

Chinese Bulletin of Botany ›› 2024, Vol. 59 ›› Issue (1): 89-98.DOI: 10.11983/CBB23021 cstr: 32102.14.CBB23021
• TECHNIQUES AND METHODS • Previous Articles Next Articles
Jiaxin Chen, Hao Mei, Caixiang Huang, Zongyuan Liang, Yitong Quan, Dongpeng Li, Buweimaieryemu·Saimaiti , Xinxin Li*( ), Hong Liao
), Hong Liao
Received:2023-02-20
Accepted:2023-05-31
Online:2024-01-10
Published:2024-01-10
Contact:
*E-mail: Jiaxin Chen, Hao Mei, Caixiang Huang, Zongyuan Liang, Yitong Quan, Dongpeng Li, Buweimaieryemu·Saimaiti , Xinxin Li, Hong Liao. A Highly Efficient Method to Generate Chimeric Soybean Plant with Transgenic Hairy Roots[J]. Chinese Bulletin of Botany, 2024, 59(1): 89-98.
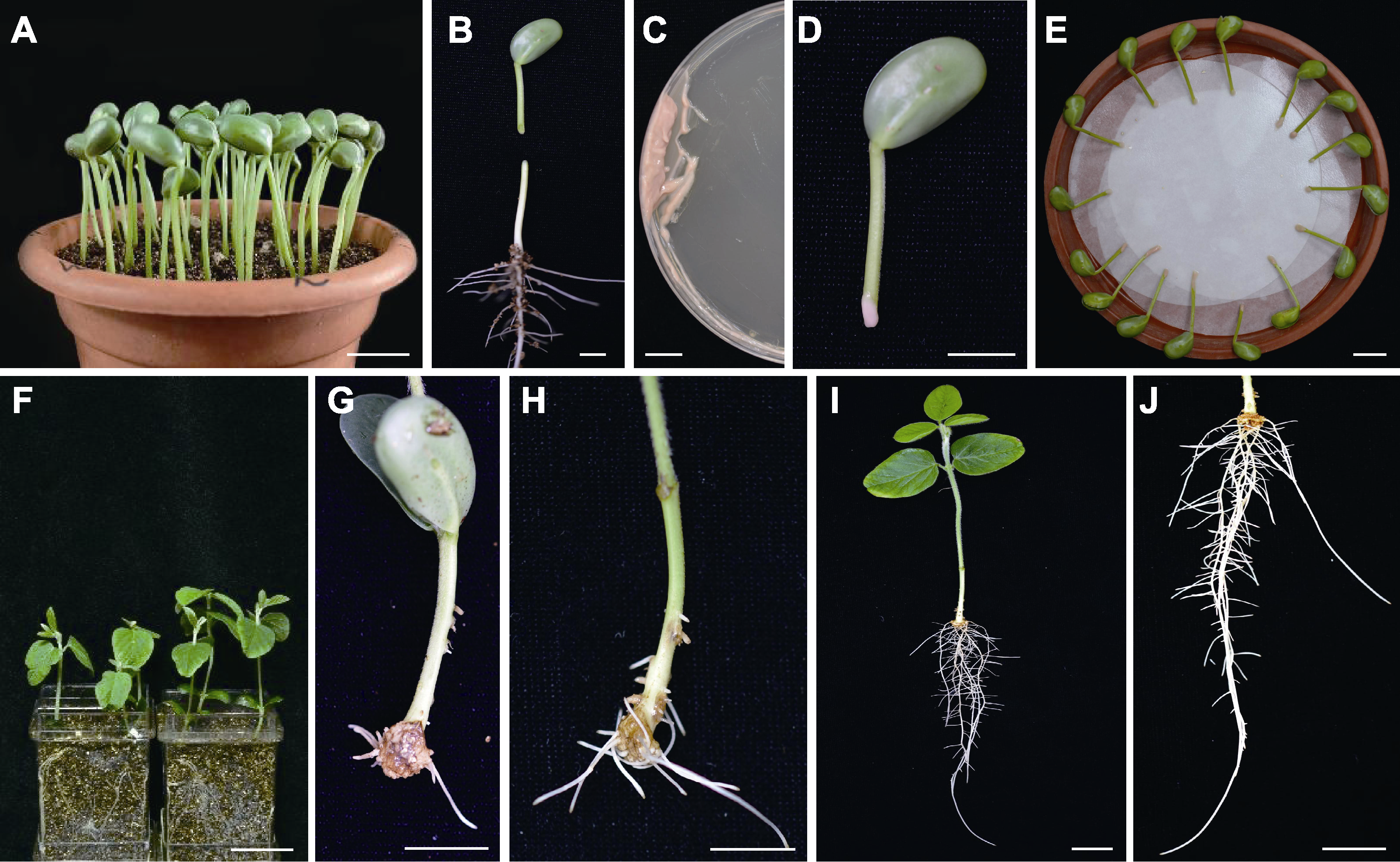
Figure 1 Procedure of generating chimeric soybean plants with Agrobacterium rhizogenes induced hypocotyl transgenic hairy roots (A) Soybean seedlings at 4 d post germination (bar=2 cm); (B) Hypocotyl was cut to form wound site with an angle of 45° (bar=1 cm); (C) A. rhizogenes were collected in petri dishes (bar=1 cm); (D) Apply bacteria to the wound (bar=1 cm); (E) Co-cultivation (bar=1 cm); (F) Inoculated explants were planted into vermiculite (bar=5 cm); (G) Callus came out after 10 d of cultivation (bar=1 cm); (H) Hairy roots emerged at 14 d post induction (bar=1 cm); (I) Growth performance of hairy roots after 20 d of growth (bar=2 cm); (J) Partial enlarged view of image I (bar=2 cm)
| Treatments | Genotype | Co-cultivation time (d) | Efficiency of induction (%) | Number of chimeric plant with HR | Surviving rate (%) | Number of surviving plants | Total number of plants |
|---|---|---|---|---|---|---|---|
| Dark | Ws82 | 0 | 87.0 | 20 | 76.7 | 23 | 30 |
| 1 | 96.2 | 25 | 86.7 | 26 | 30 | ||
| 3 | 89.7 | 26 | 96.7 | 29 | 30 | ||
| HN66 | 0 | 87.5 | 21 | 80.0 | 24 | 30 | |
| 1 | 95.7 | 22 | 76.7 | 23 | 30 | ||
| 3 | 83.3 | 15 | 60.0 | 18 | 30 | ||
| BX10 | 0 | 93.3 | 14 | 75.0 | 15 | 20 | |
| 1 | 94.7 | 18 | 95.0 | 19 | 20 | ||
| 3 | 82.4 | 14 | 85.0 | 17 | 20 | ||
| Light (100 µmol∙m-2∙s-1) | Ws82 | 0 | 82.4 | 21 | 76.7 | 23 | 30 |
| 1 | 91.3 | 21 | 76.7 | 23 | 30 | ||
| 3 | 68.2 | 15 | 73.3 | 22 | 30 | ||
| HN66 | 0 | 90.0 | 18 | 66.7 | 20 | 30 | |
| 1 | 83.3 | 20 | 80.0 | 24 | 30 | ||
| 3 | 90.5 | 19 | 70.0 | 21 | 30 | ||
| BX10 | 0 | 86.7 | 13 | 75.0 | 15 | 20 | |
| 1 | 83.3 | 15 | 90.0 | 18 | 20 | ||
| 3 | 80.0 | 12 | 75.0 | 15 | 20 |
Table 1 Comparison of hairy roots (HR) generated from different soybean genotypes under light/dark conditions
| Treatments | Genotype | Co-cultivation time (d) | Efficiency of induction (%) | Number of chimeric plant with HR | Surviving rate (%) | Number of surviving plants | Total number of plants |
|---|---|---|---|---|---|---|---|
| Dark | Ws82 | 0 | 87.0 | 20 | 76.7 | 23 | 30 |
| 1 | 96.2 | 25 | 86.7 | 26 | 30 | ||
| 3 | 89.7 | 26 | 96.7 | 29 | 30 | ||
| HN66 | 0 | 87.5 | 21 | 80.0 | 24 | 30 | |
| 1 | 95.7 | 22 | 76.7 | 23 | 30 | ||
| 3 | 83.3 | 15 | 60.0 | 18 | 30 | ||
| BX10 | 0 | 93.3 | 14 | 75.0 | 15 | 20 | |
| 1 | 94.7 | 18 | 95.0 | 19 | 20 | ||
| 3 | 82.4 | 14 | 85.0 | 17 | 20 | ||
| Light (100 µmol∙m-2∙s-1) | Ws82 | 0 | 82.4 | 21 | 76.7 | 23 | 30 |
| 1 | 91.3 | 21 | 76.7 | 23 | 30 | ||
| 3 | 68.2 | 15 | 73.3 | 22 | 30 | ||
| HN66 | 0 | 90.0 | 18 | 66.7 | 20 | 30 | |
| 1 | 83.3 | 20 | 80.0 | 24 | 30 | ||
| 3 | 90.5 | 19 | 70.0 | 21 | 30 | ||
| BX10 | 0 | 86.7 | 13 | 75.0 | 15 | 20 | |
| 1 | 83.3 | 15 | 90.0 | 18 | 20 | ||
| 3 | 80.0 | 12 | 75.0 | 15 | 20 |
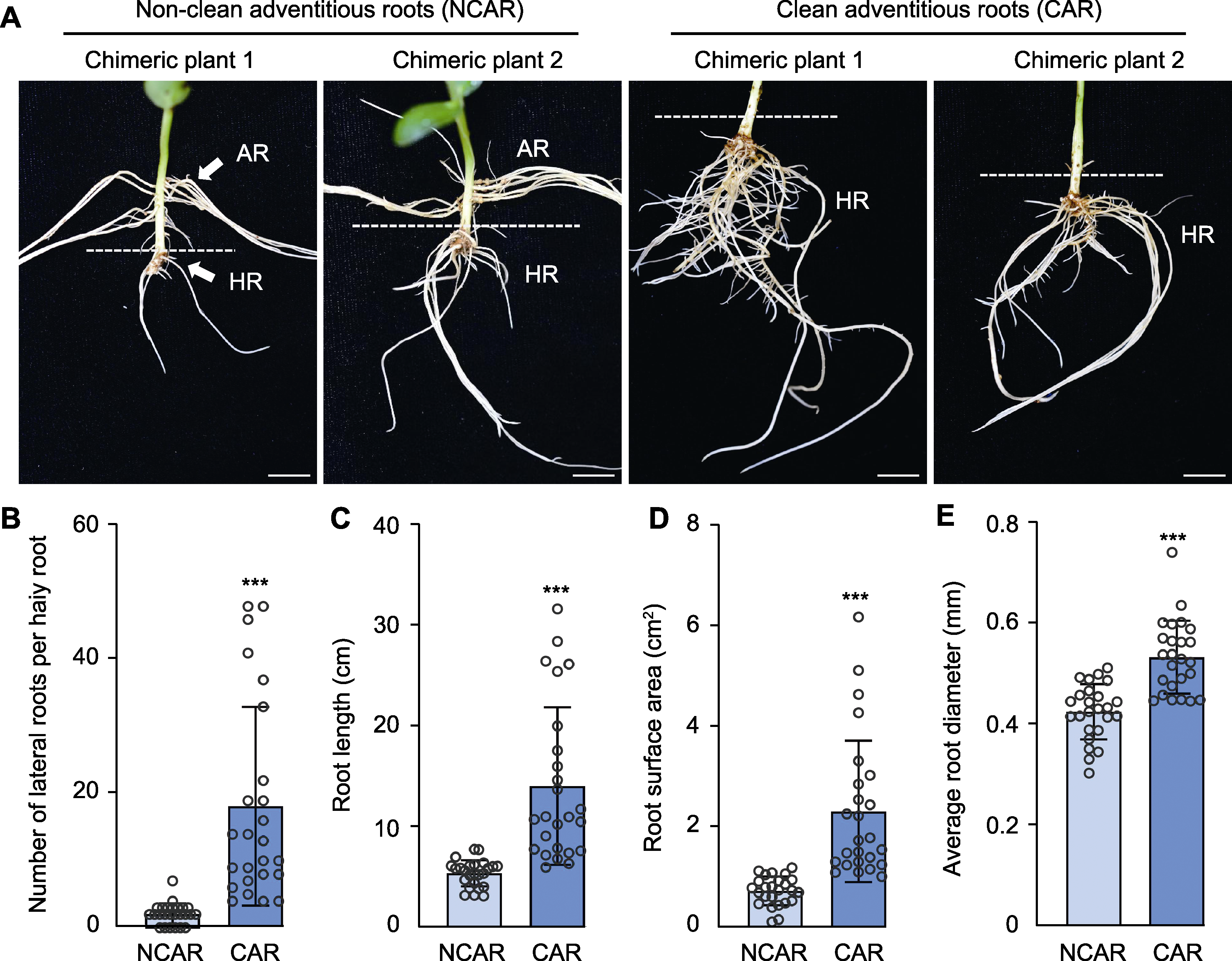
Figure 2 Effect of cleaning adventitious root at hypocotyl on induction of hairy roots (A) Comparison of hairy roots between non-clean and clean adventitious root condition (bars=1 cm); (B) Number of lateral roots per hairy root; (C) Root length; (D) Root surface area; (E) Average root diameter. Data are means±SD from 25 hairy roots. *** indicate significant differences at 0.001 level. AR: Adventitious root; HR: Hairy root

Figure 3 Effect of cleaning adventitious root at hypocotyl on positive rate of transgenic hairy roots (A) GUS staining of hairy roots under non-clean and clean adventitious roots conditions, the oval and arrows indicate non-transgenic hairy roots (bars=1 cm); (B) Total number of hairy roots generated from each explant; (C) Number of transgenic hairy roots per explant; (D) Efficiency of transgenic hairy roots. Data are means±SD from 14 explants. * and *** indicate significant differences at 0.05 and 0.001 levels, respectively.

Figure 4 Effect of cleaning adventitious root on nodulation of hairy roots (A), (D) Soybean chimeric plants without (A) or with (D) cleaning adventitious root (bars=2 cm); (B), (E) Partial enlarged view of image A and D in box (bars=1 cm); (C), (F) GUS staining of hairy roots (bars=1 cm). The red arrows indicate nodules on hairy roots. AR is the same as shown in Figure 2.
| Treatments | Chimeric plants | Hairy roots | Nodule number |
|---|---|---|---|
| NCAR | 1st | 1st | 3 |
| 2nd | 4 | ||
| 3rd | 2 | ||
| 2nd | 1st | 2 | |
| 2nd | 1 | ||
| 3rd | 3 | ||
| 3rd | 1st | 4 | |
| 2nd | 2 | ||
| 3rd | 1 | ||
| Average | 2.4 | ||
| CAR | 1st | 1st | 7 |
| 2nd | 6 | ||
| 3rd | 8 | ||
| 2nd | 1st | 10 | |
| 2nd | 8 | ||
| 3rd | 6 | ||
| 3rd | 1st | 5 | |
| 2nd | 7 | ||
| 3rd | 9 | ||
| Average | 7.3*** | ||
Table 2 Effect of non-clean (NCAR) and clean (CAR) adventitious root on nodule number in transgenic hairy roots
| Treatments | Chimeric plants | Hairy roots | Nodule number |
|---|---|---|---|
| NCAR | 1st | 1st | 3 |
| 2nd | 4 | ||
| 3rd | 2 | ||
| 2nd | 1st | 2 | |
| 2nd | 1 | ||
| 3rd | 3 | ||
| 3rd | 1st | 4 | |
| 2nd | 2 | ||
| 3rd | 1 | ||
| Average | 2.4 | ||
| CAR | 1st | 1st | 7 |
| 2nd | 6 | ||
| 3rd | 8 | ||
| 2nd | 1st | 10 | |
| 2nd | 8 | ||
| 3rd | 6 | ||
| 3rd | 1st | 5 | |
| 2nd | 7 | ||
| 3rd | 9 | ||
| Average | 7.3*** | ||
| [1] | 程凤娴, 曹桂芹, 王秀荣, 赵静, 严小龙, 廖红 (2008). 华南酸性低磷土壤中大豆根瘤菌高效株系的发现及应用. 科学通报 53, 2903-2910. |
| [2] | 杜梦柯, 连文婷, 张晓, 李欣欣 (2021). 氮处理对大豆根瘤固氮能力及GmLbs基因表达的影响. 植物学报 56, 391-403. |
| [3] | 李锦锦, 王昉, 张万科, 文自翔, 李海朝, 袁道华, 李金英, 张辉, 杨青华, 卢为国 (2012). 发根农杆菌介导不同基因型大豆转化效率的筛选. 河南农业科学 41(5), 37-41. |
| [4] | 李欣欣, 赵静, 廖红 (2011). 大豆毛状根-VA菌根真菌双重培养体系的建立. 植物生理学报 47, 475-480. |
| [5] | 栾健, 张斌, 胡钰 (2022). 中国大豆产业的发展态势、政策演进与趋势展望. 农业展望 18(8), 35-41. |
| [6] | 邱丽娟, 王昌陵, 周国安, 陈受宜, 常汝镇 (2007). 大豆分子育种研究进展. 中国农业科学 40, 2418-2436. |
| [7] | 田志喜, 刘宝辉, 杨艳萍, 李明, 姚远, 任小波, 薛勇彪 (2018). 我国大豆分子设计育种成果与展望. 中国科学院院刊 33, 915-922. |
| [8] |
Bahramnejad B, Naji M, Bose R, Jha S (2019). A critical review on use of Agrobacterium rhizogenes and their associated binary vectors for plant transformation. Biotechnol Adv 37, 107405.
DOI URL |
| [9] |
Cheng YY, Wang XL, Cao L, Ji J, Liu TF, Duan KX (2021). Highly efficient Agrobacterium rhizogenes-mediated hairy root transformation for gene functional and gene editing analysis in soybean. Plant Methods 17, 73.
DOI |
| [10] |
Cho HJ, Farrand SK, Noel GR, Widholm JM (2000). High-efficiency induction of soybean hairy roots and propagation of the soybean cyst nematode. Planta 210, 195-204.
DOI PMID |
| [11] |
Fan YL, Zhang XH, Zhong LJ, Wang XY, Jin LS, Lyu SH (2020). One-step generation of composite soybean plants with transgenic roots by Agrobacterium rhizogenes-mediated transformation. BMC Plant Biol 20, 208.
DOI |
| [12] |
Gantait S, Mukherjee E (2021). Hairy root culture technology: applications, constraints and prospect. Appl Microbiol Bio- technol 105, 35-53.
DOI |
| [13] |
Gomes C, Dupas A, Pagano A, Grima-Pettenati J, Paiva JAP (2019). Hairy root transformation: a useful tool to explore gene function and expression in Salix spp. recalcitrant to transformation. Front Plant Sci 10, 1427.
DOI URL |
| [14] |
Guo WB, Zhao J, Li XX, Qin L, Yan XL, Liao H (2011). A soybean β-expansin gene GmEXPB2 intrinsically involved in root system architecture responses to abiotic stresses. Plant J 66, 541-552.
DOI URL |
| [15] |
Herridge DF, Peoples MB, Boddey RM (2008). Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 311, 1-18.
DOI URL |
| [16] |
Huang PH, Lu MY, Li XB, Sun HY, Cheng ZY, Miao YC, Fu YF, Zhang XM (2022). An efficient Agrobacterium rhizogenes-mediated hairy root transformation method in a soybean root biology study. Int J Mol Sci 23, 12261.
DOI URL |
| [17] | Hungria M, Mendes IC (2015). Nitrogen fixation with soybean:the perfect symbiosis? In: de Bruijn FJ, ed. Biological Nitrogen Fixation. New Jersey: Wiley. pp. 1005-1019. |
| [18] |
Ke XL, Xiao H, Peng YQ, Wang J, Lv Q, Wang XL (2022). Phosphoenolpyruvate reallocation links nitrogen fixation rates to root nodule energy state. Science 378, 971-977.
DOI PMID |
| [19] |
Kereszt A, Li DX, Indrasumunar A, Nguyen CDT, Nontachaiyapoom S, Kinkema M, Gresshoff PM (2007). Agrobacterium rhizogenes-mediated transformation of soybean to study root biology. Nat Protoc 2, 948-952.
DOI PMID |
| [20] |
Kiryushkin AS, Ilina EL, Guseva ED, Pawlowski K, Demchenko KN (2021). Hairy CRISPR: genome editing in plants using hairy root transformation. Plants (Basel) 11, 51.
DOI URL |
| [21] |
Li R, Chen HF, Yang ZL, Yuan SL, Zhou XA (2020). Research status of soybean symbiosis nitrogen fixation. Oil Crop Sci 5, 6-10.
DOI URL |
| [22] | Li XX, Zhao J, Tan ZY, Zeng RS, Liao H (2015). GmEXPB2, a cell wall β-expansin, affects soybean nodulation through modifying root architecture and promoting nodule formation and development. Plant Physiol 169, 2640-2653. |
| [23] |
Li XX, Zheng JK, Yang YQ, Liao H (2018). INCREASING NODULE SIZE1 expression is required for normal rhizobial symbiosis and nodule development. Plant Physiol 178, 1233-1248.
DOI URL |
| [24] |
Liu SL, Zhang M, Feng F, Tian ZX (2020). Toward a ‘‘green revolution’’ for soybean. Mol Plant 13, 688-697.
DOI URL |
| [25] |
Morey KJ, Peebles CAM (2022). Hairy roots: an untapped potential for production of plant products. Front Plant Sci 13, 937095.
DOI URL |
| [26] |
Olhoft PM, Flagel LE, Donovan CM, Somers DA (2003). Efficient soybean transformation using hygromycin B selection in the cotyledonary-node method. Planta 216, 723-735.
DOI PMID |
| [27] |
Pistelli L, Giovannini A, Ruffoni B, Bertoli A, Pistelli L (2010). Hairy root cultures for secondary metabolites production. Adv Exp Med Biol 698, 167-184.
PMID |
| [28] |
Qin L, Zhao J, Tian J, Chen LY, Sun ZA, Guo YX, Lu X, Gu M, Xu GH, Liao H (2012). The high-affinity phosphate transporter GmPT5 regulates phosphate transport to nodules and nodulation in soybean. Plant Physiol 159, 1634-1643.
DOI PMID |
| [29] |
Wang T, Guo J, Peng YQ, Lyu X, Liu B, Sun SY, Wang XL (2021). Light-induced mobile factors from shoots regulate rhizobium-triggered soybean root nodulation. Science 374, 65-71.
DOI PMID |
| [30] |
Wang XR, Wang YX, Tian J, Lim BL, Yan XL, Liao H (2009). Overexpressing AtPAP15enhances phosphorus efficiency in soybean. Plant Physiol 151, 233-240.
DOI URL |
| [31] |
Xu HY, Li YJ, Zhang KF, Li MJ, Fu SY, Tian YZ, Qin TF, Li XX, Zhong YJ, Liao H (2021). miR169c-NFYA-C-ENOD40 modulates nitrogen inhibitory effects in soybean nodulation. New Phytol 229, 3377-3392.
DOI URL |
| [32] |
Yang ZJ, Gao Z, Zhou HW, He Y, Liu YX, Lai YL, Zheng JK, Li XX, Liao H (2021). GmPTF1 modifies root architecture responses to phosphate starvation primarily through regulating GmEXPB2 expression in soybean. Plant J 107, 525-543.
DOI URL |
| [33] |
Zhang FL, Chen C, Ge HL, Liu JM, Luo YL, Liu K, Chen L, Xu KD, Zhang Y, Tan GX, Li CW (2014). Efficient soybean regeneration and Agrobacterium-mediated transformation using a whole cotyledonary node as an explant. Biotechnol Appl Biochem 61, 620-625.
DOI PMID |
| [1] | WANG Xiu-Yuan, SHEN Lei, LIU Ting-Ting, WEI Wen-Wen, ZHANG Shuai, ZHANG Wei. Spatial and temporal distribution of root system and interspecific competition strategy in Malus pumila ‘Saiwaihong’ - Glycine max agroforestry system [J]. Chin J Plant Ecol, 2025, 49(5): 748-759. |
| [2] | Jing Gan Xiangxu Liu Xueming Lu Xing Yue. China's Large Cities in Global Biodiversity Hotspots: Conservation Policies and Optimization Directions [J]. Biodiv Sci, 2025, 33(5): 24529-. |
| [3] | Zeng Wendan, Yan Huabing, Wu Zhengdan, Shang Xiaohong, Cao Sheng, Lu Liuying, Xiao Liang, Shi Pingli, Cheng Dong, Long Ziyuan, Li Jieyu. Agrobacterium rhizogenes-mediated Transformation System of Pueraria lobata Hairy Roots [J]. Chinese Bulletin of Botany, 2025, 60(3): 425-434. |
| [4] | Jie Cao, Qiulian Lu, Jianping Zhai, Baohui Liu, Chao Fang, Shichen Li, Tong Su. Changes in the Expression of the Soybean TPS Gene Family Under Salt Stress and Haplotype Selection Analysis [J]. Chinese Bulletin of Botany, 2025, 60(2): 172-185. |
| [5] | Lin Wang, Ziyang Yin, Huifang Huang, Jing Wang. A new parameter estimation method based on the Carter-Morley Jones egg- shape model [J]. Biodiv Sci, 2025, 33(1): 24203-. |
| [6] | Tao Qin, Ronghe Cui, Rui Song, Lisha Fu. Chinese public liability insurance for wildlife accidents: Development models, realistic dilemmas, and optimization strategy [J]. Biodiv Sci, 2024, 32(5): 23431-. |
| [7] | Guofa Cui. Discussion and suggestions on several key issues in the integration and optimization of protected areas [J]. Biodiv Sci, 2023, 31(9): 22447-. |
| [8] | Tianao Chen, Xiang Li. Identifying the management system for national parks in China [J]. Biodiv Sci, 2023, 31(3): 22485-. |
| [9] | Rui Song, Jing Deng, Tao Qin. Development dilemma and optimization path of public liability insurance for wildlife accidents [J]. Biodiv Sci, 2022, 30(7): 22291-. |
| [10] | Yunhui Wang, Yifan Wang, Jiayu Lin, Jinhong Li, Shien Yao, Xiangchi Feng, Zhenlin Cao, Jun Wang, Meina Li. Plant Kinesin: from Microtubule Arrays to Physiological Regulation [J]. Chinese Bulletin of Botany, 2022, 57(3): 358-374. |
| [11] | Mengke Du, Wenting Lian, Xiao Zhang, Xinxin Li. Effects of Nitrogen Application on Nitrogen Fixation Capacity and GmLbs Expression in Soybean [J]. Chinese Bulletin of Botany, 2021, 56(4): 391-403. |
| [12] | FANG Jing-Yun. Ecological perspectives of carbon neutrality [J]. Chin J Plant Ecol, 2021, 45(11): 1173-1176. |
| [13] | WANG Yin-Liu, GENG Qian-Qian, HUANG Jian-Hui, WANG Chang-Hui, LI Lei, HASI Muqier, NIU Guo-Xiang. Effects of nitrogen addition and planting density on the growth and biological nitrogen fixation of Lespedeza davurica [J]. Chin J Plant Ecol, 2021, 45(1): 13-22. |
| [14] | Zhengjun Xia, Yuzhuo Li, Jinlong Zhu, Hongyan Wu, Kun Xu, Hong Zhai. A Rapid, Non-destructive and Continuous Sampling Technique and DNA Extraction for Soybean Seed [J]. Chinese Bulletin of Botany, 2021, 56(1): 56-61. |
| [15] | Yan Wang, Bowei Jia, Mingzhe Sun, Xiaoli Sun. Advances in Molecular Mechanisms of Stress Tolerance in Wild Soybean [J]. Chinese Bulletin of Botany, 2021, 56(1): 104-115. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||