

Chinese Bulletin of Botany ›› 2018, Vol. 53 ›› Issue (5): 603-611.DOI: 10.11983/CBB17129 cstr: 32102.14.CBB17129
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Chen Jianquan, Cheng Chen, Zhang Mengtian, Zhang Xiangqian, Zhang Yao, Wang Aiying, Zhu Jianbo*( )
)
Received:2017-07-08
Accepted:2017-10-25
Online:2018-09-01
Published:2018-11-29
Contact:
Zhu Jianbo
About author:† These authors contributed equally to this paper
Chen Jianquan, Cheng Chen, Zhang Mengtian, Zhang Xiangqian, Zhang Yao, Wang Aiying, Zhu Jianbo. Cold-tolerance Analysis of Tobacco Plants Transformed with Saussurea involucrata SiSAD and Arabidopsis thaliana AtFAB2 Gene[J]. Chinese Bulletin of Botany, 2018, 53(5): 603-611.
| Primer name | Primer sequence (5'-3') |
|---|---|
| SAD F | GTTGGAGATATGATCCACGAGGAAGC |
| SikSAD R | TTCCAGTATATCGGCATAGTCCTT |
| AtFAB2 F | GCACATGCGTGACATGCTTC |
| AtFAB2 R | CTGATCGACGGTCAATTGGC |
| GAPDH F | GTTGCTAGAGTTGCACTTCAGAGAG |
| GAPDH R | TTCCTGAAGCCGAAAACAGC |
Table 2 Primers of RT-PCR
| Primer name | Primer sequence (5'-3') |
|---|---|
| SAD F | GTTGGAGATATGATCCACGAGGAAGC |
| SikSAD R | TTCCAGTATATCGGCATAGTCCTT |
| AtFAB2 F | GCACATGCGTGACATGCTTC |
| AtFAB2 R | CTGATCGACGGTCAATTGGC |
| GAPDH F | GTTGCTAGAGTTGCACTTCAGAGAG |
| GAPDH R | TTCCTGAAGCCGAAAACAGC |
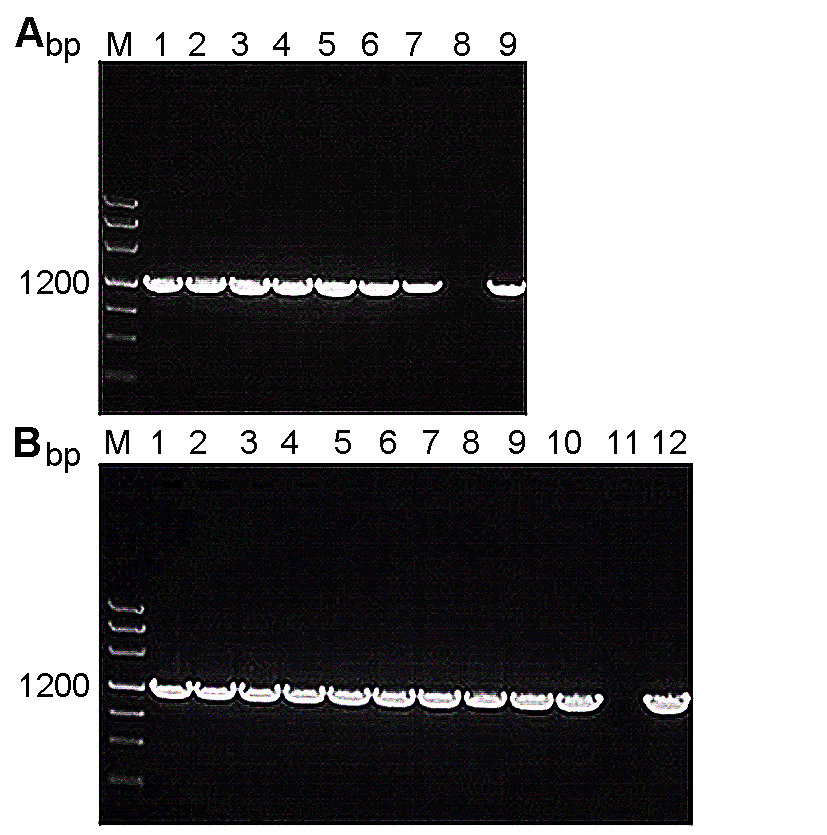
Figure 2 PCR identification of tobacco transferred with PSiSAD:SiSAD and PSiSAD:AtFAB2 recombinant plasmid, respectively (A) PCR identification of tobacco transferred with PSiSAD: SiSAD recombinant plasmid (M: Marker III DNA marker; 1-7: Different transgenic lines; 8: Wild type (negative control); 9: PSiSAD:SiSAD plasmid (positive control)); (B) PCR identification of tobacco transferred with PSiSAD:AtFAB2 recombinant plasmid (M: Marker III DNA marker; 1-10: Different transgenic lines; 11: Wild type (negative control); 12: PSiSAD:SiSAD plasmid (positive control))

Figure 4 Phenotype of wild-type and transgenic tobacco under different temperatures(A)-(E) Wild-type and transgenic tobacco plants grown at 20°C, 10°C, 5°C, 0°C, and -2°C for 2 hours, respectively; (F) After -2°C treatment recovering in 25°C for one week.s-f: PSiSAD:AtFAB2; s-s: PSiSAD:SiSAD; WT: Wild-type
| Temperature (°C) | Plant | Fatty acid (%) | |||||
|---|---|---|---|---|---|---|---|
| C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | Total desaturation products | ||
| 20 | WT | 43.70±1.10 a | 24.77±0.66 a | 14.33±0.94 a | 3.27±0.43 a | 12.97±1.56 a | 16.57 |
| s-f | 42.27±1.11 a | 20.89±1.09 b | 15.20±0.38 b | 2.77±0.92 a | 7.37±1.24 b | 17.34 | |
| s-s | 46.53±0.90 a | 20.03±0.32 c | 17.23±1.60 c | 4.61±1.15 a | 7.07±0.93 b | 21.91 | |
| 10 | WT | 38.99±0.58 a | 19.83±0.65 a | 15.87±0.92 a | 2.53±0.30 a | 8.17±1.22 a | 17.57 |
| s-f | 42.97±0.62 a | 15.87±0.86 a | 17.43±0.48 a | 2.07±0.12 a | 8.19±1.12 a | 19.69 | |
| s-s | 40.76±0.79 a | 15.70±2.80 a | 19.40±1.23 c | 1.77±0.38 a | 6.00±1.11 a | 23.17 | |
| 5 | WT | 30.30±0.80 a | 16.47±0.66 a | 17.50±0.44 a | 3.70±0.12 a | 17.20±2.02 a | 19.4 |
| s-f | 25.27±0.66 b | 13.83±0.71 b | 18.70±1.29 b | 1.90±0.17 a | 10.20±0.86 b | 20.8 | |
| s-s | 46.43±0.41 c | 12.30±0.67 a | 20.70±0.57 b | 2.67±0.09 a | 6.60±0.68 c | 31.97 | |
| 0 | WT | 40.53±0.44 a | 16.23±0.32 a | 17.90±0.76 a | 2.20±0.17 a | 8.47±0.41 a | 22.67 |
| s-f | 47.63±0.47 b | 13.67±0.73 b | 19.17±1.45 b | 2.33±0.27 a | 11.97±1.44 a | 28.47 | |
| s-s | 44.33±0.07 b | 12.47±0.45 c | 23.50±1.33 a | 2.30±0.46 a | 11.87±2.07 a | 37.67 | |
| -2 | WT | 38.20±1.07 a | 15.57±0.76 a | 15.63±2.21 a | 2.87±0.20 a | 10.23±0.72 a | 26.73 |
| s-f | 40.20±0.23 b | 11.20±1.56 b | 16.54±0.49 a | 3.13±0.59 a | 16.30±1.22 b | 31.97 | |
| s-s | 53.60±0.47 c | 10.30±0.64 b | 30.47±0.89 b | 2.33±0.48 a | 9.83±2.24 a | 49.63 | |
| Recover treatment | WT | 36.30±1.04 a | 14.46±0.68 a | 14.77±2.11 a | 2.46±0.22 a | 10.02±0.68 a | 25.94 |
| s-f | 41.40±1.03 a | 20.05±1.03 b | 14.91±0.34 b | 2.58±0.85 a | 7.19±1.18 b | 16.87 | |
| s-s | 46.33±0.87 a | 20.01±0.30 c | 17.14±1.53 c | 4.57±1.12 a | 6.98±0.90 b | 21.23 | |
Table 2 The analysis of the content of desaturation products in transgenic tobacco under different temperatures
| Temperature (°C) | Plant | Fatty acid (%) | |||||
|---|---|---|---|---|---|---|---|
| C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | Total desaturation products | ||
| 20 | WT | 43.70±1.10 a | 24.77±0.66 a | 14.33±0.94 a | 3.27±0.43 a | 12.97±1.56 a | 16.57 |
| s-f | 42.27±1.11 a | 20.89±1.09 b | 15.20±0.38 b | 2.77±0.92 a | 7.37±1.24 b | 17.34 | |
| s-s | 46.53±0.90 a | 20.03±0.32 c | 17.23±1.60 c | 4.61±1.15 a | 7.07±0.93 b | 21.91 | |
| 10 | WT | 38.99±0.58 a | 19.83±0.65 a | 15.87±0.92 a | 2.53±0.30 a | 8.17±1.22 a | 17.57 |
| s-f | 42.97±0.62 a | 15.87±0.86 a | 17.43±0.48 a | 2.07±0.12 a | 8.19±1.12 a | 19.69 | |
| s-s | 40.76±0.79 a | 15.70±2.80 a | 19.40±1.23 c | 1.77±0.38 a | 6.00±1.11 a | 23.17 | |
| 5 | WT | 30.30±0.80 a | 16.47±0.66 a | 17.50±0.44 a | 3.70±0.12 a | 17.20±2.02 a | 19.4 |
| s-f | 25.27±0.66 b | 13.83±0.71 b | 18.70±1.29 b | 1.90±0.17 a | 10.20±0.86 b | 20.8 | |
| s-s | 46.43±0.41 c | 12.30±0.67 a | 20.70±0.57 b | 2.67±0.09 a | 6.60±0.68 c | 31.97 | |
| 0 | WT | 40.53±0.44 a | 16.23±0.32 a | 17.90±0.76 a | 2.20±0.17 a | 8.47±0.41 a | 22.67 |
| s-f | 47.63±0.47 b | 13.67±0.73 b | 19.17±1.45 b | 2.33±0.27 a | 11.97±1.44 a | 28.47 | |
| s-s | 44.33±0.07 b | 12.47±0.45 c | 23.50±1.33 a | 2.30±0.46 a | 11.87±2.07 a | 37.67 | |
| -2 | WT | 38.20±1.07 a | 15.57±0.76 a | 15.63±2.21 a | 2.87±0.20 a | 10.23±0.72 a | 26.73 |
| s-f | 40.20±0.23 b | 11.20±1.56 b | 16.54±0.49 a | 3.13±0.59 a | 16.30±1.22 b | 31.97 | |
| s-s | 53.60±0.47 c | 10.30±0.64 b | 30.47±0.89 b | 2.33±0.48 a | 9.83±2.24 a | 49.63 | |
| Recover treatment | WT | 36.30±1.04 a | 14.46±0.68 a | 14.77±2.11 a | 2.46±0.22 a | 10.02±0.68 a | 25.94 |
| s-f | 41.40±1.03 a | 20.05±1.03 b | 14.91±0.34 b | 2.58±0.85 a | 7.19±1.18 b | 16.87 | |
| s-s | 46.33±0.87 a | 20.01±0.30 c | 17.14±1.53 c | 4.57±1.12 a | 6.98±0.90 b | 21.23 | |
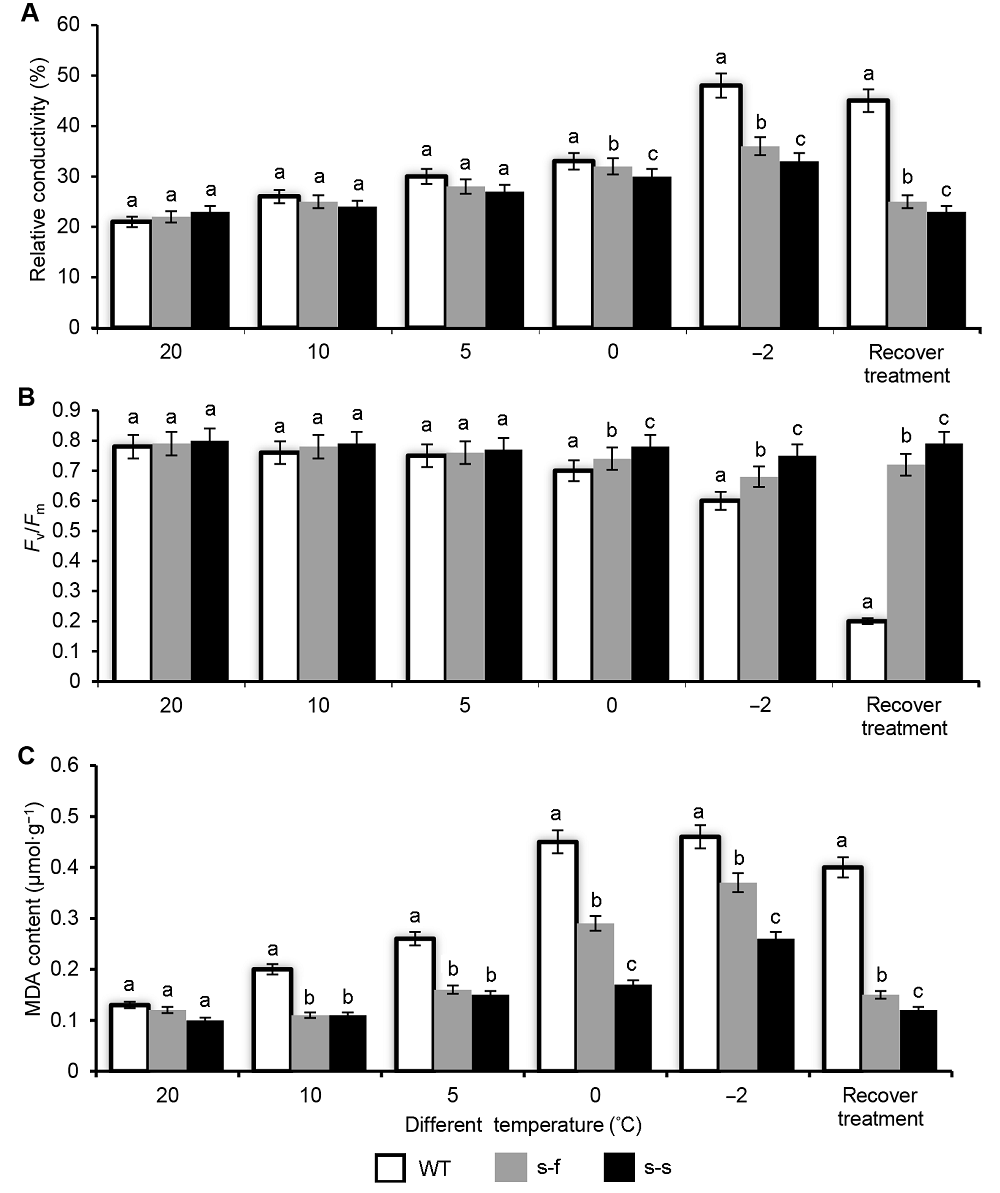
Figure 5 The physiological analysis of wild-type and transgenic tobacco plant after different processing temperature (A) Relative conductivity; (B) Maximum efficiency of photosystem II photochemistry (Fv/Fm); (C) Malondialdehyde (MDA) content; Wild-type and transgenic tobacco plants grown at 20°C, 10°C, 5°C, 0°C, and -2°C; recovery treatment: after -2°C treatment for 2 hours recovery in 25°C for 1 week. Different lowercase letters indicate significant differences at P<0.05. s-f: PSiSAD:AtFAB2; s-s: PSiSAD:SiSAD; WT: Wild-type
| 1 | 陈爱葵, 韩瑞宏, 李东洋, 凌连莲, 罗惠霞, 唐上剑 (2010). 植物叶片相对电导率测定方法比较研究. 广东教育学院学报 30(5), 88-91. |
| 2 | 陈东亮 (2010). 根癌农杆菌介导的千年桐SAD基因对产油酵母的遗传转化. 硕士论文. 北京: 中国林业科学研究院. pp. 38-50. |
| 3 | 陈发菊, 杨映根, 赵德修, 桂耀林, 郭仲琛 (1999). 我国雪莲植物的种类、生境分布及化学成分的研究进展. 植物学通报 16, 561-566. |
| 4 | 陈思羽, 刘鹏, 朱末, 夏冬冬, 李亮, 徐克章, 陈展宇, 张治安 (2016). 大豆植株不同冠层种子活力及其萌发中抗氧化酶活性. 植物学报 51, 24-30. |
| 5 | 程晨, 郭新勇, 王爱英, 祝建波 (2011). 转新疆雪莲去饱和酶基因sikSAD重组酵母低温和酒精耐受性分析. 微生物学通报 38, 1647-1656. |
| 6 | 范妙华, 李纪元, 范正琪, 田敏, 倪穗 (2008). 千年桐SAD基因克隆与分析及其丝状真菌表达载体构建. 西北植物学报 28, 18-22. |
| 7 | 桂仁意, 刘亚迪, 郭小勤, 季海宝, 贾月, 余明增, 方伟 (2010). 不同剂量137Cs-γ辐射对毛竹幼苗叶片叶绿素荧光参数的影响. 植物学报 45, 66-72. |
| 8 | 郭新勇, 程晨, 王爱英, 张煜星, 王重, 喻娜, 祝建波 (2012). 天山雪莲冷调节蛋白基因siCOR转化烟草植株的抗旱性分析. 植物学报 47, 111-119. |
| 9 | 贾艳丽, 吴磊, 卢长明 (2014). 甘蓝型油菜Δ9硬脂酰ACP脱氢酶(SAD)基因的克隆与表达分析. 中国油料作物学报 36, 135-141. |
| 10 | 李金璐, 王硕, 于婧, 王玲, 周世良 (2013). 一种改良的植物DNA提取方法. 植物学报 48, 72-78. |
| 11 | 罗华元, 董石飞, 倪明, 张峻松 (2010). 烟叶中多元酸和高级脂肪酸的分析. 安徽农业科学 38, 16212-16214. |
| 12 | 罗通 (2006). 麻疯树的抗冷性和SAD基因的克隆及表达研究. 博士论文. 成都: 四川大学. pp. 27-35. |
| 13 | 罗秀芹, 欧文军, 李开绵, 陈松笔 (2014). 抗寒蛋白硬脂酰-ACP脱饱和酶的结构与功能预测. 福建农林大学学报(自然科学版) 43, 484-489. |
| 14 | 庞磊, 周小生, 李叶云, 江昌俊 (2011). 应用叶绿素荧光法鉴定茶树品种抗寒性的研究. 茶叶科学 31, 521-524. |
| 15 | 张党权, 谭晓风, 陈鸿鹏, 曾艳玲, 蒋瑶, 李魏, 胡芳名 (2008). 油茶SAD基因的全长cDNA克隆及生物信息学分析. 林业科学 44, 155-159. |
| 16 | 甄伟, 陈溪, 孙思洋, 胡鸢雷, 林忠平 (2000). 冷诱导基因的转录因子CBF1转化油菜和烟草及抗寒性鉴定. 自然科学进展 10, 1104-1108. |
| 17 | 祝建波, 刘海亮, 王重, 周鹏 (2006). 天山雪莲叶片全长cDNA文库的构建. 西北农业学报 15(6), 170-173. |
| 18 | Aroca R, Amodeo G, Fernández-Illescas S, Herman EM, Chaumont F, Chrispeels MJ (2005). The role of aqu- aporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots.Plant Physiol 137, 341-353. |
| 19 | Barkan L, Vijayan P, Carlsson AS, Mekhedov S, Browse J (2006). A suppressor of fab1 challenges hypotheses on the role of thylakoid unsaturation in photosynthetic function. Plant Physiol 141, 1012-1020. |
| 20 | Byfield GE, Xue H, Upchurch RG (2006). Two genes from soybean encoding soluble Δ9 stearoyl-acp desaturas- es.Crop Sci 46, 840-846. |
| 21 | Craig W, Lenzi P, Scotti N, De Palma M, Saggese P, Carbone V, McGrath CN, Magee AM, Medgyesy P, Kavan- agh TA, Dix PJ, Grillo S, Cardi T (2008). Transplastomic tobacco plants expressing a fatty acid desaturase gene exhibit altered fatty acid profiles and improved cold tolerance.Transgenic Res 17, 769-782. |
| 22 | Du ZY, Bramlage WJ (1992). Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J Agric Food Chem 40, 1566-1570. |
| 23 | James DW Jr, Dooner HK (1990). Isolation of EMS-induced mutants in Arabidopsis altered in seed fatty acid composition.Theor Appl Genet 80, 241-245. |
| 24 | Jung S, Tate PL, Horn R, Kochert G, Moore K, Abbott AG (2003). The phylogenetic relationship of possible progenitors of the cultivated peanut. J Hered 94, 334-340. |
| 25 | Kachroo A, Shanklin J, Whittle E, Lapchyk L, Hildebrand D, Kachroo P (2007). The Arabidopsis stearoyl-acyl carrier protein-desaturase family and the contribution of leaf isoforms to oleic acid synthesis.Plant Mol Biol 63, 257-271. |
| 26 | Krause GH, Weis E (1991). Chlorophyll fluorescence and photosynthesis: the basics.Ann Rev Plant Physiol Plant Mol Biol 42, 313-349. |
| 27 | Lightner J, James DW Jr, Dooner HK, Browse J (1994a). Altered body morphology is caused by increased stearate levels in a mutant of Arabidopsis.Plant J 6, 401-412. |
| 28 | Lightner J, Wu JR, Browse J (1994b). A mutant of Arabidopsis with increased levels of stearic acid. Plant Physiol 106, 1443-1451. |
| 29 | Lutts S, Kinet JM, Bouharmont J (1996). Nacl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot 78, 389-398. |
| 30 | Murata N, Wada H (1995). Acyl-lipid desaturases and their importance in the tolerance and acclimatization to cold of cyanobacteria. Biochem J 308, 1-8. |
| 31 | Shilman F, Brand Y, Brand A, Hedvat I, Hovav R (2011). Identification and molecular characterization of homeologousΔ9-stearoyl acyl carrier protein desaturase 3 genes from the allotetraploid peanut(Arachis hypogaea). Plant Mol Biol Rep 29, 232-241. |
| 32 | Tasseva G, de Virville JD, Cantrel C, Moreau F, Zacho- wski A (2004). Changes in the endoplasmic reticulum lipid properties in response to low temperature in Brassica napus. Plant Physiol Biochem 42, 811-822. |
| 33 | Thompson GA, Scherer DE, Foxall-Van Aken S, Kenny JW, Young HL, Shintani DK, Kridl JC, Knauf VC (1991). Primary structures of the precursor and mature forms of stearoyl-acyl carrier protein desaturase from safflower embryos and requirement of ferredoxin for enzyme activity.Proc Natl Acad Sci USA 88, 2578-2582. |
| 34 | Uemura M, Steponkus PL (1997). Effect of cold acclimation on the lipid composition of the inner and outer membrane of the chloroplast envelope isolated from rye leaves.Plant Physiol 114, 1493-1500. |
| 35 | Whittle E, Cahoon EB, Subrahmanyam S, Shanklin J (2005). A multifunctional acyl-acyl carrier protein desaturase from Hedera helix L.(English ivy) can synthesize 16- and 18-carbon monoene and diene products. J Biol Chem 280, 28169-28176. |
| 36 | Yukawa Y, Takaiwa F, Shoji K, Masuda K, Yamada K (1996). Structure and expression of two seed-specific cDNA clones encoding stearoyl-acyl carrier protein desaturase from sesame,Sesamum indicum L. Plant Cell Phy- siol 37, 201-205. |
| 37 | Zhang P, Burton JW, Upchurch RG, Whittle E, Shanklin J, Dewey RE (2008). Mutations in a Δ9-stearoyl-acpdes- aturase gene are associated with enhanced stearic acid levels in soybean seeds.Crop Sci 48, 2305-2313. |
| [1] | Xiao-Hong YAN Wen-Hai HU. Differences in photoprotective mechanisms during winter in three evergreen broadleaf species in subtropical region [J]. Chin J Plant Ecol, 2025, 49(预发表): 0-0. |
| [2] | OUYANG Zi-Long, JIA Xiang-Lu, SHI Jing-Zhong, TENG Wei-Chao, LIU Xiu. Effects of growth regulators on photosynthetic characteristics of Rhizophora stylosa seedlings under low temperature stress and re-warming [J]. Chin J Plant Ecol, 2025, 49(4): 638-652. |
| [3] | GAO Min, GOU Qian-Qian, WANG Guo-Hua, GUO Wen-Ting, ZHANG Yu, ZHANG Yan. Effects of low temperature stress on the physiology and growth of Caragana korshinskii seedlings from different mother tree ages [J]. Chin J Plant Ecol, 2024, 48(2): 201-214. |
| [4] | SHI Sheng-Bo, ZHOU Dang-Wei, LI Tian-Cai, DE Ke-Jia, GAO Xiu-Zhen, MA Jia-Lin, SUN Tao, WANG Fang-Lin. Responses of photosynthetic function of Kobresia pygmaea to simulated nocturnal low temperature on the Qingzang Plateau [J]. Chin J Plant Ecol, 2023, 47(3): 361-373. |
| [5] | CHEN Yi-Zhu, LANG Wei-Guang, CHEN Xiao-Qiu. Process-based simulation of autumn phenology of trees and the regional differentiation attribution in northern China [J]. Chin J Plant Ecol, 2022, 46(7): 753-765. |
| [6] | Dandan Wu, Yongkun Chen, Yu Yang, Chunyan Kong, Ming Gong. Identification of the Cysteine Protease Family and Corresponding miRNAs in Jatropha curcas and Their Response to Chill-hardening [J]. Chinese Bulletin of Botany, 2021, 56(5): 544-558. |
| [7] | Yawen Zhang, Shan Liang, Guoyun Xu, Wuxia Guo, Shulin Deng. Genome-wide Identification and Analysis of CONSTANS-like Gene Family in Nicotiana tabacum [J]. Chinese Bulletin of Botany, 2021, 56(1): 33-43. |
| [8] | Xiaoqing Yang,Xiaoqin Huang,Xiaoyang Han,Tengfei Liu,Xiaowei Yue,Ran Yi. Effect of Exogenous Substances on Cold Tolerance and Key Sucrose Metabolic Gene Expression in Camellia sinensis [J]. Chinese Bulletin of Botany, 2020, 55(1): 21-30. |
| [9] | Dongfeng Liu,Yongyan Tang,Shengtao Luo,Wei Luo,Zhitao Li,Kang Chong,Yunyuan Xu. Identification of Chilling Tolerance of Rice Seedlings by Cold Water Bath [J]. Chinese Bulletin of Botany, 2019, 54(4): 509-514. |
| [10] | Li Ma, Wancang Sun, Jinhai Yuan, Zigang Liu, Junyan Wu, Yan Fang, Yaozhao Xu, Yuanyuan Pu, Jing Bai, Xiaoyun Dong, Huili He. Expression Analysis of β-1,3-Glucanase Gene from Winter Brassica rapa Under Low Temperature Stress [J]. Chinese Bulletin of Botany, 2017, 52(5): 568-578. |
| [11] | WU Hui, DAI Hai-Fang, ZHANG Ju-Song, JIAO Xiao-Ling, LIU Cui, SHI Jun-Yi, FAN Zhi-Chao, ALIYAN ·Rouzi. Responses of photosynthetic characteristics to low temperature stress and recovery treatment in cotton seedling leaves [J]. Chin J Plant Ecol, 2014, 38(10): 1124-1134. |
| [12] | Li Chen, Lei Shi, Hongxia Cui, Aiying Zhang, Deshan Zhang, Ling Wang, Fei Xia. The Response of Flowering Phenology and Growth of Viburnum to Interannual Weather Fluctuation in an Introduction habitat [J]. Chinese Bulletin of Botany, 2012, 47(6): 645-653. |
| [13] | LIU Bin-Yang, LIU Wei-Qiu, ZHANG Yi-Shun, LEI Chun-Yi. Physiological responses of bryophytes experienced low temperature stress to simulated nitrogen deposition [J]. Chin J Plant Ecol, 2011, 35(3): 268-274. |
| [14] | DAI Pan-Feng, TAN Dun-Yan. Floral biological characteristics of Saussurea involucrata in relation to ecological adaptation [J]. Chin J Plant Ecol, 2011, 35(1): 56-65. |
| [15] | Xingfu Yan;Min Cao. Influence of Light and Temperature on the Germination of Shorea wantianshuea (Dipterocarpaceae) Seeds [J]. Chinese Bulletin of Botany, 2006, 23(6): 642-650. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||