

Chinese Bulletin of Botany ›› 2025, Vol. 60 ›› Issue (6): 875-887.DOI: 10.11983/CBB24150 cstr: 32102.14.CBB24150
• RESEARCH ARTICLES • Previous Articles Next Articles
Linlin Dou1, Yu Zhu1, Cuicui Liu1, Yunping Zang2, Zhengguo Tao3, Manzhu Bao1, Yanhong He1,*( )
)
Received:2024-10-02
Accepted:2025-01-20
Online:2025-11-10
Published:2025-01-21
Contact:
Yanhong He
Linlin Dou, Yu Zhu, Cuicui Liu, Yunping Zang, Zhengguo Tao, Manzhu Bao, Yanhong He. Establishment of an Efficient Transient Transformation System for Tagetes erecta Corollas and Analysis on the Promoter Activity of TeCYC2c Gene[J]. Chinese Bulletin of Botany, 2025, 60(6): 875-887.
| Primer name | Sequences (5′→3′) | Function |
|---|---|---|
| pTeCYC2c-F (-1735) | ACGACAATGAAGTTTGAAGACACAG | Promoter cloning |
| pTeCYC2c-F (-1406) | ATTTGAAGTTGCAACTCTGTCATAAAT | Promoter deletion fragments cloning |
| pTeCYC2c-F (-1000) | AACCATTAGGGCCGTGTCG | Promoter deletion fragments cloning |
| pTeCYC2c-F (-650) | GGTGTCTTGGTAATTTTAAAAAAACG | Promoter deletion fragments cloning |
| pTeCYC2c-R | TGTATTTTGGAATTGAAATGTGAAATAA | Promoter deletion fragments cloning |
Table 1 Primer sequences
| Primer name | Sequences (5′→3′) | Function |
|---|---|---|
| pTeCYC2c-F (-1735) | ACGACAATGAAGTTTGAAGACACAG | Promoter cloning |
| pTeCYC2c-F (-1406) | ATTTGAAGTTGCAACTCTGTCATAAAT | Promoter deletion fragments cloning |
| pTeCYC2c-F (-1000) | AACCATTAGGGCCGTGTCG | Promoter deletion fragments cloning |
| pTeCYC2c-F (-650) | GGTGTCTTGGTAATTTTAAAAAAACG | Promoter deletion fragments cloning |
| pTeCYC2c-R | TGTATTTTGGAATTGAAATGTGAAATAA | Promoter deletion fragments cloning |
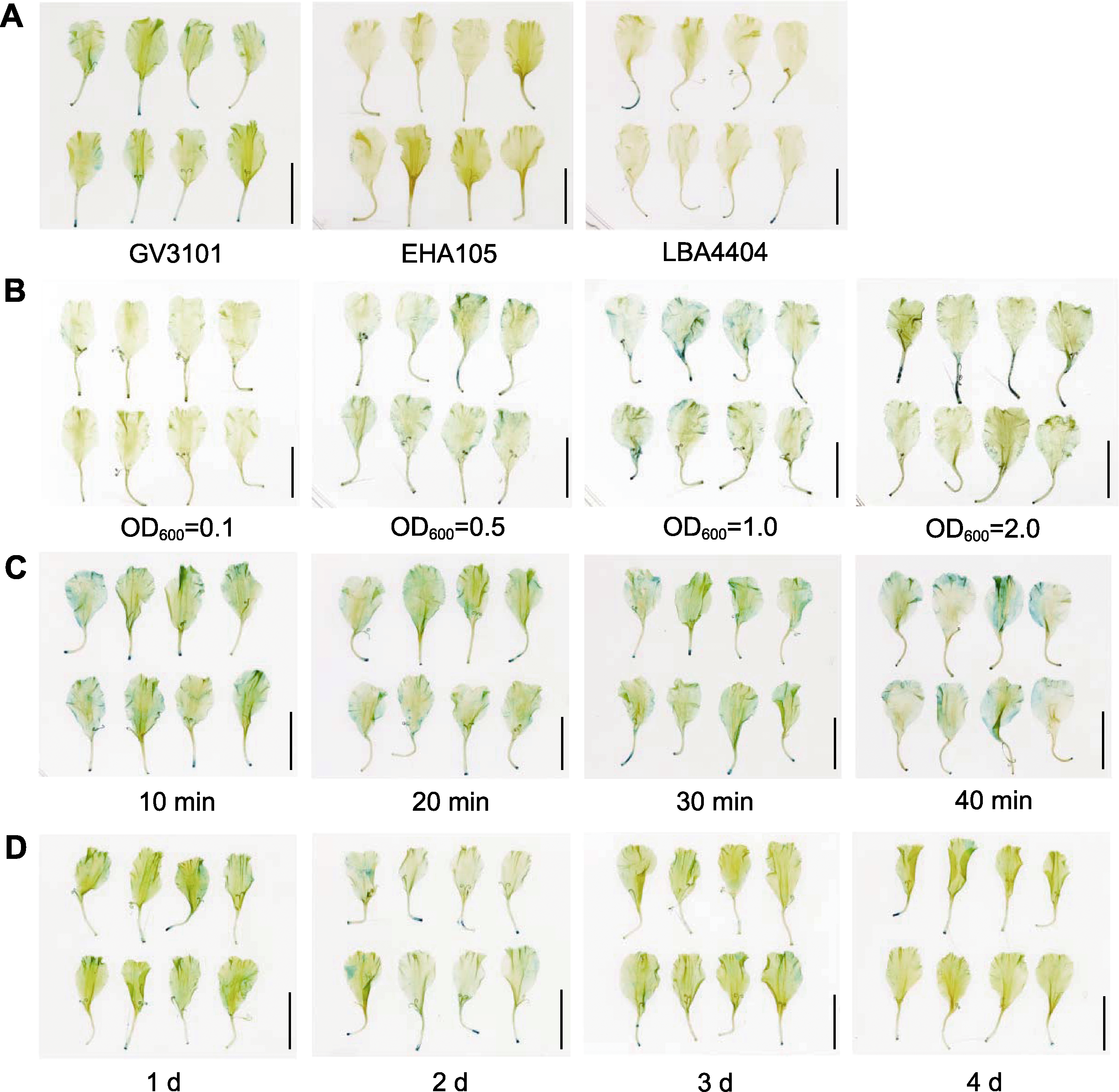
Figure 1 GUS staining of marigold (Tagetes erecta) corollas under different transient transformation conditions (A) GUS staining of marigold corollas infected by three different bacterial strains; (B) GUS staining under four different concentrations (OD600) of bacterial suspension; (C) GUS staining under four different infection duration; (D) GUS staining under four different co-culture time. (A)-(D) Bars=1 cm
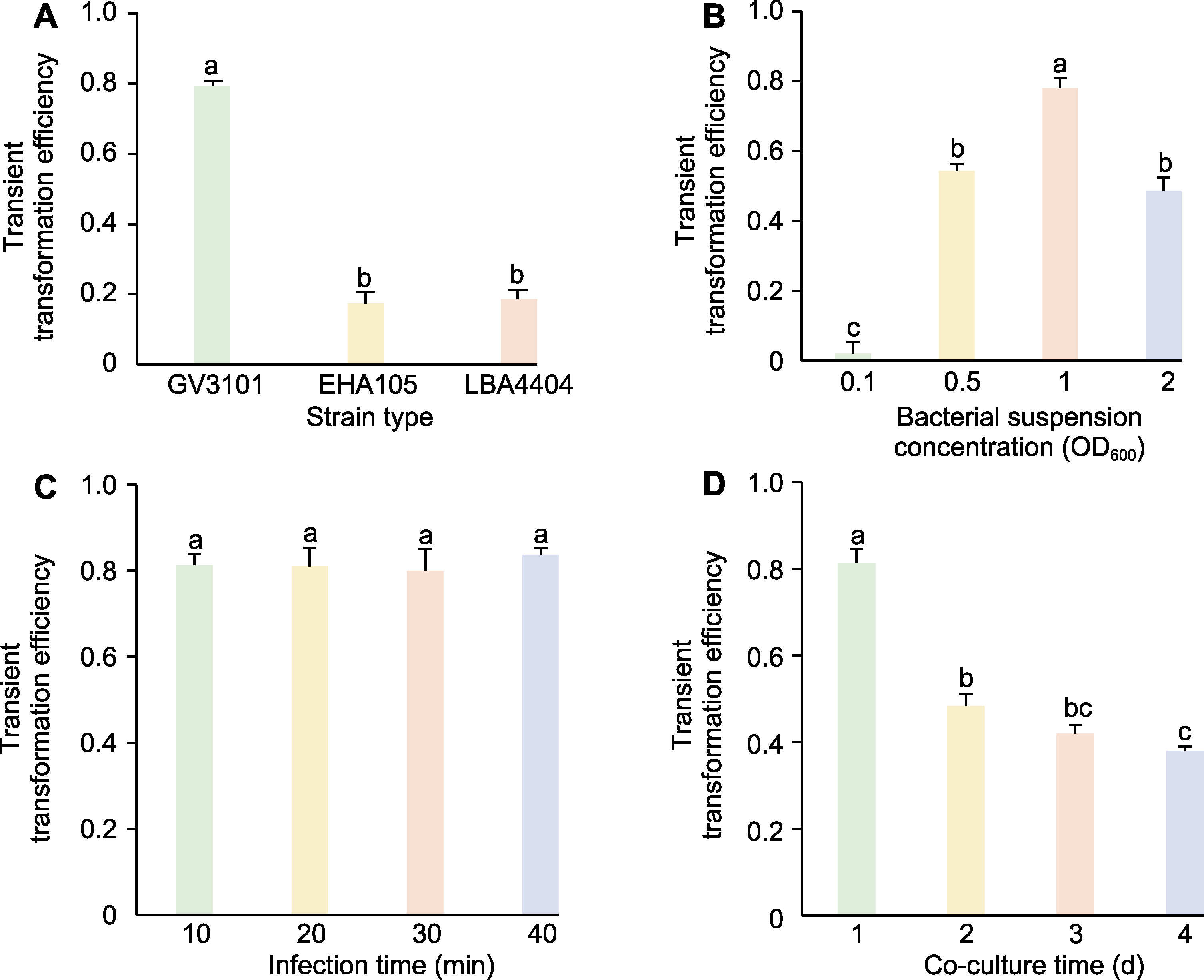
Figure 2 The influence of strain type (A), bacterial suspension concentration (B), infection time (C), and co-culture time (D) on the transient transformation efficiency of Tagetes erecta corollas Different lowercase letters on the histogram indicate significant differences at the P<0.05 level.
| Cis-regulatory elements | Sequences and position | Number | Description |
|---|---|---|---|
| ARE | AAACCA: -1359, -1296 | 2 | Cis-acting regulatory element essential for the anaerobic induction |
| LAMP-element | CTTTATCA: -1661 | 1 | Part of a light responsive element |
| MBS | CAACTG: -1712 | 1 | MYB binding site involved in drought-inducibility |
| TCT-motif | TCTTAC: -603 | 1 | Part of a light responsive element |
| Box 4 | ATTAAT: -1420, -429, -413, -409 | 4 | Part of a conserved DNA module involved in light responsiveness |
| ACE | CTAACGTATT: -630 | 1 | Cis-acting element involved in light responsiveness |
| CGTCA-motif | CGTCA: -1690, -1347, -1235, -797, -713 | 5 | Cis-acting regulatory element involved in the MeJA- responsiveness |
| MBSI | aaaAaaC(G/C)GTTA: -633 | 1 | MYB binding site involved in flavonoid biosynthetic genes regulation |
| GT1-motif | GGTTAA: -1002, -67 | 2 | Light responsive element |
| ABRE | ACGTG: -1349 | 1 | Cis-acting element involved in the abscisic acid responsiveness |
| TATC-box | TATCCCA: -1708 | 1 | Cis-acting element involved in gibberellin-responsiveness |
| G-box | CACGAC: -1655, -1504 | 2 | Cis-acting regulatory element involved in light responsiveness |
| O2-site | GATGATGTGG: -981 | 1 | Cis-acting regulatory element involved in zein metabolism regulation |
| AE-box | AGAAACAA: -74 | 1 | Part of a module for light response |
| LTR | CCGAAA: -1496 | 1 | Cis-acting element involved in low-temperature responsiveness |
Table 2 Analysis of the cis-acting elements in the TeCYC2c promoter
| Cis-regulatory elements | Sequences and position | Number | Description |
|---|---|---|---|
| ARE | AAACCA: -1359, -1296 | 2 | Cis-acting regulatory element essential for the anaerobic induction |
| LAMP-element | CTTTATCA: -1661 | 1 | Part of a light responsive element |
| MBS | CAACTG: -1712 | 1 | MYB binding site involved in drought-inducibility |
| TCT-motif | TCTTAC: -603 | 1 | Part of a light responsive element |
| Box 4 | ATTAAT: -1420, -429, -413, -409 | 4 | Part of a conserved DNA module involved in light responsiveness |
| ACE | CTAACGTATT: -630 | 1 | Cis-acting element involved in light responsiveness |
| CGTCA-motif | CGTCA: -1690, -1347, -1235, -797, -713 | 5 | Cis-acting regulatory element involved in the MeJA- responsiveness |
| MBSI | aaaAaaC(G/C)GTTA: -633 | 1 | MYB binding site involved in flavonoid biosynthetic genes regulation |
| GT1-motif | GGTTAA: -1002, -67 | 2 | Light responsive element |
| ABRE | ACGTG: -1349 | 1 | Cis-acting element involved in the abscisic acid responsiveness |
| TATC-box | TATCCCA: -1708 | 1 | Cis-acting element involved in gibberellin-responsiveness |
| G-box | CACGAC: -1655, -1504 | 2 | Cis-acting regulatory element involved in light responsiveness |
| O2-site | GATGATGTGG: -981 | 1 | Cis-acting regulatory element involved in zein metabolism regulation |
| AE-box | AGAAACAA: -74 | 1 | Part of a module for light response |
| LTR | CCGAAA: -1496 | 1 | Cis-acting element involved in low-temperature responsiveness |
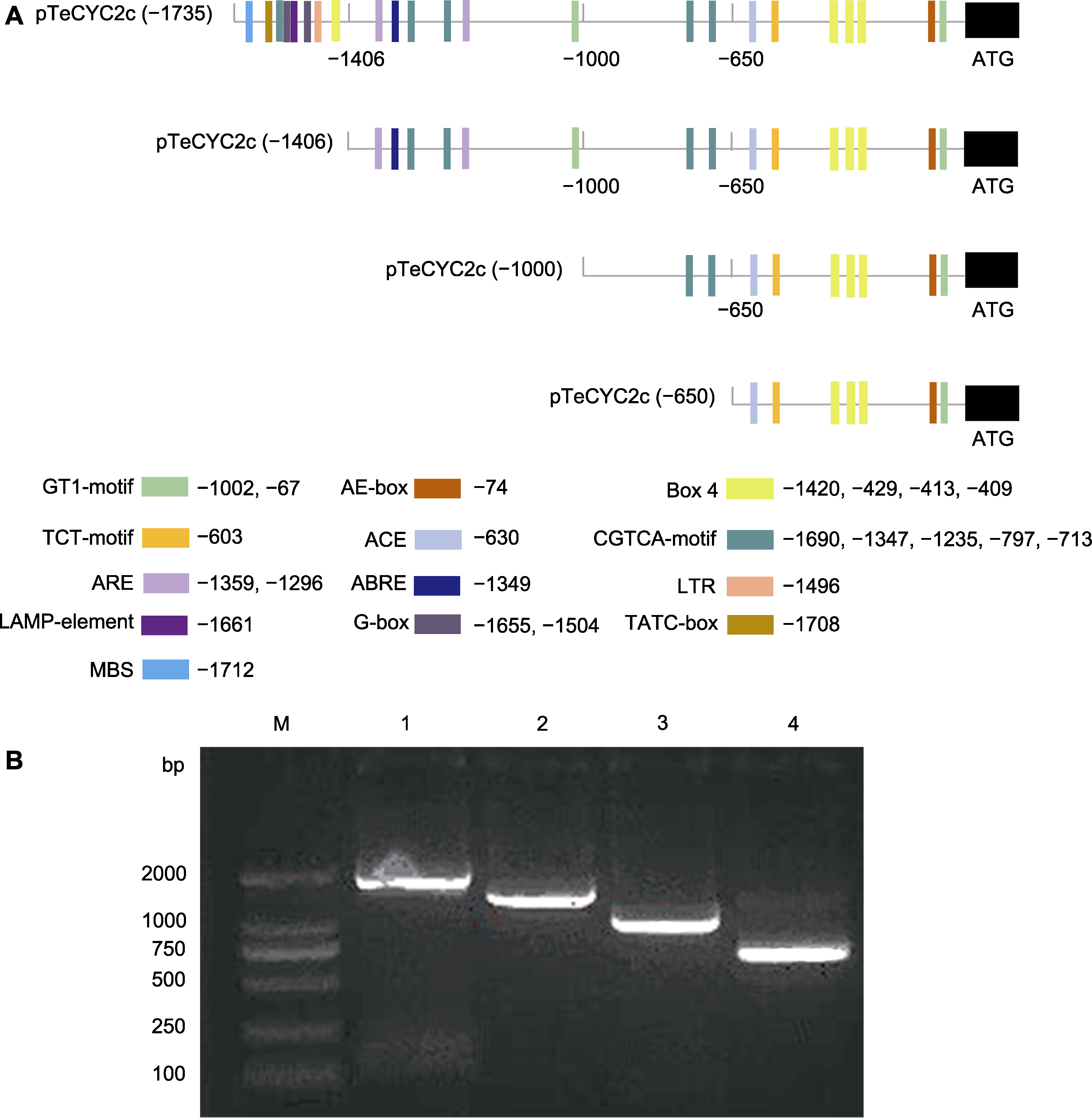
Figure 3 Schematic diagram of the design of TeCYC2c gene promoter deletion fragments and cloning of different length fragments of the promoter (A) Schematic diagram of the design of TeCYC2c gene promoter deletion fragments (Box 4: Light-responsive element; ACE: Light-responsive element; TCT-motif: Light-responsive element; GT1-motif: Light-responsive element; AE-box: Light-responsive element; ARE: Anaerobic induction element; MBS: Drought induction element; CGTCA-motif: MeJA-responsive element; ABRE: ABA-responsive element; TATC-box: GA-responsive element; LTR: Low-temperature responsive element; the numerical values in the figure are in bp); (B) Cloning of different length fragments of the TeCYC2c gene promoter (M: DL2000 marker; 1-4: Representing pTeCYC2c (-1 735), pTeCYC2c (-1 406), pTeCYC2c (-1 000), and pTeCYC2c (-650), respectively).
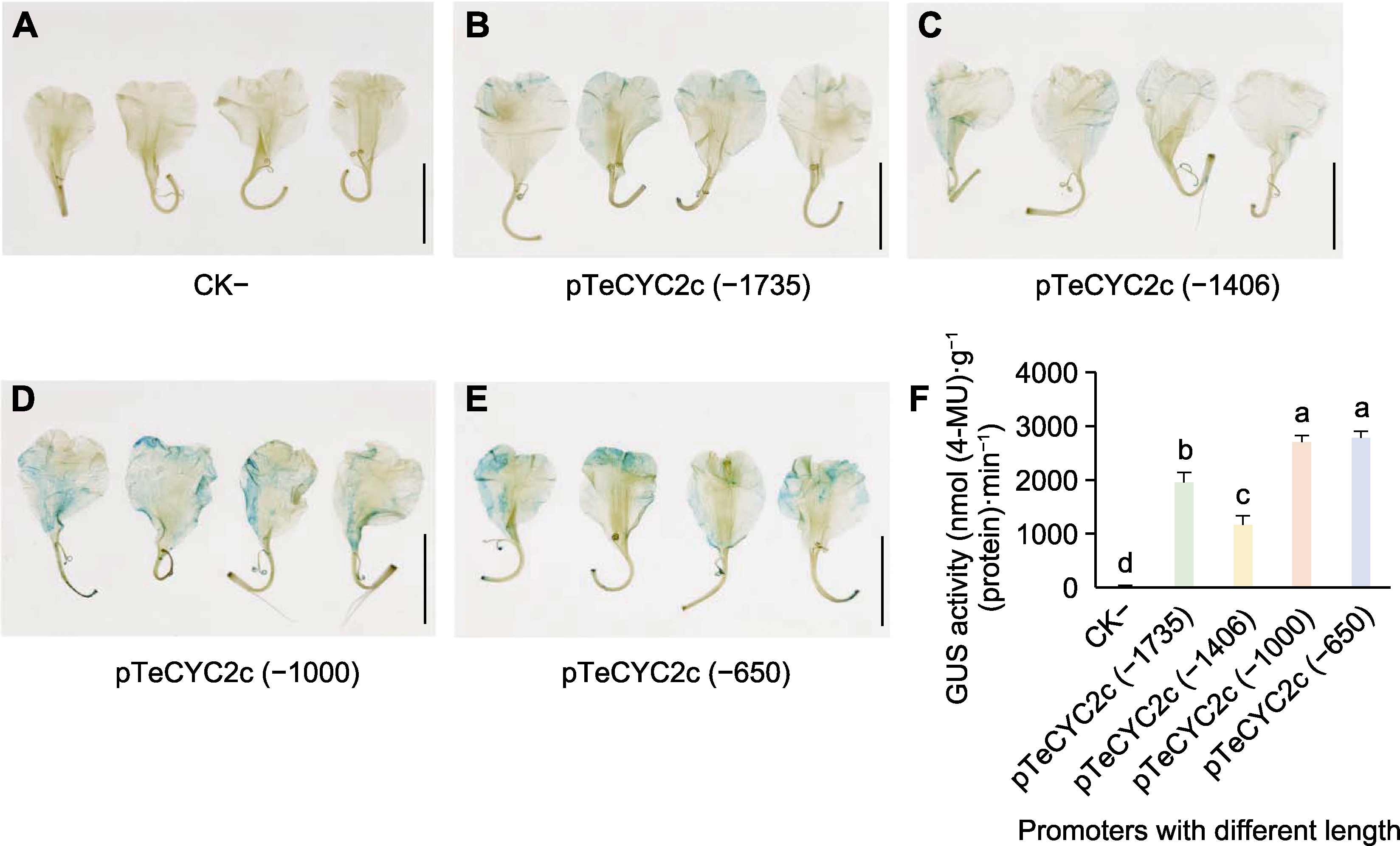
Figure 4 GUS staining and GUS activity assay driven by the promoters with different lengths (A) Negative control; (B) GUS staining under the influence of pTeCYC2c (-1 735); (C) GUS staining under the influence of pTeCYC2c (-1 406); (D) GUS staining under the influence of pTeCYC2c (-1 000); (E) GUS staining under the influence of pTeCYC2c (-650); (F) Determination of GUS activity driven by the promoters with different length. Different lowercase letters on the bar graph indicate significant differences at the P<0.05 level. (A)-(E) Bars=1 cm
| [1] | Ai Y, Zhang QH, Wang WN, Zhang CL, Cao Z, Bao MZ, He YH (2016). Transcriptomic analysis of differentially expressed genes during flower organ development in genetic male sterile and male fertile Tagetes erecta by digital gene-expression profiling. PLoS One 11, e0150892. |
| [2] |
Broholm SK, Tähtiharju S, Laitinen RAE, Albert VA, Teeri TH, Elomaa P (2008). A TCP domain transcription factor controls flower type specification along the radial axis of the Gerbera (Asteraceae) inflorescence. Proc Natl Acad Sci USA 105, 9117-9122.
DOI PMID |
| [3] |
Fambrini M, Pugliesi C (2017). Mobilization of the Tetu1 transposable element of Helianthus annuus: evidence for excision in different developmental stages. Biol Plant 61, 55-63.
DOI URL |
| [4] |
Fukuda N, Ajima C, Yukawa T, Olsen JE (2016). Antagonistic action of blue and red light on shoot elongation in petunia depends on gibberellin, but the effects on flowering are not generally linked to gibberellin. Environ Exp Bot 121, 102-111.
DOI URL |
| [5] |
Godoy-Hernández G, Berzunza EA, Concha LC, de Lourdes Miranda-Ham M (2006). Agrobacterium-mediated transient transformation of marigold (Tagetes erecta). Plant Cell Tissue Organ Cult 84, 365-368.
DOI URL |
| [6] | Goto N, Pharis RP (1999). Role of gibberellins in the development of floral organs of the gibberellin-deficient mutant, ga1-1, of Arabidopsis thaliana. Can J Bot 77, 944-954. |
| [7] | He YH (2010). Genetic Analysis of the Male Sterility of Tagetes erecta and Its Application in Breeding. Doctoral dissertation. Wuhan: Huazhong Agricultural University. pp. 148. (in Chinese) |
| 何燕红 (2010). 万寿菊雄性不育性状的遗传分析及其育种应用. 博士论文. 武汉: 华中农业大学. pp. 148. | |
| [8] |
Hileman LC (2014). Bilateral flower symmetry-how, when and why? Curr Opin Plant Biol 17, 146-152.
DOI PMID |
| [9] |
Howarth DG, Donoghue MJ (2006). Phylogenetic analysis of the “ECE” (CYC/TB1) clade reveals duplications predating the core eudicots. Proc Natl Acad Sci USA 103, 9101-9106.
DOI URL |
| [10] |
Hu JH, Mitchum MG, Barnaby N, Ayele BT, Ogawa M, Nam E, Lai WC, Hanada A, Alonso JM, Ecker JR, Swain SM, Yamaguchi S, Kamiya Y, Sun TP (2008). Potential sites of bioactive gibberellin production during reproductive growth in Arabidopsis. Plant Cell 20, 320-336.
DOI URL |
| [11] |
Huang D, Li XW, Sun M, Zhang TX, Pan HT, Cheng TR, Wang J, Zhang QX (2016). Identification and characterization of CYC-like genes in regulation of ray floret development in Chrysanthemum morifolium. Front Plant Sci 7, 1633.
PMID |
| [12] |
Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K (2001). The DEFECTIVE IN ANTHER DEHISCENCE1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 13, 2191-2209.
DOI PMID |
| [13] |
Jefferson RA, Kavanagh TA, Bevan MW (1987). GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6, 3901-3907.
DOI PMID |
| [14] |
Juntheikki-Palovaara I, Tähtiharju S, Lan TY, Broholm SK, Rijpkema AS, Ruonala R, Kale L, Albert VA, Teeri TH, Elomaa P (2014). Functional diversification of duplicated CYC2 clade genes in regulation of inflorescence development in Gerbera hybrida (Asteraceae). Plant J 79, 783-796.
DOI URL |
| [15] | Moënne-Loccoz Y, Mavingui P, Combes C, Normand P, Steinberg C (2015). Microorganisms and biotic interactions. In: Bertrand JC, Caumette P, Lebaron P, Matheron R, Normand P, Sime-Ngando T, eds. Environmental Microbiology: Fundamentals and Applications. Dordrecht: Springer. pp. 395-444. |
| [16] |
Rieu I, Ruiz-Rivero O, Fernandez-Garcia N, Griffiths J, Powers SJ, Gong F, Linhartova T, Eriksson S, Nilsson O, Thomas SG, Phillips AL, Hedden P (2008). The gibberellin biosynthetic genes AtGA20ox1 and AtGA20ox2 act, partially redundantly, to promote growth and development throughout the Arabidopsis life cycle. Plant J 53, 488-504.
DOI PMID |
| [17] |
Su WR, Huang KL, Shen RS, Chen WS (2002). Abscisic acid affects floral initiation in Polianthes tuberosa. J Plant Physiol 159, 557-559.
DOI URL |
| [18] | Sun M, Yang HH, Wang AQ, Zhang YJ, Da XW, Zhang J, Sun K, Wu JP, Feng HQ (2023). Effects of different vectors and Agrobacterium tumefaciens on transient expression of alfalfa. Bull Bot Res 43, 835-845. (in Chinese) |
|
孙敏, 杨红红, 王安琪, 张悦婧, 达晓伟, 张继, 孙坤, 吴建平, 冯汉青 (2023). 不同载体和农杆菌对苜蓿瞬时表达影响的研究. 植物研究 43, 835-845.
DOI |
|
| [19] |
Tahtiharju S, Rijpkema AS, Vetterli A, Albert VA, Teeri TH, Elomaa P (2012). Evolution and diversification of the CYC/TB1 gene family in Asteraceae—a comparative study in gerbera (Mutisieae) and sunflower (Heliantheae). Mol Biol Evol 29, 1155-1166.
DOI URL |
| [20] |
Tanimoto S, Miyazaki A, Harada H (1985). Regulation by abscisic acid of in vitro flower formation in Torenia stem segments. Plant Cell Physiol 26, 675-682.
DOI URL |
| [21] |
Tong Z, Wang T, Xu Y (1990). Evidence for involvement of phytochrome regulation in male sterility of a mutant of Oryza sativa L. Photochem Photobiol 52, 161-164.
DOI URL |
| [22] |
van der Meer IM, Spelt CE, Mol JNM, Stuitje AR (1990). Promoter analysis of the chalcone synthase (chsA) gene of Petunia hybrida: a 67 bp promoter region directs flower- specific expression. Plant Mol Biol 15, 95-109.
PMID |
| [23] | Wang RP, Liu Q, Yang ZQ, Zhang BK, Huang Z, Niu XL (2023). Cloning and analysis of carotene hydroxylase gene TeCHYE in Tagetes erecta L. J Hefei Univ Technol (Nat Sci) 46, 535-540. (in Chinese) |
| 王瑞鹏, 刘茜, 杨智强, 张博昆, 黄姿, 牛向丽 (2023). 万寿菊胡萝卜素羟化酶基因TeCHYE克隆与分析. 合肥工业大学学报(自然科学版) 46, 535-540. | |
| [24] | Wang WJ, Zhu Y, Zhang HM, Wei LD, Yi QP, Yu XM, Liu YH, Zhang LX, Cheng WH, He YH (2023). Morphological identification and development of linkage markers for lobed ray floret mutants in marigold (Tagetes erecta). Chin Bull Bot 58, 893-904. (in Chinese) |
|
王文静, 朱钰, 张洪铭, 韦陆丹, 易庆平, 余晓敏, 刘雨菡, 张莉雪, 程文翰, 何燕红 (2023). 万寿菊舌状花花冠裂片突变体的形态鉴定及连锁标记开发. 植物学报 58, 893-904.
DOI |
|
| [25] | Wang YQ, Wei LD, Wang WJ, Liu BJ, Zhang CL, Zhang JW, He YH (2020). The establishment and optimization of a regeneration system for marigold (Tagetes erecta). Chin Bull Bot 55, 749-759. (in Chinese) |
| 王亚琴, 韦陆丹, 王文静, 刘宝骏, 张春玲, 张俊卫, 何燕红 (2020). 万寿菊再生体系的建立及优化. 植物学报 55, 749-759. | |
| [26] | Wen Y, Li B, Zhao DG, Zhao YC (2020). Cloning and expression analysis of the promoter of glycosyltransferase gene in Eucommia ulmoides. J Agr Biotechnol 28, 223-231. (in Chinese) |
| 文永, 李彪, 赵德刚, 赵懿琛 (2020). 杜仲糖基转移酶基因的启动子克隆及表达分析. 农业生物技术学报 28, 223-231. | |
| [27] | Yang F (2011). Establishment of PSY Plant Expression Vector Harboring Two T-DNAs and Agrobacterium-mediated Transformation System and Optimization in SSR- PCR Reaction System for Tagetes erecta L. Master’s thesis. Shanghai: Shanghai Jiao Tong University. pp. 58. (in Chinese) |
| 杨帆 (2011). 色素万寿菊PSY双边界载体及遗传转化体系建立和SSR-PCR体系的优化. 硕士论文. 上海: 上海交通大学. pp. 58. | |
| [28] | Yu XM, Wang YQ, Liu YH, Yi QP, Cheng WH, Zhu Y, Duan F, Zhang LX, He YH (2023). Establishment of Agrobacterium tumefaciens-mediated genetic transformation system of marigold (Tagetes erecta). Chin Bull Bot 58, 760-769. (in Chinese) |
|
余晓敏, 王亚琴, 刘雨菡, 易庆平, 程文翰, 朱钰, 段枫, 张莉雪, 何燕红 (2023). 根癌农杆菌介导万寿菊遗传转化体系的建立. 植物学报 58, 760-769.
DOI |
|
| [29] |
Yuan CQ, Huang D, Yang Y, Sun M, Cheng TR, Wang J, Pan HT, Zhang QX (2020). CmCYC2-like transcription factors may interact with each other or bind to the promoter to regulate floral symmetry development in Chrysanthemum morifolium. Plant Mol Biol 103, 159-171.
DOI |
| [30] | Zhang B (2012). The Study of Karyotype on Genus Tagetes L. and Factors in the Genetic Transformation System of psy Gene for Tagetes erecta L. Master’s thesis. Shanghai: Shanghai Jiao Tong University. pp. 64. (in Chinese) |
| 张嫔 (2012). 万寿菊属植物染色体核型分析及万寿菊psy基因遗传转化体系影响因素的研究. 硕士论文. 上海: 上海交通大学. pp. 64. | |
| [31] |
Zheng L, Liu GF, Meng XN, Li YB, Wang YC (2012). A versatile Agrobacterium-mediated transient gene expression system for herbaceous plants and trees. Biochem Genet 50, 761-769.
DOI PMID |
| [32] |
Zhu Y, Liu YH, Wang WJ, Li H, Liu CC, Dou LL, Wei LD, Cheng WH, Bao MZ, Yi QP, He YH (2023). Identification and characterization of CYC2-like genes related to floral symmetric development in Tagetes erecta (Asteraceae). Gene 889, 147804.
DOI URL |
| [1] | Xiang Song, Luyao Wang, Boxiao Fu, Shuangda Li, Yuanyuan Wei, Yan Hong, Silan Dai. Advances in Identification and Synthesis of Promoter Elements in Higher Plants [J]. Chinese Bulletin of Botany, 2024, 59(5): 691-708. |
| [2] | Fei Zhao, Liuyi Dang, Minhui Wei, Chunying Liu, Wei Leng, Chenjing Shang. Expression of Amaranthin-like Lectins Gene and Responses to Abiotic Stresses in Cucumber [J]. Chinese Bulletin of Botany, 2021, 56(2): 183-190. |
| [3] | Yaqin Wang, Ludan Wei, Wenjing Wang, Baojun Liu, Chunling Zhang, Junwei Zhang, Yanhong He. The Establishment and Optimization of a Regeneration System for Marigold (Tagetes erecta) [J]. Chinese Bulletin of Botany, 2020, 55(6): 749-759. |
| [4] | Huijin Fan, Kangming Jin, Renying Zhuo, Guirong Qiao. Cloning and Expression Analysis of Different Truncated U3 Promoters in Phyllostachys edulis [J]. Chinese Bulletin of Botany, 2020, 55(3): 299-307. |
| [5] | Hu Tianyuan, Wang Rui, Chen Shang, Ma Baowei, Gao Wei, Huang Luqi. Protoplast Isolation and Establishment of Transient Expression System of Tripterygium wilfordii Suspension Culture Cells [J]. Chinese Bulletin of Botany, 2017, 52(6): 774-782. |
| [6] | Xinli Zan, Ying Gao, Yuling Chen, Kaijun Zhao. Pathogen-responsive Cis-acting Elements and Their Interactive Transcription Factors [J]. Chinese Bulletin of Botany, 2013, 48(2): 219-229. |
| [7] | Weiwei Liu, Hailei Zhang, Caifeng Liu, Xiaochun Ge. Construction and Activity Analysis of an Antibiotic-inducible Promoter [J]. Chinese Bulletin of Botany, 2011, 46(5): 560-568. |
| [8] | Xuanyu Liu, Qingyun Wang, Shujun Liu, Songquan Song. Advances in the Genetic Transformation of Sorghum bicolor [J]. Chinese Bulletin of Botany, 2011, 46(2): 216-223. |
| [9] | Xiaobo Qin;Jihai Gao;Ying Xu*;Jinping Zhang;Caixia Shao;Sha Lin;Shuwen Zhang;Luding Jiang;Yueqin Li;Fang Chen . Isolation of Curcin Promoter from Jatropha curcas and Analysis in Transgenic Tobacco Plants [J]. Chinese Bulletin of Botany, 2008, 25(04): 407-414. |
| [10] | Wei Xie Chaoyin Yue Zhenghong Guo Zhipeng Dai Min Liu Wei Yao. Transient Expression of GUS Gene Controlled by Different Regulator Sequences of Tobacco [J]. Chinese Bulletin of Botany, 2007, 24(04): 452-458. |
| [11] | Xiaona Liu;Chang Fu;Yongfen Huang*. Advances in Studies of Seed-specific Gene Promoters [J]. Chinese Bulletin of Botany, 2007, 24(02): 218-225. |
| [12] | I Xin-Qi YUAN Long-PingDENG Qi-Yun XIAO Jin-Hua. Potential Ways to Use Spontaneous Genic Male Sterility in the Molecular Breeding of Hybrid Crops [J]. Chinese Bulletin of Botany, 2003, 20(05): 625-631. |
| [13] | SU NingSUN Meng LI Yi-Nü NI Pi-Chong SHEN Gui-Fang. Isolation and Modification of Rice Chloroplast 16S Promoter,Construction of Expression Vector and Transformation [J]. Chinese Bulletin of Botany, 2003, 20(03): 295-301. |
| [14] | LI Li ZHANG Jing-Yu DU Gui-Sen SONG Yan-Ru. Isolation of Seed Specific Promoter (napinB promoter),Construction of Expression Vector and Obtainmentof Transgenic Tobacco Plants [J]. Chinese Bulletin of Botany, 2001, 18(02): 216-220. |
| [15] | LI Yi-Kun and WANG Jin-Fa. Advances of the Studies on Plant Promoter [J]. Chinese Bulletin of Botany, 1998, 15(增刊): 1-6. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||