

植物学报 ›› 2023, Vol. 58 ›› Issue (3): 486-498.DOI: 10.11983/CBB22079 cstr: 32102.14.CBB22079
孙永江1, 王琪1, 邵琪雯1, 辛智鸣2, 肖辉杰1, 程瑾1( )
)
收稿日期:2022-04-20
接受日期:2022-07-06
出版日期:2023-05-01
发布日期:2023-05-17
通讯作者:
*E-mail: chengjin@bjfu.edu.cn
基金资助:
Yongjiang Sun1, Qi Wang1, Qiwen Shao1, Zhiming Xin2, Huijie Xiao1, Jin Cheng1( )
)
Received:2022-04-20
Accepted:2022-07-06
Online:2023-05-01
Published:2023-05-17
Contact:
*E-mail: chengjin@bjfu.edu.cn
摘要: 随着人为活动产生的大气CO2浓度的增加, 全球气候持续变暖。过去5年是自有温度记录以来最热的5年。高温胁迫已经成为影响植物生长发育的主要逆境因子之一。光合作用是地球生命活动的基础, 对环境波动高度敏感。解析植物在高温环境下光合作用的响应特性, 可为探索植物抵御高温的生理生态机制、培育抗高温新品种以及采取合理措施适应未来极端气候提供科学依据。该文论述了高温胁迫对植物光合电子传递及碳固定过程的影响, 从光质和光强角度综合分析了光照对高温胁迫下光合作用的影响; 从植物自身及外源缓解物质等方面阐述了植物增强抗高温胁迫的途径和机制。同时, 对植物光合作用响应高温胁迫的研究方向及多组学联合分析在揭示植物抵御高温胁迫机制中的应用进行了展望。
孙永江, 王琪, 邵琪雯, 辛智鸣, 肖辉杰, 程瑾. 高温胁迫对植物光合作用的影响研究进展. 植物学报, 2023, 58(3): 486-498.
Yongjiang Sun, Qi Wang, Qiwen Shao, Zhiming Xin, Huijie Xiao, Jin Cheng. Research Advances on the Effect of High Temperature Stress on Plant Photosynthesis. Chinese Bulletin of Botany, 2023, 58(3): 486-498.
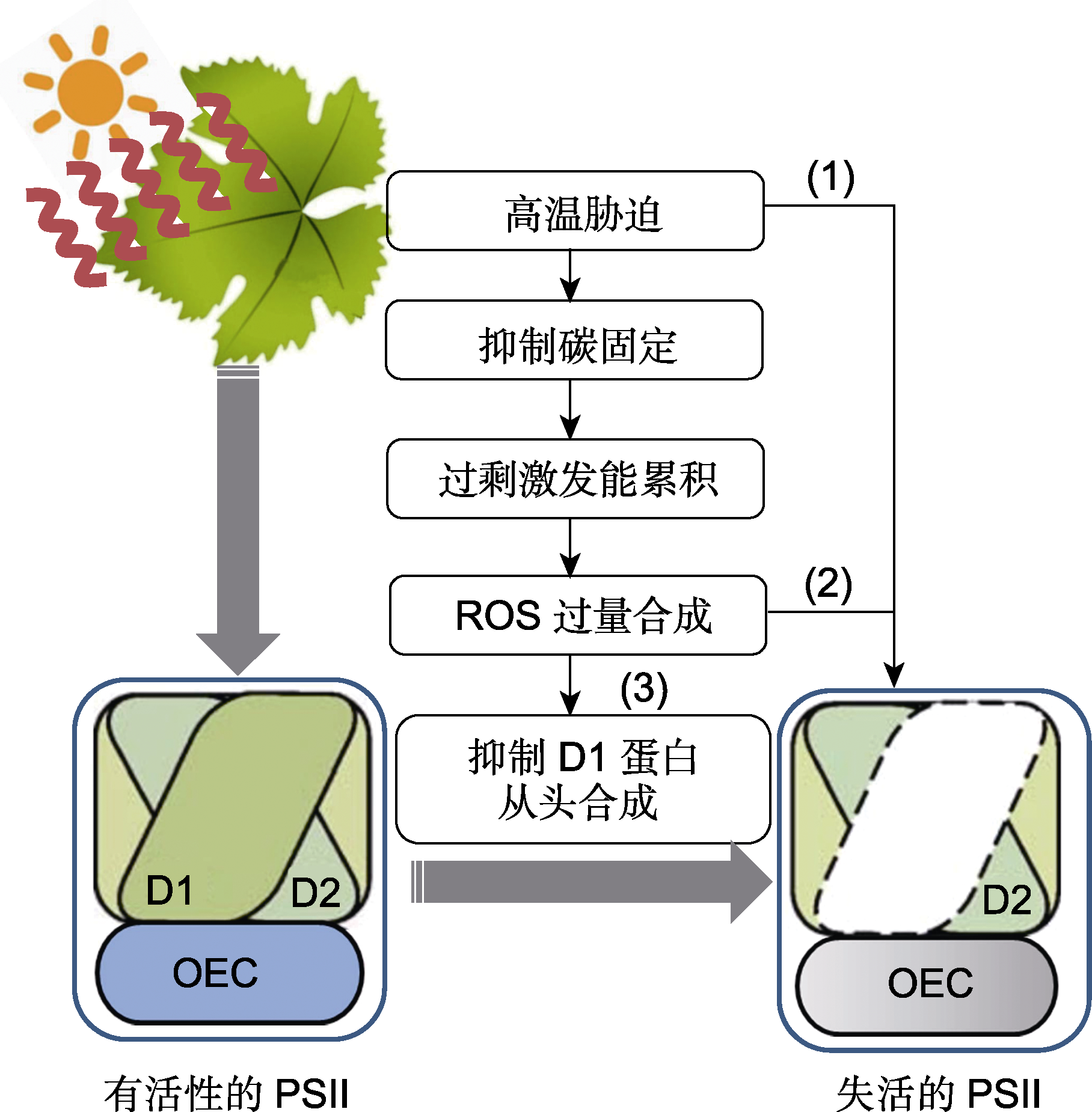
图1 高温胁迫诱导植物PSII发生光抑制 高温胁迫导致PSII处的放氧复合体及光合电子传递链失活(1), 或者通过抑制碳固定, 使过剩激发能引发ROS大量积累, ROS一方面直接损伤光合机构组分(2), 另一方面通过抑制D1蛋白的从头合成, 导致D1蛋白的净损失(3), 引起PSII光抑制。D1: D1蛋白; D2: D2蛋白; OEC: 放氧复合体; PSII: 光系统II; ROS: 活性氧
Figure 1 High temperature stress induces PSII photoinhibition in plants High temperature stress can lead to the inactivation of oxygen evolving complex and photosynthetic electron transport chain at PSII (1), It can also result in excess excitation and accumulation by inhibiting the process of carbon fixation, resulting in excess excitation and accumulation, resulting in a large amount of ROS accumulation. On the one hand, ROS directly damage the photosynthetic apparatus components (2), on the other hand, ROS cause the net loss of the D1 protein by inhibiting its de novo synthesis (3), thereby inducing PSII photoinhibition. D1: D1 protein; D2: D2 protein; OEC: Oxygen evolving complex; PSII: Photosystem II; ROS: Reactive oxygen species
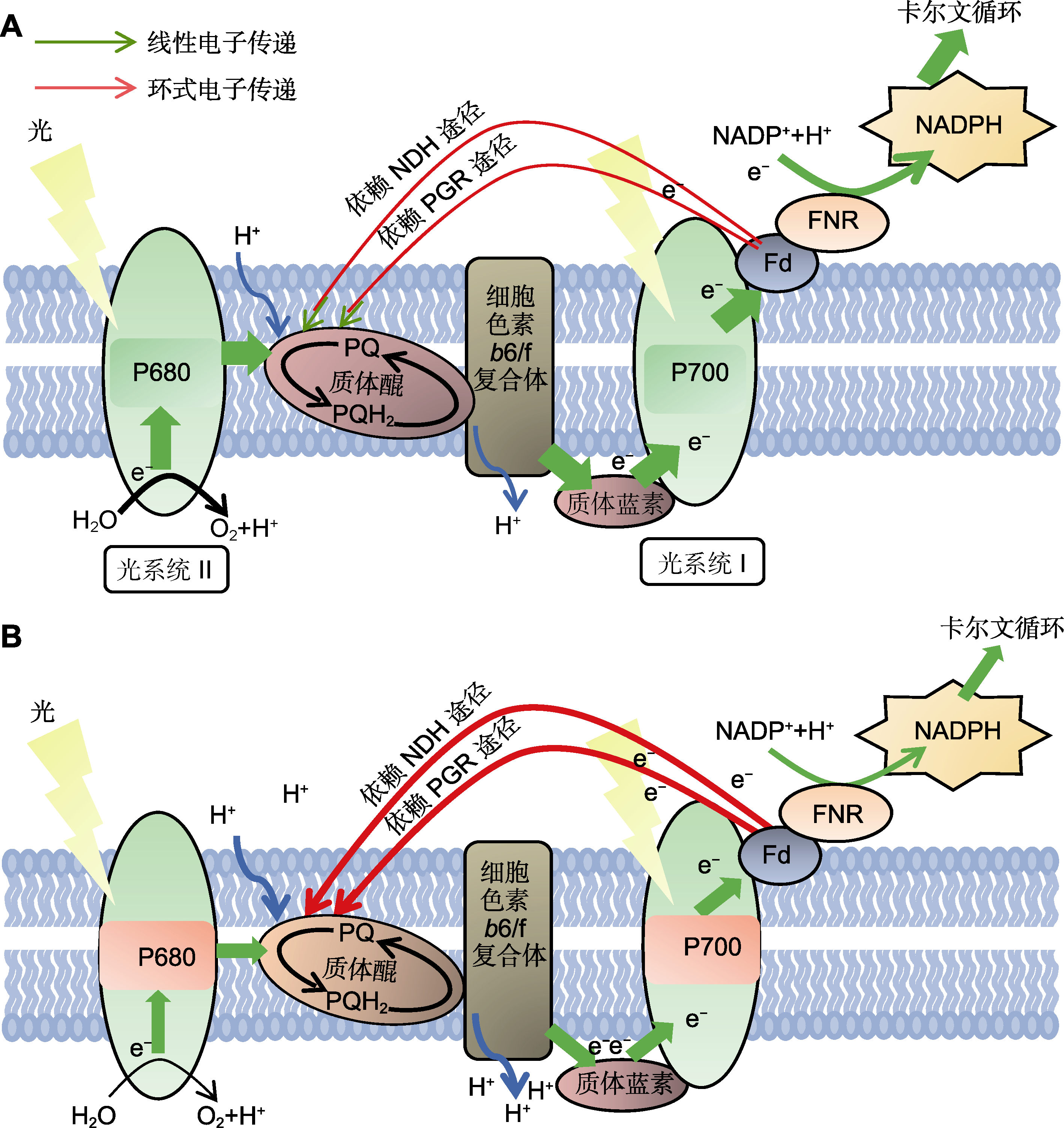
图2 高温胁迫下植物光合电子传递链的变化 (A) 光合电子和质子的定向转移依赖于类囊体膜中的关键蛋白复合体; 适宜环境温度下, PSII裂解H2O中的电子主要通过线性电子传递(LEF)至光系统I, 在Fd和FNR的作用下, 将NADP+还原成NADPH, 形成固定CO2所需的还原力; (B) 高温胁迫下, LEF被抑制, PQ库被还原的同时, 依赖NDH及质子梯度调节蛋白/质子梯度调节类似蛋白(PGR5/PGRL1)的环式电子传递(CEF)被激活; LEF的下调可以通过降低传递至光系统I处的电子促进P700的氧化, 而CEF的上调可以通过增加跨膜质子梯度来产生更多的ATP, 也可以通过激发非光化学淬灭来保护光合机构; 因此, 高温胁迫下, LEF和CEF协同作用调节PQ库及P700的氧化还原状态及热耗散, 起到光保护作用。P680和P700分别表示PSII和PSI反应中心叶绿素在可见光谱中的波长吸收峰值。绿色箭头表示LEF转移, 红线表示CEF转移。Fd: 铁氧还蛋白; FNR: 黄素蛋白铁氧还蛋白-NADP还原酶; NADP+: 氧化型烟酰胺腺嘌呤二核苷磷酸; NDH: NA(P)H脱氢酶复合体; PQ: 质体醌; PQH2: 质体醌醇
Figure 2 Changes in the photosynthetic electron transfer chain in plants under high temperature stress (A) The directional transfer of electrons and protons depends on the key protein complexes in thylakoid membranes; under suitable temperature conditions, electrons in H2O oxidized by PSII are mainly transferred to PSI through linear electron flow (LEF), and NADP+ is reduced to NADPH under the action of Fd and FNR, forming the reducing force needed to fix CO2; (B) Under high temperature stress condition, LEF is inhibited, PQ-pool is reduced, and NDH and PGR5 (proton gradient regulation 5)/PGRL1 (proton gradient regulation like 1)-dependent cyclic electron flow (CEF) is activated; the down-regulation of LEF can promote the oxidation of P700 by reducing the electrons transferred to PSI, while the activation of CEF can produce more ATP by increasing the transmembrane proton gradient and protect the photosynthetic apparatus by stimulating non-photochemical quenching. Therefore, under high temperature stress, LEF and CEF synergistically regulate the redox state and heat dissipation of PQ-pool and P700, and thus play a role in photoprotection. P680 and P700 refer to the wavelength absorption peak of chlorophyll in the visible spectrum of PSII and PSI reaction centers, respectively. The green arrow indicates LEF flow, and the red line indicates CEF flow. Fd: Ferredoxin; FNR: Ferredoxin-NADP reductase; NADP+: Oxidized nicotinamide adenine dinucleoside phosphate; NDH: NA(P)H dehydrogenase complex; PQ: Plastoquinone; PQH2: Plastoquinol
| 植物种名 | 处理温度/时间 | 酶 | 酶活性 | 参考文献 |
|---|---|---|---|---|
| 橡树(Quercus pubescens) | 35°C/0.5小时 | Rubisco | 降低 | Haldimann and Feller, |
| 豌豆(Pisum sativum) | 50°C/0.5小时 | PEPC | 降低 | Chinthapalli et al., |
| 剪股颖(Agrostis palustris) | 35°C/10天 | Rubisco | 降低 | Xu and Huang, |
| 水稻(Oryza sativa) | 35°C/2小时 | SBPase | 降低 | Feng et al., |
| 水稻(O. sativa) 水稻(O. sativa) | 42°C/24小时 42°C/24小时 | TK PRK | 升高 降低 | Lee et al., Lee et al., |
表1 高温胁迫对植物光合作用碳同化过程酶活性的影响
Table 1 Effects of high temperature stress on the activities of enzymes involved in carbon assimilation processes of plant pho- tosynthesis
| 植物种名 | 处理温度/时间 | 酶 | 酶活性 | 参考文献 |
|---|---|---|---|---|
| 橡树(Quercus pubescens) | 35°C/0.5小时 | Rubisco | 降低 | Haldimann and Feller, |
| 豌豆(Pisum sativum) | 50°C/0.5小时 | PEPC | 降低 | Chinthapalli et al., |
| 剪股颖(Agrostis palustris) | 35°C/10天 | Rubisco | 降低 | Xu and Huang, |
| 水稻(Oryza sativa) | 35°C/2小时 | SBPase | 降低 | Feng et al., |
| 水稻(O. sativa) 水稻(O. sativa) | 42°C/24小时 42°C/24小时 | TK PRK | 升高 降低 | Lee et al., Lee et al., |
| 植物种名 | 外源物质 | 处理温度/时间 | 浓度/方式 | 参考文献 |
|---|---|---|---|---|
| 烟草(Nicotiana tabacum) | 氯化钙 | 40°C/24小时 | 20 mmol?L-1/叶面喷施 | Tan et al., |
| 水稻(Oryza sativa) | 表油菜素内酯 | 40°C/7天 | 0.1 μmol?L-1/叶面喷施 | Thussagunpanit et al., |
| 马铃薯(Solanum tuberosum) | 亚磷酸钾 | 37°C/24小时 | 1%/土壤施入 | Xi et al., |
| 番茄(S. lycopersicum) | 褪黑素 | 42°C/24小时 | 100 mmol?L-1/叶面喷施 | Jahan et al., |
| 小麦(Triticum aestivum) | 海藻糖 | 40°C/24小时 | 1.5 mmol?L-1/水培施入 | Luo et al., |
| 水稻(O. sativa) | 亚精胺 | 37.5°C/22天 | 1 mmol?L-1/叶面喷施 | Tang et al., |
| 高羊茅(Lolium arundinaceum) | 柠檬酸 | 35°C/15天 | 20 mmol?L-1/叶面喷施 | Hu et al., |
| 番茄(S. lycopersicum) | 抗坏血酸 | 40°C/8小时 | 0.5 mmol?L-1/叶面喷施 | Alayafi, |
表2 高温胁迫下外源物质对植物光合作用的缓解
Table 2 Alleviation of exogenous substances to photosynthesis under high temperature stress
| 植物种名 | 外源物质 | 处理温度/时间 | 浓度/方式 | 参考文献 |
|---|---|---|---|---|
| 烟草(Nicotiana tabacum) | 氯化钙 | 40°C/24小时 | 20 mmol?L-1/叶面喷施 | Tan et al., |
| 水稻(Oryza sativa) | 表油菜素内酯 | 40°C/7天 | 0.1 μmol?L-1/叶面喷施 | Thussagunpanit et al., |
| 马铃薯(Solanum tuberosum) | 亚磷酸钾 | 37°C/24小时 | 1%/土壤施入 | Xi et al., |
| 番茄(S. lycopersicum) | 褪黑素 | 42°C/24小时 | 100 mmol?L-1/叶面喷施 | Jahan et al., |
| 小麦(Triticum aestivum) | 海藻糖 | 40°C/24小时 | 1.5 mmol?L-1/水培施入 | Luo et al., |
| 水稻(O. sativa) | 亚精胺 | 37.5°C/22天 | 1 mmol?L-1/叶面喷施 | Tang et al., |
| 高羊茅(Lolium arundinaceum) | 柠檬酸 | 35°C/15天 | 20 mmol?L-1/叶面喷施 | Hu et al., |
| 番茄(S. lycopersicum) | 抗坏血酸 | 40°C/8小时 | 0.5 mmol?L-1/叶面喷施 | Alayafi, |
| [1] | 毕焕改, 李福德, 董绪兵, 艾希珍 (2017). 转酮醇酶基因沉默对高温胁迫下黄瓜幼苗光合作用的影响. 植物生理学报 53, 1859-1866. |
| [2] | 程超华, 唐蜻, 邓灿辉, 戴志刚, 许英, 杨泽茂, 刘婵, 吴姗, 粟建光 (2020). 表型组学及多组学联合分析在植物种质资源精准鉴定中的应用. 分子植物育种 18, 2747-2753. |
| [3] | 黄伟, 张石宝, 曹坤芳 (2012). 高等植物环式电子传递的生理作用. 植物科学学报 30, 100-106. |
| [4] | 姜振升, 孙晓琦, 艾希珍, 王美玲, 毕焕改, 王洪涛 (2010). 低温弱光对黄瓜幼苗Rubisco与Rubisco活化酶的影响. 应用生态学报 21, 2045-2050. |
| [5] | 刘玉凤, 鹿嘉智, 孟思达, 王珍琪, 张耀丰, 王峰, 齐明芳, 李天来 (2019). PGR5/PGRL1介导的环式电子传递研究进展. 植物生理学报 55, 433-443. |
| [6] |
王浩, 王明, 梁婷, 姚玉新, 杜远鹏, 高振 (2022). 气温和根区温度对葡萄叶片光合荧光特性的影响. 植物学报 57, 209-216.
DOI |
| [7] | 许大全 (2002). 光合作用效率. 上海: 上海科学技术出版社. pp. 4-5. |
| [8] |
Aihara Y, Takahashi S, Minagawa J (2016). Heat induction of cyclic electron flow around photosystem I in the symbio- tic dinoflagellate Symbiodinium. Plant Physiol 171, 522-529.
DOI URL |
| [9] |
Alayafi AAM (2020). Exogenous ascorbic acid induces systemic heat stress tolerance in tomato seedlings: transcriptional regulation mechanism. Environ Sci Pollut Res Int 27, 19186-19199.
DOI |
| [10] |
Allen JF (1995). Thylakoid protein phosphorylation, state 1- state 2 transitions, and photosystem stoichiometry adjustment: redox control at multiple levels of gene expression. Physiol Plant 93, 196-205.
DOI URL |
| [11] |
Arico D, Legris M, Castro L, Garcia CF, Laino A, Casal JJ, Mazzella MA (2019). Neighbour signals perceived by phytochrome B increase thermotolerance in Arabidopsis. Plant Cell Environ 42, 2554-2566.
DOI URL |
| [12] |
Atkin OK, Bruhn D, Hurry VM, Tjoelker MG (2005). The hot and the cold: unravelling the variable response of plant respiration to temperature. Funct Plant Biol 32, 87-105.
DOI PMID |
| [13] |
Berry J, Bjorkman O (1980). Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol 31, 491-543.
DOI URL |
| [14] |
Bracher A, Whitney SM, Hartl FU, Hayer-Hartl M (2017). Biogenesis and metabolic maintenance of Rubisco. Annu Rev Plant Biol 68, 29-60.
DOI PMID |
| [15] |
Buchner O, Stoll M, Karadar M, Kranner I, Neuner G (2015). Application of heat stress in situ demonstrates a protective role of irradiation on photosynthetic perfor-mance in alpine plants. Plant Cell Environ 38, 812-826.
DOI URL |
| [16] |
Cao YY, Zhao H (2008). Protective roles of brassinolide on rice seedlings under high temperature stress. Rice Sci 15, 63-68.
DOI URL |
| [17] | Charng YY, Liu HC, Liu NY, Chi WT, Wang CN, Chang SH, Wang TT (2007). A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiol 143, 251-262. |
| [18] |
Chen JH, Chen ST, He NY, Wang QL, Zhao Y, Gao W, Guo FQ (2020). Nuclear-encoded synthesis of the D1 subunit of photosystem II increases photosynthetic efficiency and crop yield. Nat Plants 6, 570-580.
DOI |
| [19] |
Chen JH, Tang M, Jin XQ, Li H, Chen LS, Wang QL, Sun AZ, Yi Y, Guo FQ (2022). Regulation of Calvin-Benson cycle enzymes under high temperature stress. aBIOTECH 3, 65-77.
DOI |
| [20] |
Chen ST, He NY, Chen JH, Guo FQ (2017). Identification of core subunits of photosystem II as action sites of HSP21, which is activated by the GUN5-mediated retrograde path- way in Arabidopsis. Plant J 89, 1106-1118.
DOI URL |
| [21] |
Chinthapalli B, Murmu J, Raghavendra AS (2003). Dramatic difference in the responses of phosphoenolpyruvate carboxylase to temperature in leaves of C3 and C4 plants. J Exp Bot 54, 707-714.
PMID |
| [22] |
Crafts-Brandner SJ, Salvucci ME (2000). Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proc Natl Acad Sci USA 97, 13430-13435.
DOI PMID |
| [23] |
Demirevska-Kepova K, Holzer R, Simova-Stoilova L, Feller U (2005). Heat stress effects on ribulose-1,5- bisphosphate carboxylase/oxygenase, Rubisco binding protein and Rubisco activase in wheat leaves. Biol Plant 49, 521-525.
DOI URL |
| [24] |
DeRidder BP, Salvucci ME (2007). Modulation of Rubisco activase gene expression during heat stress in cotton (Gossypium hirsutum L.) involves post-transcriptional mechanisms. Plant Sci 172, 246-254.
DOI URL |
| [25] |
Essemine J, Qu MN, Mi HL, Zhu XG (2016). Response of chloroplast NAD(P)H dehydrogenase-mediated cyclic elec- tron flow to a shortage or lack in ferredoxin-quinone oxidoreductase-dependent pathway in rice following short- term heat stress. Front Plant Sci 7, 383.
DOI PMID |
| [26] |
Farquhar GD, Von Caemmerer S, Berry JA (1980). A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78-90.
DOI PMID |
| [27] |
Fauset S, Freitas HC, Galbraith DR, Sullivan MJP, Aidar MPM, Joly CA, Phillips OL, Vieira SA, Gloor MU (2018). Differences in leaf thermoregulation and water use strategies between three co-occurring Atlantic forest tree species. Plant Cell Environ 41, 1618-1631.
DOI URL |
| [28] |
Feng LL, Wang K, Li Y, Tan YP, Kong J, Li H, Li YS, Zhu YG (2007). Overexpression of SBPase enhances photosynthesis against high temperature stress in transgenic rice plants. Plant Cell Rep 26, 1635-1646.
PMID |
| [29] |
Foyer CH, Neukermans J, Queval G, Noctor G, Harbinson J (2012). Photosynthetic control of electron transport and the regulation of gene expression. J Exp Bot 63, 1637-1661.
DOI PMID |
| [30] |
Gounaris K, Brain ARR, Quinn PJ, Williams WP (1984). Structural reorganisation of chloroplast thylakoid membranes in response to heat-stress. Biochim Biophys Acta Bioenerg 766, 198-208.
DOI URL |
| [31] |
Grabsztunowicz M, Koskela MM, Mulo P (2017). Post- translational modifications in regulation of chloroplast function: recent advances. Front Plant Sci 8, 240.
DOI PMID |
| [32] |
Haldimann P, Feller U (2004). Inhibition of photosynthesis by high temperature in oak (Quercus pubescens L.) lea-ves grown under natural conditions closely correlates with a reversible heat-dependent reduction of the activation state of ribulose-1,5-bisphosphate carboxylase/oxy- genase. Plant Cell Environ 27, 1169-1183.
DOI URL |
| [33] |
Hasanuzzaman M, Nahar K, Alam MM, Roychowdhury R, Fujita M (2013). Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14, 9643-9684.
DOI PMID |
| [34] |
Hashimoto K, Kudla J (2011). Calcium decoding mechanisms in plants. Biochimie 93, 2054-2059.
DOI PMID |
| [35] |
Havaux M, Greppin H, Strasser RJ (1991). Functioning of photosystems I and II in pea leaves exposed to heat stress in the presence or absence of light: analysis using in vivo fluorescence, absorbance, oxygen and photoa- coustic measurements. Planta 186, 88-98.
DOI PMID |
| [36] |
Hill R, Bendall FAY (1960). Function of the two cytochrome components in chloroplasts: a working hypothesis. Nature 186, 136-137.
DOI |
| [37] |
Hu LX, Zhang ZF, Xiang ZX, Yang ZJ (2016). Exogenous application of citric acid ameliorates the adverse effect of heat stress in tall fescue (Lolium arundinaceum). Front Plant Sci 7, 179.
DOI PMID |
| [38] |
Hüve K, Bichele I, Rasulov B, Niinemets Ü (2011). When it is too hot for photosynthesis: heat-induced instability of photosynthesis in relation to respiratory burst, cell permeability changes and H2O2 formation. Plant Cell Environ 34, 113-126.
DOI URL |
| [39] | IPCC (2021). Climate Change 2021: the Physical Science Basis. Cambridge: Cambridge University Press. pp. 41. |
| [40] |
Jahan MS, Guo SR, Sun J, Shu S, Wang Y, El-Yazied AA, Alabdallah NM, Hikal M, Mohamed MHM, Ibrahim MFM, Hasan MM (2021). Melatonin-mediated photosynthetic performance of tomato seedlings under high-tem- perature stress. Plant Physiol Biochem 167, 309-320.
DOI URL |
| [41] |
Jiang Y, Feng X, Wang H, Chen YQ, Sun YJ (2021). Heat-induced down-regulation of photosystem II protects photosystem I in honeysuckle (Lonicera japonica). J Plant Res 134, 1311-1321.
DOI PMID |
| [42] |
Joly D, Carpentier R (2007). Regulation of energy dissipation in photosystem I by the redox state of the plasto- quinone pool. Biochemistry 46, 5534-5541.
DOI URL |
| [43] |
Jung JH, Domijan M, Klose C, Biswas S, Ezer D, Gao MJ, Khattak AK, Box MS, Charoensawan V, Cortijo S, Kumar M, Grant A, Locke JCW, Schäfer E, Jaeger KE, Wigge PA (2016). Phytochromes function as thermosensors in Arabidopsis. Science 354, 886-889.
PMID |
| [44] |
Kallis RP, Ewy RG, Portis AR Jr (2000). Alteration of the adenine nucleotide response and increased Rubisco activation activity of Arabidopsis Rubisco activase by site- directed mutagenesis. Plant Physiol 123, 1077-1086.
PMID |
| [45] |
Kao WY, Forseth IN (1992). Dirunal leaf movement, chlorophyll fluorescence and carbon assimilation in soybean grown under different nitrogen and water availabilities. Plant Cell Environ 15, 703-710.
DOI URL |
| [46] |
Lascano HR, Casano LM, Martin M, Sabater B (2003). The activity of the chloroplastic NDH complex is regulated by phosphorylation of the NDH-F subunit. Plant Physiol 132, 256-262.
PMID |
| [47] |
Lau OS, Song ZJ, Zhou ZM, Davies KA, Chang J, Yang X, Wang SQ, Lucyshyn D, Tay IHZ, Wigge PA, Bergmann DC (2018). Direct control of SPEECHLESS by PIF4 in the high-temperature response of stomatal development. Curr Biol 28, 1273-1280.
DOI |
| [48] |
Le Quéré C, Jackson RB, Jones MW, Smith AJP, Abernethy S, Andrew RM, De-Gol AJ, Willis DR, Shan YL, Canadell JG, Friedlingstein P, Creutzig F, Peters GP (2020). Temporary reduction in daily global CO2 emissions during the COVID-19 forced confinement. Nat Clim Change 10, 647-653.
DOI |
| [49] |
Lee DG, Ahsan N, Lee SH, Kang KY, Bahk JD, Lee IJ, Lee BH (2007). A proteomic approach in analyzing heat- responsive proteins in rice leaves. Proteomics 7, 3369-3383.
DOI URL |
| [50] |
Legris M, Nieto C, Sellaro R, Prat S, Casal JJ (2017). Perception and signaling of light and temperature cues in plants. Plant J 90, 683-697.
DOI URL |
| [51] |
Li N, Bo CP, Zhang YY, Wang L (2021). PHYTOCHROME INTERACTING FACTORS PIF4 and PIF5 promote heat stress induced leaf senescence in Arabidopsis. J Exp Bot 72, 4577-4589.
DOI URL |
| [52] |
Li PM, Cheng LL, Gao HY, Jiang CD, Peng T (2009). Hete- rogeneous behavior of PSII in soybean (Glycine max) leaves with identical PSII photochemistry efficiency under different high temperature treatments. J Plant Physiol 166, 1607-1615.
DOI URL |
| [53] |
Luo Y, Xie Y, He D, Wang W, Yuan S (2021). Exogenous trehalose protects photosystem II by promoting cyclic electron flow under heat and drought stresses in winter wheat. Plant Biol 23, 770-776.
DOI URL |
| [54] |
Marutani Y, Yamauchi Y, Kimura Y, Mizutani M, Sugimoto Y (2012). Damage to photosystem II due to heat stress without light-driven electron flow: involvement of enhanced introduction of reducing power into thylakoid membranes. Planta 236, 753-761.
DOI PMID |
| [55] |
Mathur S, Allakhverdiev SI, Jajoo A (2011). Analysis of high temperature stress on the dynamics of antenna size and reducing side heterogeneity of photosystem II in wheat (Triticum aestivum) leaves. Biochim Biophys Acta Bioenerg 1807, 22-29.
DOI URL |
| [56] |
Maxwell DP, Laudenbach DE, Huner N (1995). Redox regulation of light-harvesting complex II and cab mRNA abundance in Dunaliella salina. Plant Physiol 109, 787-795.
DOI PMID |
| [57] |
Mayton H, Myers K, Fry WE (2008). Potato late blight in tubers—the role of foliar phosphonate applications in suppressing pre-harvest tuber infections. Crop Prot 27, 943-950.
DOI URL |
| [58] |
Mittler R, Finka A, Goloubinoff P (2012). How do plants feel the heat? Trends Biochem Sci 37, 118-125.
DOI PMID |
| [59] |
Mohanty S, Verma SK, Nayak SK (2006). Dynamic mechanical and thermal properties of MAPE treated jute/ HDPE composites. Compos Sci Technol 66, 538-547.
DOI URL |
| [60] | Müller P, Li XP, Niyogi KK (2001). Non-photochemical quenching. A response to excess light energy. Plant Phy- siol 125, 1558-1566. |
| [61] |
Munekage Y, Hashimoto M, Miyake C, Tomizawa KI, Endo T, Tasaka M, Shikanai T (2004). Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 429, 579-582.
DOI |
| [62] |
Murchie EH, Hubbart S, Peng S, Horton P (2005). Acclimation of photosynthesis to high irradiance in rice: gene expression and interactions with leaf development. J Exp Bot 56, 449-460.
PMID |
| [63] |
Nishiyama Y, Allakhverdiev SI, Murata N (2006). A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim Biophys Acta Bioenerg 1757, 742-749.
DOI URL |
| [64] |
Nishiyama Y, Yamamoto H, Allakhverdiev SI, Inaba M, Yokota A, Murata N (2001). Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J 20, 5587-5594.
DOI PMID |
| [65] |
Ogweno JO, Song XS, Shi K, Hu WH, Mao WH, Zhou YH, Yu JQ, Nogués S (2008). Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicon esculentum. J Plant Growth Regul 27, 49-57.
DOI URL |
| [66] |
Osei-Bonsu I, McClain AM, Walker BJ, Sharkey TD, Kramer DM (2021). The roles of photorespiration and alternative electron acceptors in the responses of photosynthesis to elevated temperatures in cowpea. Plant Cell Environ 44, 2290-2307.
DOI URL |
| [67] |
Oyarburo NS, Machinandiarena MF, Feldman ML, Daleo GR, Andreu AB, Olivieri FP (2015). Potassium phos- phite increases tolerance to UV-B in potato. Plant Physiol Biochem 88, 1-8.
DOI URL |
| [68] |
Perkins-Kirkpatrick SE, Lewis SC (2020). Increasing trends in regional heatwaves. Nat Commun 11, 3357.
DOI PMID |
| [69] |
Pollastri S, Sukiran NA, Jacobs BCIC, Knight MR (2021). Chloroplast calcium signaling regulates thermomemory. J Plant Physiol 264, 153470.
DOI URL |
| [70] |
Pshybytko NL, Kruk J, Kabashnikova LF, Strzalka K (2008). Function of plastoquinone in heat stress reactions of plants. Biochim Biophys Acta Bioenerg 1777, 1393-1399.
DOI URL |
| [71] | Qazi HA, Jan N, Ramazan S, John R (2019). Protein modification in plants in response to abiotic stress. In: Dar TA, Singh LR, eds. Protein Modificomics. Amsterdam: Else- vier. pp. 171-201. |
| [72] |
Qu AL, Ding YF, Jiang Q, Zhu C (2013). Molecular mechanisms of the plant heat stress response. Biochem Bioph Res Commun 432, 203-207.
DOI URL |
| [73] |
Quint M, Delker C, Franklin KA, Wigge PA, Halliday KJ, Van Zanten M (2016). Molecular and genetic control of plant thermomorphogenesis. Nat Plants 2, 15190.
DOI PMID |
| [74] |
Richter K, Haslbeck M, Buchner J (2010). The heat shock response: life on the verge of death. Mol Cell 40, 253-266.
DOI PMID |
| [75] |
Ruban AV (2016). Nonphotochemical chlorophyll fluorescence quenching: mechanism and effectiveness in protecting plants from photodamage. Plant Physiol 170, 1903-1916.
DOI PMID |
| [76] |
Russell AW, Critchley C, Robinson SA, Franklin LA, Seaton GGR, Chow WS, Anderson JM, Osmond CB (1995). Photosystem II regulation and dynamics of the chloroplast D1 protein in Arabidopsis leaves during photosynthesis and photoinhibition. Plant Physiol 107, 943-952.
PMID |
| [77] |
Rytz TC, Miller MJ, McLoughlin F, Augustine RC, Marshall RS, Juan YT, Charng YY, Scalf M, Smith LM, Vierstra RD (2018). SUMOylome profiling reveals a diverse array of nuclear targets modified by the SUMO liga- se SIZ1 during heat stress. Plant Cell 30, 1077-1099.
DOI URL |
| [78] |
Sadura I, Libik-Konieczny M, Jurczyk B, Gruszka D, Janeczko A (2020). HSP transcript and protein accumulation in brassinosteroid barley mutants acclimated to low and high temperatures. Int J Mol Sci 21, 1889.
DOI URL |
| [79] |
Sajid M, Rashid B, Ali Q, Husnain T (2018). Mechanisms of heat sensing and responses in plants. It is not all about Ca2+ ions. Biol Plant 62, 409-420.
DOI URL |
| [80] |
Shekhawat K, Almeida-Trapp M, García-Ramírez GX, Hirt H (2022). Beat the heat: plant- and microbe-mediated strategies for crop thermotolerance. Trends Plant Sci 27, 802-813.
DOI URL |
| [81] |
Sherstneva O, Khlopkov A, Gromova E, Yudina L, Vetrova Y, Pecherina A, Kuznetsova D, Krutova E, Sukhov V, Vodeneev V (2022). Analysis of chlorophyll fluorescence parameters as predictors of biomass accumulation and tolerance to heat and drought stress of wheat (Triticum aestivum) plants. Funct Plant Biol 49, 155-169.
DOI URL |
| [82] |
Siebers MH, Slattery RA, Yendrek CR, Locke AM, Drag D, Ainsworth EA, Bernacchi CJ, Ort DR (2017). Simulated heat waves during maize reproductive stages alter reproductive growth but have no lasting effect when applied during vegetative stages. Agric Ecosyst Environ 240, 162-170.
DOI URL |
| [83] |
Slattery RA, Ort DR (2019). Carbon assimilation in crops at high temperatures. Plant Cell Environ 42, 2750-2758.
DOI |
| [84] |
Song JY, Liu QJ, Hu BR, Wu WJ (2017). Photoreceptor phyB involved in Arabidopsis temperature perception and heat-tolerance formation. Int J Mol Sci 18, 1194.
DOI URL |
| [85] |
Sonoike K (2011). Photoinhibition of photosystem I. Physiol Plant 142, 56-64.
DOI PMID |
| [86] |
Stotz M, Mueller-Cajar O, Ciniawsky S, Wendler P, Hartl FU, Bracher A, Hayer-Hartl M (2011). Structure of green- type Rubisco activase from tobacco. Nat Struct Mol Biol 18, 1366-1370.
DOI |
| [87] |
Strand DD, Livingston AK, Satoh-Cruz M, Froehlich JE, Maurino VG, Kramer DM (2015). Activation of cyclic electron flow by hydrogen peroxide in vivo. Proc Natl Acad Sci USA 112, 5539-5544.
DOI URL |
| [88] |
Sun YJ, Geng QW, Du YP, Yang XH, Zhai H (2017). Induction of cyclic electron flow around photosystem I du- ring heat stress in grape leaves. Plant Sci 256, 65-71.
DOI URL |
| [89] |
Suorsa M, Rossi F, Tadini L, Labs M, Colombo M, Jahns P, Kater MM, Leister D, Finazzi G, Aro EM, Barbato R, Pesaresi P (2016). PGR5-PGRL1-dependent cyclic electron transport modulates linear electron transport rate in Arabidopsis thaliana. Mol Plant 9, 271-288.
DOI URL |
| [90] |
Suzuki K, Nagasuga K, Okada M (2008). The chilling injury induced by high root temperature in the leaves of rice seedlings. Plant Cell Physiol 49, 433-442.
DOI PMID |
| [91] |
Takahashi S, Badger MR (2011). Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci 16, 53-60.
DOI PMID |
| [92] |
Takahashi S, Murata N (2008). How do environmental stresses accelerate photoinhibition? Trends Plant Sci 13, 178-182.
DOI PMID |
| [93] |
Tan SL, Yang YJ, Huang W (2020). Moderate heat stress accelerates photoinhibition of photosystem I under fluct- uating light in tobacco young leaves. Photosynth Res 144, 373-382.
DOI |
| [94] |
Tan W, Meng QW, Brestic M, Olsovska K, Yang XH (2011). Photosynthesis is improved by exogenous calcium in heat-stressed tobacco plants. J Plant Physiol 168, 2063-2071.
DOI URL |
| [95] |
Tang S, Zhang HX, Li L, Liu X, Chen L, Chen WZ, Ding YF (2018). Exogenous spermidine enhances the photosynthetic and antioxidant capacity of rice under heat stress during early grain-filling period. Funct Plant Biol 45, 911-921.
DOI PMID |
| [96] |
Tarvainen L, Wittemann M, Mujawamariya M, Manishim- we A, Zibera E, Ntirugulirwa B, Ract C, Manzi OJL, Andersson MX, Spetea C, Nsabimana D, Wallin G, Uddling J (2022). Handling the heat-photosynthetic thermal stress in tropical trees. New Phytol 233, 236-250.
DOI URL |
| [97] |
Teicher HB, Scheller HV (1998). The NAD(P)H dehydrogenase in barley thylakoids is photoactivatable and uses NADPH as well as NADH. Plant Physiol 117, 525-532.
DOI URL |
| [98] |
Thussagunpanit J, Jutamanee K, Kaveeta L, Chai-Arree W, Pankean P, Homvisasevongsa S, Suksamrarn A (2015). Comparative effects of brassinosteroid and brass- inosteroid mimic on improving photosynthesis, lipid peroxidation, and rice seed set under heat stress. J Plant Growth Regul 34, 320-331.
DOI URL |
| [99] |
Tikkanen M, Mekala NR, Aro EM (2014). Photosystem II photoinhibition-repair cycle protects photosystem I from irreversible damage. Biochim Biophys Acta Bioenerg 1837, 210-215.
DOI URL |
| [100] | Tollefson J (2021). IPCC climate report:earth is warmer than it's been in 125,000 years. Nature 596, 171-172. |
| [101] | Tserej O, Feeley KJ (2021). Variation in leaf temperatures of tropical and subtropical trees are related to leaf thermoregulatory traits and not geographic distributions. Biotro- pica 53, 868-878. |
| [102] | Tyystjärvi E (2012). Photoinhibition of photosystem II. Int Rev Cell Mol Biol 300, 243-303. |
| [103] |
Tyystjärvi E, Aro EM (1996). The rate constant of photoinhibition, measured in lincomycin-treated leaves, is directly proportional to light intensity. Proc Natl Acad Sci USA 93, 2213-2218.
DOI PMID |
| [104] |
Vierling E (1991). The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 42, 579-620.
DOI URL |
| [105] |
Von Caemmerer S (2020). Rubisco carboxylase/oxyge- nase: from the enzyme to the globe: a gas exchange perspective. J Plant Physiol 252, 153240.
DOI URL |
| [106] |
Wada M, Kagawa T, Sato Y (2003). Chloroplast movement. Annu Rev Plant Biol 54, 455-468.
PMID |
| [107] |
Wang ML, Zhang XY, Li QH, Chen X, Li XH (2019). Comparative transcriptome analysis to elucidate the enhanced thermotolerance of tea plants (Camellia sinensis) treated with exogenous calcium. Planta 249, 775-786.
DOI PMID |
| [108] |
Wang P, Duan W, Takabayashi A, Endo T, Shikanai T, Ye JY, Mi HL (2006). Chloroplastic NAD(P)H dehydrogenase in tobacco leaves functions in alleviation of oxidative da- mage caused by temperature stress. Plant Physiol 141, 465-474.
DOI URL |
| [109] |
Wang QL, Chen JH, He NY, Guo FQ (2018). Metabolic reprogramming in chloroplasts under heat stress in plants. Int J Mol Sci 19, 849.
DOI URL |
| [110] |
Wise RR, Olson AJ, Schrader SM, Sharkey TD (2004). Electron transport is the functional limitation of photosynthesis in field-grown pima cotton plants at high temperature. Plant Cell Environ 27, 717-724.
DOI URL |
| [111] |
Wu TY, Juan YT, Hsu YH, Wu SH, Liao HT, Fung RWM, Charng YY (2013). Interplay between heat shock proteins HSP101 and HSA32 prolongs heat acclimation memory posttranscriptionally in Arabidopsis. Plant Physiol 161, 2075-2084.
DOI URL |
| [112] |
Xi YP, Han XY, Zhang ZZ, Joshi J, Borza T, Mohammad Aqa M, Zhang BB, Yuan HM, Wang-Pruski G (2020). Exogenous phosphite application alleviates the adverse effects of heat stress and improves thermotolerance of potato (Solanum tuberosum L.) seedlings. Ecotoxicol Environ Saf 190, 110048.
DOI URL |
| [113] |
Xia ZQ, Si LY, Jin Y, Fu YF, Wang Q, Lu HD (2021). Effects of root zone temperature increase on physiological indexes and photosynthesis of different genotype maize seedlings. Russ J Plant Physiol 68, 169-178.
DOI |
| [114] |
Xu HG, Liu GJ, Liu GT, Yan BF, Duan W, Wang LJ, Li SH (2014). Comparison of investigation methods of heat injury in grapevine (Vitis) and assessment to heat tolerance in different cultivars and species. BMC Plant Biol 14, 156.
DOI PMID |
| [115] |
Xu QZ, Huang BR (2001). Morphological and physiological characteristics associated with heat tolerance in creeping bentgrass. Crop Sci 41, 127-133.
DOI URL |
| [116] |
Yamamoto H, Shikanai T (2019). PGR5-dependent cyclic electron flow protects photosystem I under fluctuating light at donor and acceptor sides. Plant Physiol 179, 588-600.
DOI PMID |
| [117] |
Yamamoto Y (2016). Quality control of photosystem II: the mechanisms for avoidance and tolerance of light and heat stresses are closely linked to membrane fluidity of the thylakoids. Front Plant Sci 7, 1136.
DOI PMID |
| [118] |
Yamori N, Levine CP, Mattson NS, Yamori W (2022). Optimum root zone temperature of photosynthesis and plant growth depends on air temperature in lettuce plants. Plant Mol Biol 110, 385-395.
DOI |
| [119] | Yamori W, Hikosaka K, Way DA (2014). Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation. Pho- tosynth Res 119, 101-117. |
| [120] | Yamori W, Shikanai T, Makino A (2015). Photosystem I cyclic electron flow via chloroplast NADH dehydrogenase- like complex performs a physiological role for photosyn- thesis at low light. Sci Rep 5, 13908. |
| [121] | Yin YL, Qin KZ, Song XW, Zhang QH, Zhou YH, Xia XJ, Yu JQ (2018). BZR1 transcription factor regulates heat stress tolerance through FERONIA receptor-like kinase- mediated reactive oxygen species signaling in tomato. Plant Cell Physiol 59, 2239-2254. |
| [122] |
Zhang HM, Zhu JH, Gong ZZ, Zhu JK (2022). Abiotic stress responses in plants. Nat Rev Genet 23, 104-119.
DOI |
| [123] |
Zheng XT, Wang CJ, Lin WX, Lin CF, Han DL, Xie Q, Lai JB, Yang CW (2022). Importation of chloroplast proteins under heat stress is facilitated by their SUMO conjugations. New Phytol 235, 173-187.
DOI URL |
| [124] |
Zhong LL, Zhou W, Wang HJ, Ding SH, Lu QT, Wen XG, Peng LW, Zhang LX, Lu CM (2013). Chloroplast small heat shock protein HSP21 interacts with plastid nucleoid protein pTAC5 and is essential for chloroplast development in Arabidopsis under heat stress. Plant Cell 25, 2925-2943.
DOI URL |
| [1] | 王涛, 冯敬磊, 张翠. 高温胁迫影响玉米生长发育的分子机制研究进展[J]. 植物学报, 2024, 59(6): 963-977. |
| [2] | 闫恒宇, 李朝霞, 李玉斌. 高温对玉米生长的影响及中国耐高温玉米筛选研究进展[J]. 植物学报, 2024, 59(6): 1007-1023. |
| [3] | 叶洁泓, 于成龙, 卓少菲, 陈新兰, 杨科明, 文印, 刘慧. 木兰科植物叶片光合系统耐热性与叶片形态及温度生态位的关系[J]. 植物生态学报, 2023, 47(10): 1432-1440. |
| [4] | 刘晓龙, 季平, 杨洪涛, 丁永电, 付佳玲, 梁江霞, 余聪聪. 脱落酸对水稻抽穗开花期高温胁迫的诱抗效应[J]. 植物学报, 2022, 57(5): 596-610. |
| [5] | 李颖, 龚吉蕊, 刘敏, 侯向阳, 丁勇, 杨波, 张子荷, 王彪, 朱趁趁. 不同放牧强度下内蒙古温带典型草原优势种植物防御策略[J]. 植物生态学报, 2020, 44(6): 642-653. |
| [6] | 汪俊宇, 王小东, 马元丹, 傅卢成, 周欢欢, 王彬, 张汝民, 高岩. ‘波叶金桂’对干旱和高温胁迫的生理生态响应[J]. 植物生态学报, 2018, 42(6): 681-691. |
| [7] | 闫霜,张黎,景元书,何洪林,于贵瑞. 植物叶片最大羧化速率与叶氮含量关系的变异性[J]. 植物生态学报, 2014, 38(6): 640-652. |
| [8] | 张超, 占东霞, 张鹏鹏, 张亚黎, 罗宏海, 张旺锋. 棉花苞叶光呼吸和PSII热耗散对土壤水分的响应[J]. 植物生态学报, 2014, 38(4): 387-395. |
| [9] | 郝海平, 姜闯道, 石雷, 唐宇丹, 姚涓, 李志强. 根系温度对光核桃幼苗光合机构热稳定性的影响[J]. 植物生态学报, 2009, 33(5): 984-992. |
| [10] | 焦健, 李朝周, 黄高宝. 乙烯产生抑制剂对高温胁迫下蚕豆幼苗叶片的保护作用[J]. 植物生态学报, 2006, 30(3): 465-471. |
| [11] | 王利军 黄卫东. 高温胁迫及其信号转导[J]. 植物学报, 2000, 17(02): 114-120. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||