling time used in proximity labeling (A) Wild-type (WT) and transgenic Arabidopsis seedlings overexpressing TurboID-EYFP were treated with different concentrations of biotin solution for 1 h, followed by immunoblots with SA-HRP and anti-GFP antibodies to detect the proximity ligation activity and protein expression level of TurbolD-EYFP; (B) Wild-type (WT) and transgenic Arabidopsis seedings overexpressing TurboID-EYFP were treated with 50 µmol∙L-1 biotin for different times, followed by immunoblots with appropriate antibodies. SA-HRP: Streptavidin-HRP; CBB: Coomassie Brilliant Blue
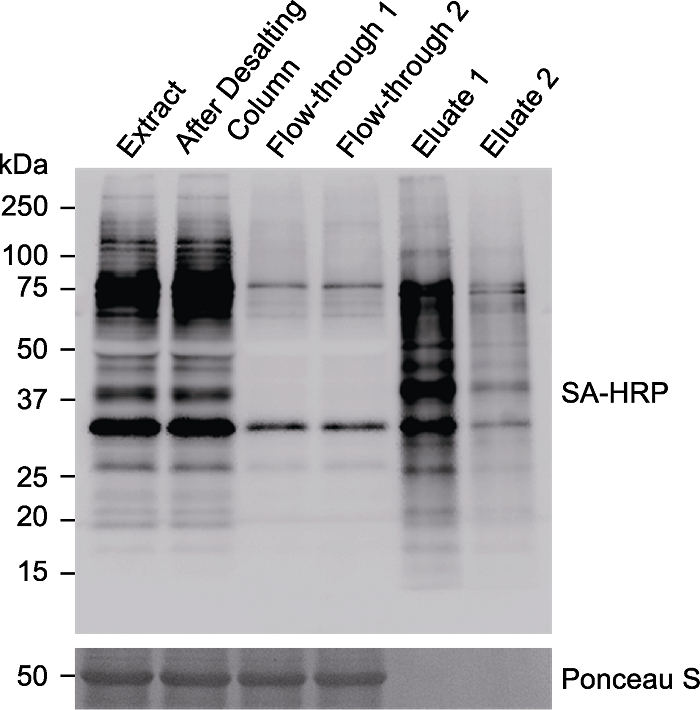
图5 免疫印迹检测被磁珠富集的生物素化蛋白 对表达TurboID-EYFP-ATG8a的拟南芥植株进行生物素处理(50 µmol∙L-1, 3小时), 提取蛋白后使用脱盐柱进行蛋白脱盐, 将脱盐后的样品等体积分成2份, 分别与20 μL Streptavidin Magnetic Beads过夜孵育, 最后分别用20 μL或200 μL 4× SDS Sample Buffer进行洗脱。Extract: 离心后的蛋白上清; After Desalting Column: 脱盐后的蛋白样品; Flow-through 1/2: 与链霉亲和素磁珠过夜孵育后的上清; Eluate 1: 使用20 μL buffer洗脱的蛋白样品; Eluate 2: 使用200 μL buffer洗脱的蛋白样品; SA-HRP: 辣根过氧化物酶标记链霉亲和素
Figure 5 Immunoblotting analyses of the biotinylated proteins enriched by Streptavidin Magnetic Beads Arabidopsis seedlings overexpressing TurboID-EYFP-ATG8a were treated with 50 µmol∙L-1 biotin for 3 hours. The protein was extracted and desalted through Desalting Column. The desalted sample was split into two parts and incubated with 20 μL Streptavidin Magnetic Beads overnight, followed by protein elution with 20 μL or 200 μL 4× SDS Sample Buffer, respectively. Extract: Protein supernatant after centrifugation; After Desalting Column: Protein sample after desalting; Flow-through 1 or 2: Supernatant after overnight incubation with streptavidin (SA) beads; Eluate 1: Protein sample eluated with 20 μL buffer; Eluate 2: Protein sample eluated with 200 μL buffer; SA-HRP: Streptavidin-HRP
| [1] | 李喜豹, 赖敏怡, 梁山, 王小菁, 高彩吉, 杨超 (2021). 植物细胞自噬基因的功能与转录调控机制. 植物学报 56, 201-217. |
| [2] | 刘洋, 张静, 王秋玲, 侯岁稳 (2018). 植物细胞自噬研究进展. 植物学报 53, 5-16. |
| [3] | 苏田, 韩笑, 刘华东 (2020). 邻近标记在蛋白质组学中的发展及应用. 中国生物化学与分子生物学报 36, 36-41. |
| [4] |
Alban C, Job D, Douce R (2000). Biotin metabolism in plants. Annu Rev Plant Physiol Plant Mol Biol 51, 17-47.
DOI URL |
| [5] |
Arora D, Abel NB, Liu C, Van Damme P, Yperman K, Eeckhout D, Vu LD, Wang J, Tornkvist A, Impens F, Korbei B, Van Leene J, Goossens A, De Jaeger G, Ott T, Moschou PN, Van Damme D (2020). Establishment of proximity-dependent biotinylation approaches in different plant model systems. Plant Cell 32, 3388-3407.
DOI URL |
| [6] |
Branon TC, Bosch JA, Sanchez AD, Udeshi ND, Svinkina T, Carr SA, Feldman JL, Perrimon N, Ting AY (2018). Efficient proximity labeling in living cells and organisms with TurboID. Nat Biotechnol 36, 880-887.
DOI PMID |
| [7] |
Cho KF, Branon TC, Rajeev S, Svinkina T, Udeshi ND, Thoudam T, Kwak C, Rhee HW, Lee IK, Carr SA, Ting AY (2020). Split-TurboID enables contact-dependent proximity labeling in cells. Proc Natl Acad Sci USA 117, 12143-12154.
DOI URL |
| [8] |
Kim TW, Park CH, Hsu CC, Zhu JY, Hsiao Y, Branon T, Xu SL, Ting AY, Wang ZY (2019). Application of TurboID mediated proximity labeling for mapping a GSK3 kinase signaling network in Arabidopsis. bioRxiv doi: https://doi.org/10.1101/636324.
DOI |
| [9] |
Lin QP, Zhou ZJ, Luo WB, Fang MC, Li MR, Li HQ (2017). Screening of proximal and interacting proteins in rice protoplasts by proximity-dependent biotinylation. Front Plant Sci 8, 749.
DOI URL |
| [10] |
Mair A, Xu SL, Branon TC, Ting AY, Bergmann DC (2019). Proximity labeling of protein complexes and cell- type-specific organellar proteomes in Arabidopsis enabled by TurboID. eLife 8, e47864.
DOI URL |
| [11] |
Martell JD, Deerinck TJ, Sancak Y, Poulos TL, Mootha VK, Sosinsky GE, Ellisman MH, Ting AY (2012). Engineered ascorbate peroxidase as a genetically encoded reporter for electron microscopy. Nat Biotechnol 30, 1143-1148.
DOI URL |
| [12] |
Roux KJ, Kim DI, Raida M, Burke B (2012). A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J Cell Biol 196, 801-810.
DOI URL |
| [13] |
Yang C, Shen WJ, Yang LM, Sun Y, Li XB, Lai MY, Wei J, Wang CJ, Xu YC, Li FQ, Liang S, Yang CW, Zhong SW, Luo M, Gao CJ (2020). HY5-HDA9 module transcriptionally regulates plant autophagy in response to light-to-dark conversion and nitrogen starvation. Mol Plant 13, 515-531.
DOI URL |
| [14] |
Yang XX, Wen ZY, Zhang DL, Li Z, Li DW, Nagalakshmi U, Dinesh-Kumar SP, Zhang YL (2021). Proximity labeling: an emerging tool for probing in planta molecular interactions. Plant Commun 2, 100137.
DOI URL |
| [15] |
Zhang YL, Song GY, Lal NK, Nagalakshmi U, Li YY, Zheng WJ, Huang PJ, Branon TC, Ting AY, Walley JW, Dinesh-Kumar SP (2019). TurboID-based proximity labeling reveals that UBR7 is a regulator of N NLR immune receptor-mediated immunity. Nat Commun 10, 3252.
DOI URL |
| [1] | 徐重益. 植物中验证蛋白相互作用的Pull-down和Co-IP技术[J]. 植物学报, 2020, 55(1): 62-68. |
| [2] | 陈立超,詹妮,李彦莎,冯健,左建儒. 植物蛋白质S-亚硝基化修饰的检测与分析[J]. 植物学报, 2019, 54(4): 497-502. |
| [3] | 宋爱华, 张文斌, 孙姝兰, 李凌飞, 王小菁. 非洲菊原生质体制备及瞬时转化系统的建立[J]. 植物学报, 2017, 52(4): 511-519. |
| [4] | 景艳军, 林荣呈. 我国植物光信号转导研究进展概述[J]. 植物学报, 2017, 52(3): 257-270. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||