

Chinese Bulletin of Botany ›› 2018, Vol. 53 ›› Issue (1): 72-81.DOI: 10.11983/CBB17004 cstr: 32102.14.CBB17004
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Xinlu Xu, Dandan Li, Yuandan Ma*( ), Jianyun Zhai, Jianfei Sun, Yan Gao, Rumin Zhang
), Jianyun Zhai, Jianfei Sun, Yan Gao, Rumin Zhang
Received:2017-01-06
Accepted:2017-05-04
Online:2018-01-01
Published:2018-08-10
Contact:
Yuandan Ma
Xinlu Xu, Dandan Li, Yuandan Ma, Jianyun Zhai, Jianfei Sun, Yan Gao, Rumin Zhang. Responses of the Antioxidant Defense System of Osmanthus fragrans cv. ‘Tian Xiang TaiGe’ to Drought, Heat and the Synergistic Stress[J]. Chinese Bulletin of Botany, 2018, 53(1): 72-81.
| Temperature | Treatment intensity | O2-· (nmol·g-1 FW) | H2O2 (μmol·g-1 FW) | MDA (μmol·g-1 FW) |
|---|---|---|---|---|
| 28°C | CK | 7.56±0.77 C | 20.11±1.01 D | 4.09±0.64 C |
| Light drought | 14.04±0.44 B | 33.08±2.33 C | 7.42±1.32 B | |
| Moderate drought | 15.49±0.45 B | 40.60±2.30 B | 14.29±2.55 A | |
| Heavy drought | 18.84±2.05 A | 49.24±1.74 A | 15.39±1.69 A | |
| Sum of squares | Between groups (d.f.1=3) | 402.69 | 2743.44 | 532.18 |
| Within groups (d.f.2=20) | 25.94 | 73.89 | 57.58 | |
| 40°C | CK | 10.92±0.81 b | 26.52±0.51 c | 9.14±0.67 b |
| Light drought | 12.79±0.78 a | 37.52±3.73 b | 14.11±1.51 a | |
| Moderate drought | 11.92±0.64 ab | 45.60±0.92 a | 16.36±2.29 a | |
| Heavy drought | 11.30±1.69 ab | 34.64±4.18 b | 8.48±1.74 b | |
| Sum of squares | Between groups (d.f.1=3) | 11.98 | 1117.58 | 264.48 |
| Within groups (d.f.2=20) | 22.59 | 162.38 | 55.13 | |
| P: Ft | ** | ns | ** | |
| P: Fd | ** | ** | * | |
| P: Ft×Fd | ** | ** | ** |
Table 1 Effect of drought and heat stress on reactive oxygen species and malondialdehyde (MDA) content in Osmanthus fragrans cv. ‘Tian Xiang TaiGe’
| Temperature | Treatment intensity | O2-· (nmol·g-1 FW) | H2O2 (μmol·g-1 FW) | MDA (μmol·g-1 FW) |
|---|---|---|---|---|
| 28°C | CK | 7.56±0.77 C | 20.11±1.01 D | 4.09±0.64 C |
| Light drought | 14.04±0.44 B | 33.08±2.33 C | 7.42±1.32 B | |
| Moderate drought | 15.49±0.45 B | 40.60±2.30 B | 14.29±2.55 A | |
| Heavy drought | 18.84±2.05 A | 49.24±1.74 A | 15.39±1.69 A | |
| Sum of squares | Between groups (d.f.1=3) | 402.69 | 2743.44 | 532.18 |
| Within groups (d.f.2=20) | 25.94 | 73.89 | 57.58 | |
| 40°C | CK | 10.92±0.81 b | 26.52±0.51 c | 9.14±0.67 b |
| Light drought | 12.79±0.78 a | 37.52±3.73 b | 14.11±1.51 a | |
| Moderate drought | 11.92±0.64 ab | 45.60±0.92 a | 16.36±2.29 a | |
| Heavy drought | 11.30±1.69 ab | 34.64±4.18 b | 8.48±1.74 b | |
| Sum of squares | Between groups (d.f.1=3) | 11.98 | 1117.58 | 264.48 |
| Within groups (d.f.2=20) | 22.59 | 162.38 | 55.13 | |
| P: Ft | ** | ns | ** | |
| P: Fd | ** | ** | * | |
| P: Ft×Fd | ** | ** | ** |

Figure 1 The effect of drought and heat stress on the activity of antioxidant enzymes in Osmanthus fragrans cv. ‘Tian Xiang TaiGe’(A) Superoxide dismutase (SOD) activity; (B) Peroxidase (POD) activity; (C) Catalase (CAT) activity. Ft: Effect of different temperature; Fd: Effect of different drought treatment intensity; Ft×Fd: Different responses of plant tissues to drought and heat stress. Each value is the mean±SE (n=6). Different capital letters indicate statistically significant differences of drought stress, different lowercase letters indicate statistically significant differences of heat stress. According to Tukey test, * P<0.05; ** P<0.01; ns: Non-significant
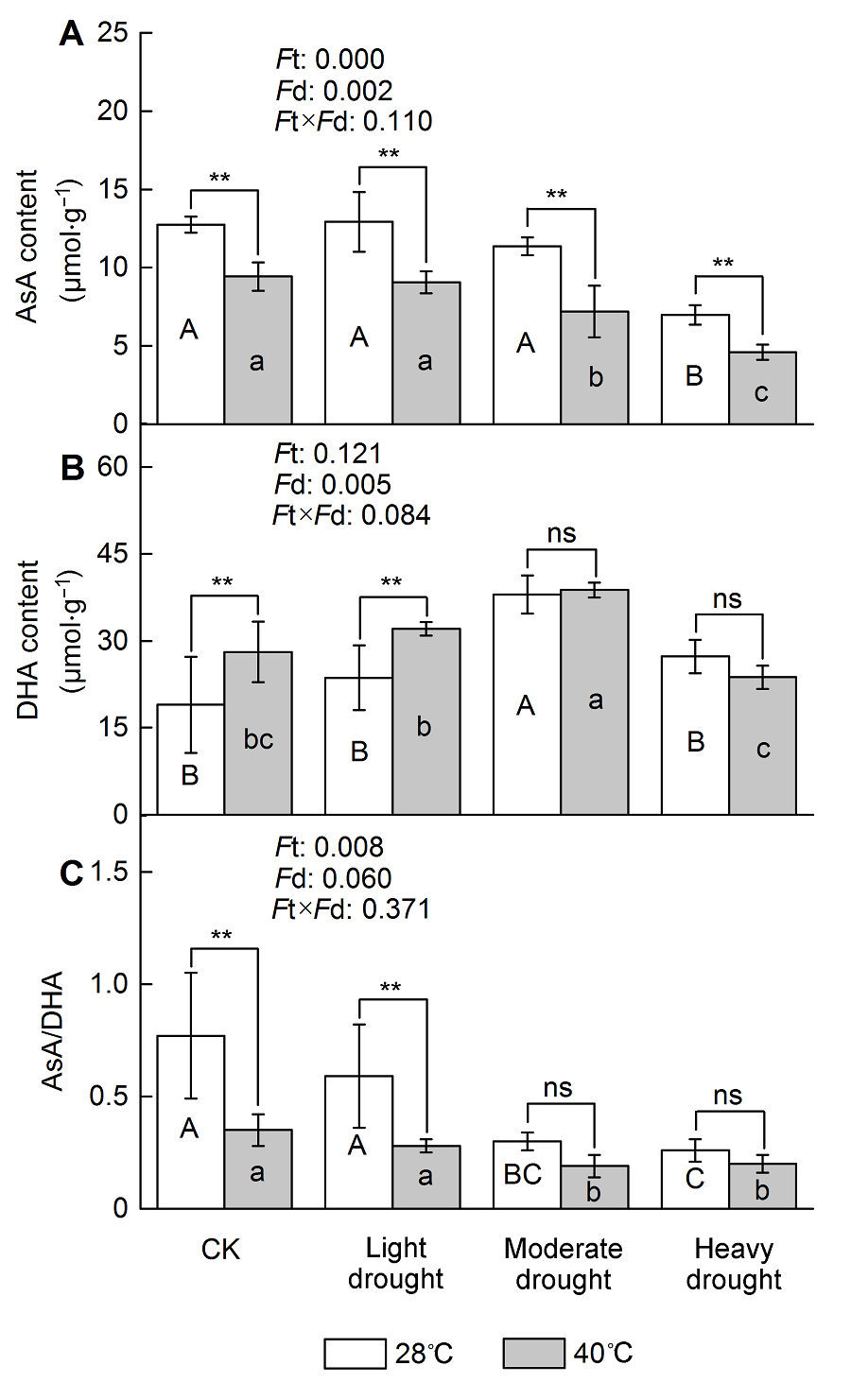
Figure 2 The effect of drought and heat stress on the AsA content in Osmanthus fragrans cv. ‘Tian Xiang TaiGe’ (A) Ascorbic acid (AsA) content; (B) Dehydroascorbate (DHA) content; (C) AsA/DHA. Ft: Effect of different temperature; Fd: Effect of different drought treatment intensity; Ft×Fd: Different responses of plant tissues to drought and heat stress. Each value is the mean±SE (n=6). Different capital letters indicate statistically significant differences of drought stress, different lowercase letters indicate statistically significant differences of heat stress. According to Tukey test, * P<0.05; ** P<0.01; ns: Non-significant
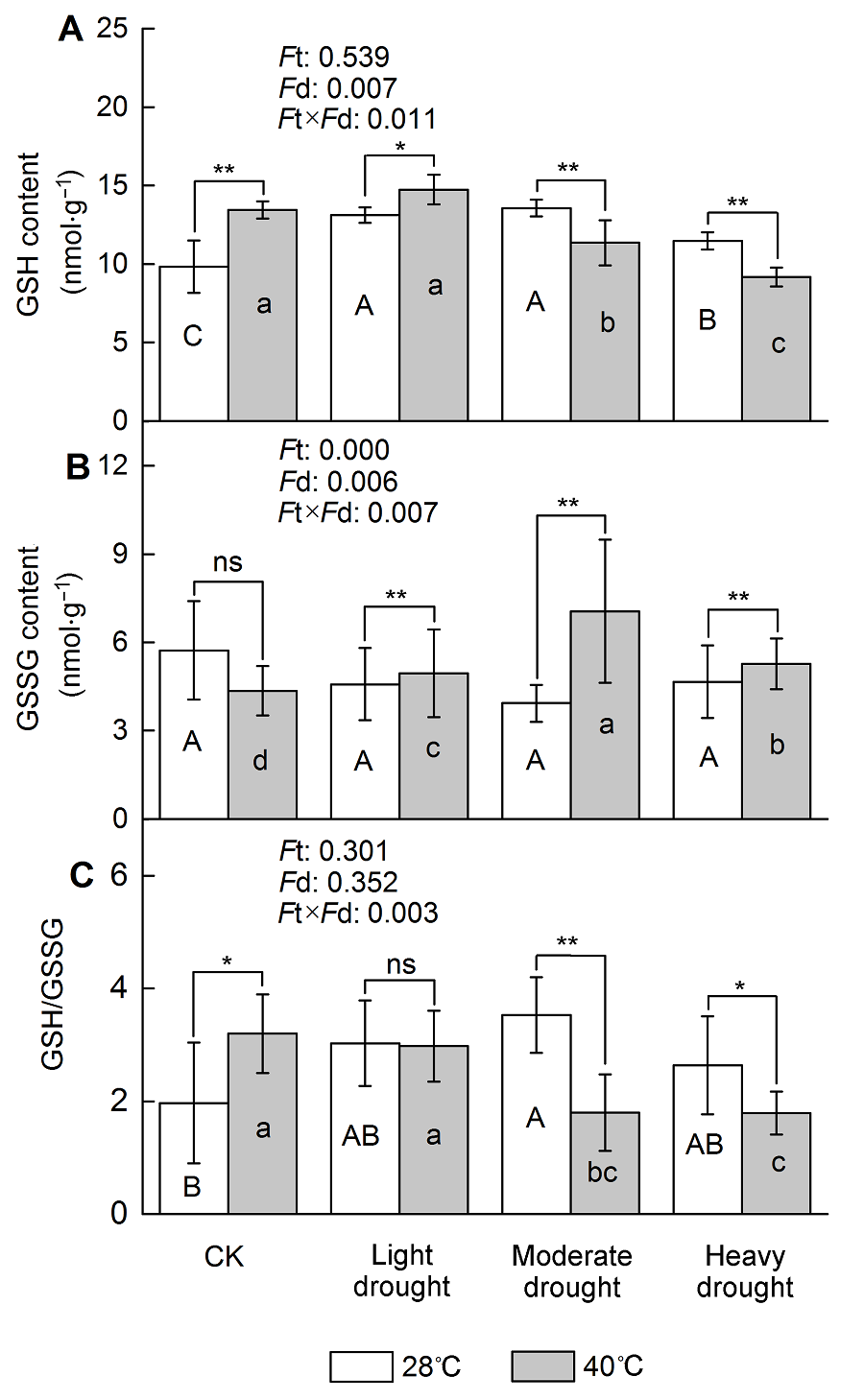
Figure 3 The effect of drought and heat stress on the GSH content in Osmanthus fragrans cv. ‘Tian Xiang TaiGe’ (A) Glutathione (GSH) content; (B) Oxidized glutathione (GSSG) content; (C) GSH/GSSG. Ft: Effect of different temperature; Fd: Effect of different drought treatment intensity; Ft×Fd: Different responses of plant tissues to drought and heat stress. Each value is the mean±SE (n=6). Different capi- tal letters indicate statistically significant differences of drought stress, different lowercase letters indicate statistically significant differences of heat stress. According to Tukey test, * P<0.05; ** P<0.01; ns: Non-significant
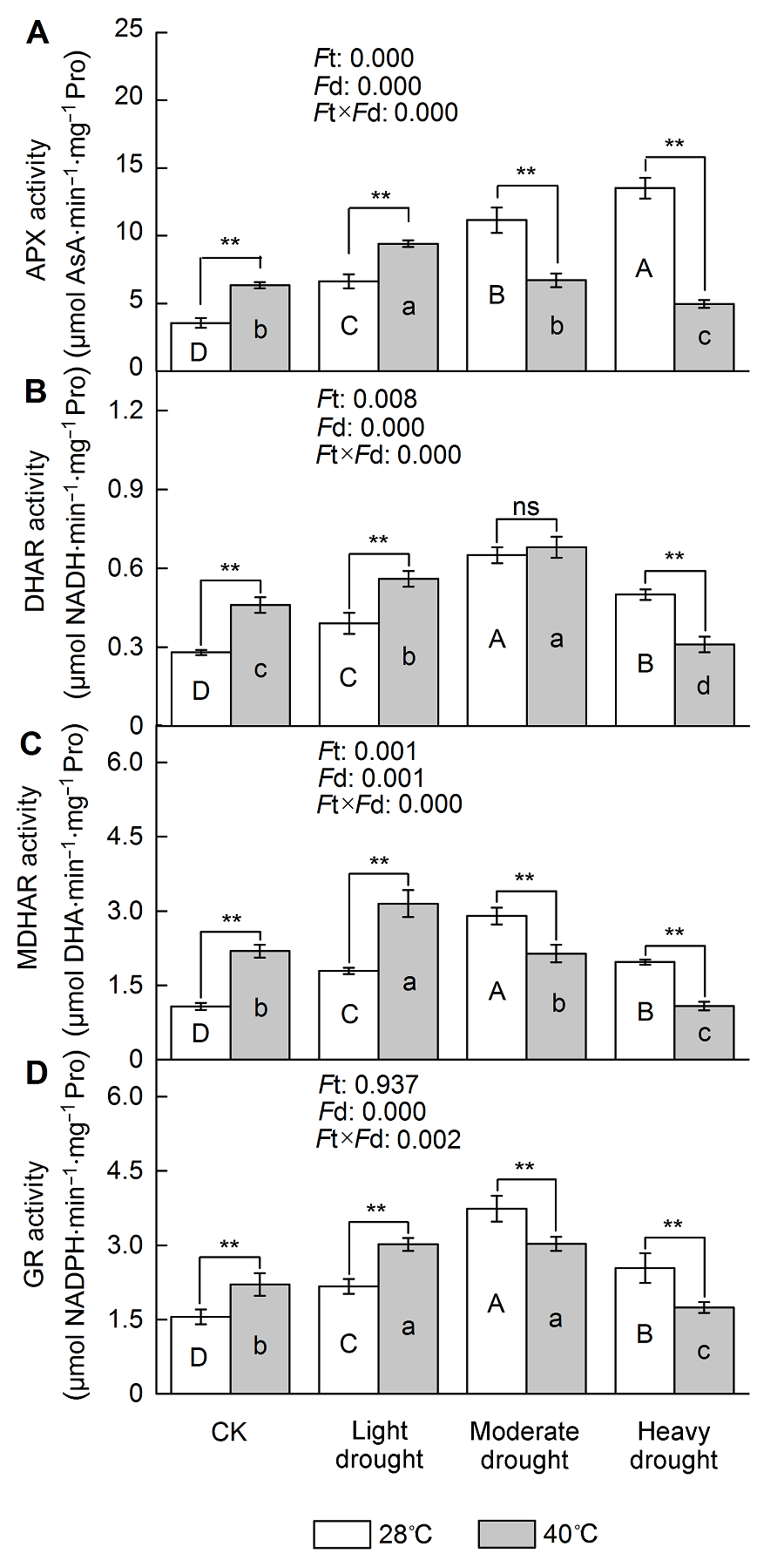
Figure 4 The effect of drought and heat stress on the enzymes activity of AsA-GSH cycle in Osmanthus fragrans cv. ‘Tian Xiang TaiGe’ (A) Ascorbate peroxidase (APX) activity; (B) Dehydroascorbate reductase (DHAR) activity; (C) Monodehydroascobate reductase (MDHAR) activity; (D) Glutathione reductase (GR) activity. Ft: Effect of different temperature; Fd: Effect of different drought treatment intensity; Ft×Fd: Different responses of plant tissues to drought and heat stress. Each value is the mean±SE (n=6). Different capital letters indicate statistically significant differences of drought stress, different lowercase letters indicate statistically significant differences of heat stress. According to Tukey test, * P<0.05; ** P<0.01; ns: Non- significant
| [1] | 陈晓峰, 江洪, 牛晓栋, 张金梦, 刘玉莉, 方成圆 (2016). 季节性高温和干旱对亚热带毛竹林碳通量的影响. 应用生态学报 27, 335-344. |
| [2] | 李忠光, 龚明 (2005). 植物中超氧阴离子自由基测定方法的改进. 云南植物研究 27, 211-216. |
| [3] | 吴永波, 叶波 (2016). 高温干旱复合胁迫对构树幼苗抗氧化酶活性和活性氧代谢的影响. 生态学报 36, 403-410. |
| [4] | 谢华英, 马均, 代邹, 李玥, 孙加威, 赵建红, 徐徽, 孙永健 (2016). 抽穗期高温干旱胁迫对杂交水稻产量及生理特性的影响. 杂交水稻 31, 62-69. |
| [5] | Arab L, Kreuzwieser J, Kruse J, Zimmer I, Ache P, Alfarraj S, Al-rasheid KAS, Schnitzler JP, Hedrich R, Rennenberg H (2016). Acclimation to heat and drought- lessons to learn from the date palm ( Phoenix dactylifera). Environ Exp Bot 125, 20-30. |
| [6] | Doulis AG, Debian N, Kingston-Smith AH, Foyer CH (1997). Differential localization of antioxidants in maize leaves.Plant Physiol 114, 1031-1037. |
| [7] | Foyer CH, Noctor G (2011). Ascorbate and glutathione: the heart of the redox hub.Plant Physiol 155, 2-18. |
| [8] | Giannopolitis CN, Ries SK (1977). Superoxide dismutases: I. Occurrence in higher plants.Plant Physiol 59, 309-314. |
| [9] | Hijioka Y, Lin E, Pereira J, Corlett R, Cui X, Insarov G, Lasco R, Lindgren E, Surjan A (2014).Climate Change 2014: Impacts, Adaptation, and Vulnerability, Part B: Regional Aspects, Contribution of Working Group II, Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press. pp.1327-1370. |
| [10] | Hodges DM, Delong JM, Forney CF, Prange RK (1999). Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds.Pl- anta 207, 604-611. |
| [11] | Hossain MA, Nakano Y, Asada K (1984). Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide.Plant Cell Physiol 25, 385-395. |
| [12] | Imahori Y, Takemura M, Bai JH (2008). Chilling-induced oxidative stress and antioxidant responses in mume ( Pru- nus mume) fruit during low temperature storage. Postharvest Biol Technol 49, 54-60. |
| [13] | Kumari GJ, Reddy AM, Naik ST, Kumar SG, Prasanthi J, Sriranganayakulu G, Reddy PC, Sudhakar C (2006). Jasmonic acid induced changes in protein pattern, antioxidative enzyme activities and peroxidase isozymes in peanut seedlings.Biol Plant 50, 219-226. |
| [14] | Lei P, Xu ZQ, Ding Y, Tang B, Zhang YX, Li HS, Feng XH, Xu H (2015). Effect of poly (γ-glutamic acid) on the physiological responses and calcium signaling of rape seedlings ( Brassica napus L.) under cold stress. J Agric Food Chem 63, 10399-10406. |
| [15] | Lei P, Xu ZQ, Liang JF, Luo XH, Zhang YX, Feng XH, Xu H (2016). Poly (γ-glutamic acid) enhanced tolerance to salt stress by promoting proline accumulation in Brassica napus L. Plant Growth Regul 78, 233-241. |
| [16] | Li H, Xu HL, Zhang PJ, Gao MQ, Wang D, Zhao HJ (2017). High temperature effects on D1 protein turnover in three wheat varieties with different heat susceptibility.Plant Grow- th Regul 78, 1-9. |
| [17] | Liu CC, Liu YG, Guo K, Fan DY, Li GQ, Zheng YR, Yu LF, Yang R (2011). Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China.Environ Exp Bot 71, 174-183. |
| [18] | Ma YH, Ma FW, Wang YH, Zhang JK (2011). The responses of the enzymes related with ascorbate-gluta- thione cycle during drought stress in apple leaves.Acta Physiol Plant 33, 173-180. |
| [19] | Nahar K, Hasanuzzaman M, Alam MM, Fujita M (2015). Exogenous glutathione confers high temperature stress tolerance in mung bean ( Vigna radiata L.) by modulating antioxidant defense and methylglyoxal detoxification system. Environ Exp Bot 112, 44-54. |
| [20] | Nakano Y, Asada K (1981). Hydrogen peroxide is scaven- ged by ascorbate-specific peroxidase in spinach chloroplasts.Plant Cell Physiol 22, 867-880. |
| [21] | Rai AC, Singh M, Shah K (2012). Effect of water withdrawal on formation of free radical, proline accumulation and activities of antioxidant enzymes in ZAT12-transformed transgenic tomato plants. Plant Physiol Biochem 61, 108-114. |
| [22] | Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA (2003). Fingerprints of global warming on wild animals and plants.Nature 421, 57-60. |
| [23] | Schaedle M, Bassham JA (1977). Chloroplast glutathione reductase.Plant Physiol 59, 1011-1012. |
| [24] | Sharma P, Jha AB, Dubey RS, Pessarakli M (2012). Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions.J Bot 2012, 217037. |
| [25] | Silva EN, Ferreira-Silva SL, Fontenele ADV, Ribeiro RV, Viégasc RA, Silveira JAG (2010). Photosynthetic chan- ges and protective mechanisms against oxidative damage subjected to isolated and combined drought and heat stresses in Jatropha curcas plants. J Plant Physiol 167, 1157-1164. |
| [26] | Suzuki N, Koussevitzky S, Mittler R, Miller G (2012). ROS and redox signaling in the response of plants to abiotic stress.Plant Cell Environ 35, 259-270. |
| [27] | Wang SC, Liang D, Li C, Hao Yl, Ma FW, Shu HR (2012). Influence of drought stress on the cellular ultrastructure and antioxidant systemin leaves of drought-tolerant and drought-sensitive apple rootstocks.Plant Physiol Biochem 51, 81-89. |
| [28] | Wu XX, He J, Ding HD, Zhu ZW, Chen JL, Xu S, Zha DS (2015). Modulation of zinc-induced oxidative damage in Solanum melongena by 6-benzylaminopurine involves ascorbate-glutathione cyclemetabolism. Environ Exp Bot 116, 1-11. |
| [29] | Zou MQ, Yuan LY, Zhu SD, Liu S, Ge JT, Wang CG (2016). Response of osmotic adjustment and ascorbate-glutathione cycle to heat stress in a heat-sensitive and a heat-tolerant genotype of wucai ( Brassica campestris L.). Sci Hortic 211, 87-94. |
| [1] | Xu Tingyang, Liu Yuchen, Wang Wanpeng, Su Hang, Su Kunlong, Wu Zhenying, Lϋ Ming, Li Fuli, Wang Xiaoshan, Fu Chunxiang. Effects of Different Plant Growth Regulators on Wheat Growth and Development in the Saline-alkali Land [J]. Chinese Bulletin of Botany, 2025, 60(3): 354-362. |
| [2] | LIU Ke-Yan, HAN Lu, SONG Wu-Ye, ZHANG Chu-Rui, HU Xu, XU Hang, CHEN Li-Xin. Detection of drought effects on photosynthetic stability of vegetation on the Loess Plateau based on solar-induced chlorophyll fluorescence [J]. Chin J Plant Ecol, 2025, 49(3): 415-431. |
| [3] | Zhao Ling, Guan Ju, Liang Wenhua, Zhang Yong, Lu Kai, Zhao Chunfang, Li Yusheng, Zhang Yadong. Mapping of QTLs for Heat Tolerance at the Seedling Stage in Rice Based on a High-density Bin Map [J]. Chinese Bulletin of Botany, 2025, 60(3): 342-353. |
| [4] | Bei Fan, Min Ren, Yanfeng Wang, Fengfeng Dang, Guoliang Chen, Guoting Cheng, Jinyu Yang, Huiru Sun. Functions of SlWRKY45 in Response to Low-temperature and Drought Stress in Tomato [J]. Chinese Bulletin of Botany, 2025, 60(2): 186-203. |
| [5] | WANG Kun-Ying, QIU Gui-Fu, LIU Zi-He, MENG Jun, LIU Yu-Xuan, JIA Guo-Dong. Climate change regulate tree growth and intrinsic water use efficiency of Populus simonii at different levels of degradation [J]. Chin J Plant Ecol, 2025, 49(2): 343-355. |
| [6] | SHAO Chang-Chang, DUAN Hong-Lang, ZHAO Xi-Zhou, DING Gui-Jie. Research progress on the prediction of drought death point and the mechanism of drought- induced tree mortality [J]. Chin J Plant Ecol, 2025, 49(2): 221-231. |
| [7] | Ruoyue Li, Xiaochao Yang, Zhanqing Hao, Shihong Jia. The intensity of heat waves and insect herbivory on campus plants and their relationship with leaf functional traits [J]. Biodiv Sci, 2025, 33(1): 24283-. |
| [8] | Jianhong Tian, Yan Liu, Mengqi Yin, Jing Wang, Ting Chen, Yan Wang, Xiaocheng Jiang. OsWAK16 Regulates Seed Anti-aging Ability by Modulating Antioxidant Enzyme Activity in Rice [J]. Chinese Bulletin of Botany, 2025, 60(1): 17-32. |
| [9] | WANG Yin, TONG Xiao-Juan, ZHANG Jin-Song, LI Jun, MENG Ping, LIU Pei-Rong, ZHANG Jing-Ru. Impact of drought on carbon and water fluxes and their coupling in a Quercus variabilis plantation [J]. Chin J Plant Ecol, 2024, 48(9): 1157-1171. |
| [10] | ZHANG Peng, JIAO Liang, XUE Ru-Hong, WEI Meng-Yuan, DU Da-Shi, WU Xuan, WANG Xu-Ge, LI Qian. Drought intensity affected the growth recovery of Picea crassifolia across different altitudes in western Qilian Mountains [J]. Chin J Plant Ecol, 2024, 48(8): 977-987. |
| [11] | LONG Ji-Lan, JIANG Zheng, LIU Ding-Qin, MIAO Yu-Xuan, ZHOU Ling-Yan, FENG Ying, PEI Jia-Ning, LIU Rui-Qiang, ZHOU Xu-Hui, FU Yu-Ling. Effects of drought on plant root exudates and associated rhizosphere priming effect: review and prospect [J]. Chin J Plant Ecol, 2024, 48(7): 817-827. |
| [12] | Ziyang Wang, Shengxue Liu, Zhirui Yang, Feng Qin. Genetic Dissection of Drought Resistance in Maize [J]. Chinese Bulletin of Botany, 2024, 59(6): 883-902. |
| [13] | Hengyu Yan, Zhaoxia Li, Yubin Li. Research Progress on Heat Stress Impact on Maize Growth and Heat-Tolerant Maize Screening in China [J]. Chinese Bulletin of Botany, 2024, 59(6): 1007-1023. |
| [14] | Tao Wang, Jinglei Feng, Cui Zhang. Research Progress on Molecular Mechanisms of Heat Stress Affecting the Growth and Development of Maize [J]. Chinese Bulletin of Botany, 2024, 59(6): 963-977. |
| [15] | BAI Hao-Ran, HOU Meng, LIU Yan-Jie. Mechanisms of the invasion of Cenchrus spinifex and drought effects on productivity of Leymus chinensis community [J]. Chin J Plant Ecol, 2024, 48(5): 577-589. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||