

Chinese Bulletin of Botany ›› 2018, Vol. 53 ›› Issue (4): 447-450.DOI: 10.11983/CBB18104 cstr: 32102.14.CBB18104
• COMMENTARIES • Previous Articles Next Articles
Zhao Xijuan, Qian Lichao, Liu Yule*( )
)
Received:2018-04-25
Accepted:2018-05-21
Online:2018-07-01
Published:2018-09-11
Contact:
Liu Yule
About author:† These authors contributed equally to this paper
Zhao Xijuan, Qian Lichao, Liu Yule. Chinese Scientists Made Breakthrough Progresses in Plant Programmed Cell Death[J]. Chinese Bulletin of Botany, 2018, 53(4): 447-450.
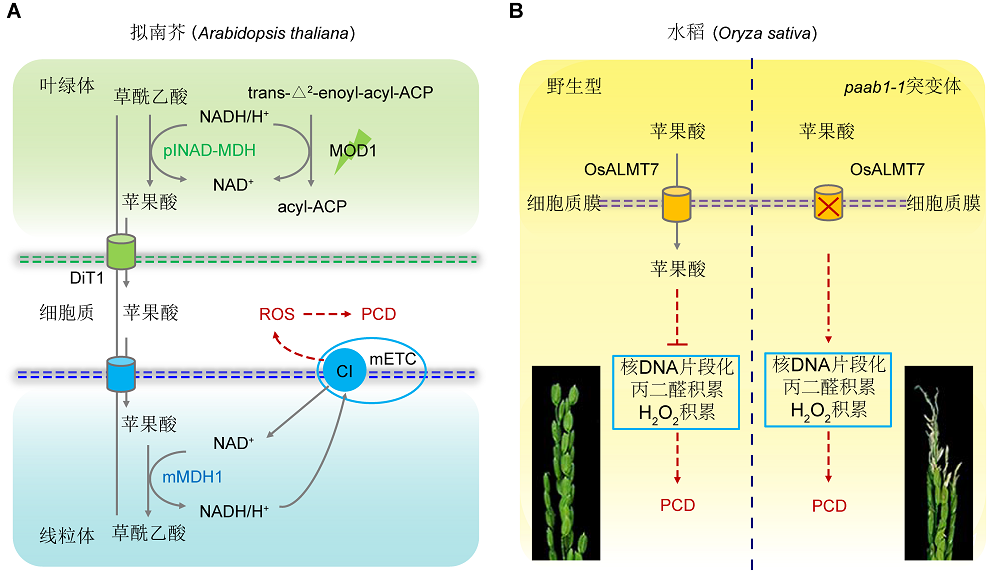
Figure 1 A proposed model of malate-regulated programmed cell death in plants (modified from Zhao et al., 2018; Heng et al., 2018)(A) In Arabidopsis thaliana, MOD1 encodes an enoyl-acyl carrier protein reductase, the deficiency of MOD1 causes an increased level of NADH in the chloroplasts, which drives oxaloacetate (OAA) to be converted to malate by plNAD-MDH. Malate is transported out of the chloroplast into the cytosol by DiT1, and then be transported into the mitochondrion by an unidentified transporter or transporters. In the mitochondrion, malate is converted to OAA by mMDH1, and simultaneously NADH is generated to provide electrons for mETC to induce ROS formation and initiate programmed cell death (PCD) process in the mod1 cells. (B) In Oryza sativa, OsALMT7 encodes a putative aluminum-activated plasma membrane localized malate transporter, which could transport malate into cells. Malate is involved in nuclear DNA stability and inhibits excessive accumulation of H2O2 and malondialdehyde, which protects cells from PCD. The paab1-1 mutant harbors a mutation in OsALMT7, and its panicle contained less malate than wild type, particularly at the apical portions. The apical spikelets in the paab1-1 mutant undergo PCD accompanied by nuclear DNA fragmentation and accumulation of higher levels of H2O2 and malondialdehyde.
| 1 | Beers EP (1997). Programmed cell death during plant growth and development.Cell Death Differ 4, 649-661. |
| 2 | Heng Y, Wu C, Long Y, Luo S, Ma J, Chen J, Liu J, Zhang H, Ren Y, Wang M, Tan J, Zhu S, Wang J, Lei C, Zhang X, Guo X, Wang H, Cheng Z, Wan J (2018). OsALMT7 maintains panicle size and grain yield in rice by mediating malate transport.Plant Cell 30, 889-906. |
| 3 | Mou Z, He Y, Dai Y, Liu X, Li J (2000). Deficiency in fatty acid synthase leads to premature cell death and dramatic alterations in plant morphology. Plant Cell 12, 405-418. |
| 4 | Noctor G, Veljovic-Jovanovic S, Driscoll S, Novitskaya L, Foyer CH (2002). Drought and oxidative load in the leaves of C3 plants: a predominant role for photorespiration?Ann Bot 89, 841-850. |
| 5 | Van Aken O, Van Breusegem F (2015). Licensed to kill: mitochondria, chloroplasts, and cell death.Trends Plant Sci 20, 754-766. |
| 6 | Vianello A, Zancani M, Peresson C, Petrussa E, Casolo V, Krajňáková J, Patui S, Braidot E, Macrì F (2007). Plant mitochondrial pathway leading to programmed cell death.Physiol Plant 129, 242-252. |
| 7 | Wu J, Sun Y, Zhao Y, Zhang J, Luo L, Li M, Wang J, Yu H, Liu G, Yang S, Xiong G, Zhou J, Zuo J, Wang Y, Li J (2015). Deficient plastidic fatty acid synthesis triggers cell death by modulating mitochondrial reactive oxygen species.Cell Res 25, 621-633. |
| 8 | Zhao Y, Luo L, Xu J, Xin P, Guo H, Wu J, Bai L, Wang G, Chu J, Zuo J, Yu H, Huang X, Li J (2018). Malate transported from chloroplast to mitochondrion triggers production of ROS and PCD in Arabidopsis thaliana. Cell Res 28, 448-461. |
| [1] | Yuejing Zhang, Hetian Sang, Hanqi Wang, Zhenzhen Shi, Li Li, Xin Wang, Kun Sun, Ji Zhang, Hanqing Feng. Research Progress of Plant Signaling in Systemic Responses to Abiotic Stresses [J]. Chinese Bulletin of Botany, 2024, 59(1): 122-133. |
| [2] | Zhou Yuping, Yan Jiahao, Tian Chang’en. Research Progress on the Regulatory Mechanisms of ABA Signal Transduction in Guard Cells [J]. Chinese Bulletin of Botany, 2022, 57(5): 684-696. |
| [3] | Liu Xiaolong, Ji Ping, Yang Hongtao, Ding Yongdian, Fu Jialing, Liang Jiangxia, Yu Congcong. Priming Effect of Abscisic Acid on High Temperature Stress During Rice Heading-flowering Stage [J]. Chinese Bulletin of Botany, 2022, 57(5): 596-610. |
| [4] | Haitao Hu, Tingting Qian, Ling Yang. Detection of Reactive Oxygen Species Using H2DCFDA Probe in Plant [J]. Chinese Bulletin of Botany, 2022, 57(3): 320-326. |
| [5] | Wei Wang, Mengxiang Sun. POLLENCOAT PROTEIN B-class Peptides (PCP-Bs), a Key of Compatible Pollen to Open the Gate of Stigma [J]. Chinese Bulletin of Botany, 2021, 56(2): 147-150. |
| [6] | Wei Wang, Dingzhong Tang. Synergistic Cooperation Between Cell Surface and Intracellular Immune Receptors Potentiates to Activate Robust Plant Defense [J]. Chinese Bulletin of Botany, 2021, 56(2): 142-146. |
| [7] | Yujia Dai,Xiaofeng Luo,Wenguan Zhou,Feng Chen,Haiwei Shuai,Wenyu Yang,Kai Shu. Plant Systemic Signaling Under Biotic and Abiotic Stresses Conditions [J]. Chinese Bulletin of Botany, 2019, 54(2): 255-264. |
| [8] | Danying Ma,Dongchao Ji,Yong Xu,Tong Chen,Shiping Tian. Advances in the Regulation on Autophagy by Reactive Oxygen Species in Plant Cells [J]. Chinese Bulletin of Botany, 2019, 54(1): 81-92. |
| [9] | He Guangming, Deng Xingwang. Death Signal Transduction: Chloroplast-to-Mitochondrion Communication Regulates Programmed Cell Death in Plants [J]. Chinese Bulletin of Botany, 2018, 53(4): 441-444. |
| [10] | Zhang Xian-sheng. Chinese Scientists Have Made a Great Breakthrough in the Mechanism of Programmed Cell Death [J]. Chinese Bulletin of Botany, 2018, 53(4): 445-446. |
| [11] | Xinlu Xu, Dandan Li, Yuandan Ma, Jianyun Zhai, Jianfei Sun, Yan Gao, Rumin Zhang. Responses of the Antioxidant Defense System of Osmanthus fragrans cv. ‘Tian Xiang TaiGe’ to Drought, Heat and the Synergistic Stress [J]. Chinese Bulletin of Botany, 2018, 53(1): 72-81. |
| [12] | Shengchun Zhang, Qingming Li, Chengwei Yang. Arabidopsis Metalloprotease FtSH4 Regulates Leaf Senescence Through Auxin and Reactive Oxygen Species [J]. Chinese Bulletin of Botany, 2017, 52(4): 453-464. |
| [13] | Kai Huo, Wei Lu, Xia Li, Pingbo Chen. Recent Progress in Understanding Extracellular ATP Signal Molecule in Plants [J]. Chinese Bulletin of Botany, 2014, 49(5): 618-625. |
| [14] | Pengtao Wang, Jing Zhao, Huanhuan Yu. Reactive Oxygen Species Signaling in Stomata [J]. Chinese Bulletin of Botany, 2014, 49(4): 490-503. |
| [15] | WANG Wei-Qing, CHENG Hong-Yan, LIU Shu-Jun, SONG Song-Quan. Response of respiratory rate and reactive oxygen species scavenging enzyme activity in seed mitochondria of Clausena lansium dehydration and its ecological significance [J]. Chin J Plant Ecol, 2012, 36(8): 870-879. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||