

植物学报 ›› 2023, Vol. 58 ›› Issue (3): 475-485.DOI: 10.11983/CBB22227 cstr: 32102.14.CBB22227
钱虹萍1,2,3,4, 罗鹏云1,2,3,4, 刘帅5, 徐昌文1,2,3,4, 殷金环1,2,3,4, 崔亚宁1,2,3,4( ), 林金星1,2,3,4(
), 林金星1,2,3,4( )
)
收稿日期:2022-09-29
接受日期:2023-01-28
出版日期:2023-05-01
发布日期:2023-05-17
通讯作者:
*E-mail: cuiyaning@bjfu.edu.cn; linjx@bjfu.edu.cn
基金资助:
Hongping Qian1,2,3,4, Pengyun Luo1,2,3,4, Shuai Liu5, Changwen Xu1,2,3,4, Jinhuan Yin1,2,3,4, Yaning Cui1,2,3,4( ), Jinxing Lin1,2,3,4(
), Jinxing Lin1,2,3,4( )
)
Received:2022-09-29
Accepted:2023-01-28
Online:2023-05-01
Published:2023-05-17
Contact:
*E-mail: cuiyaning@bjfu.edu.cn; linjx@bjfu.edu.cn
摘要: 细胞是生物体的基本结构和功能单位, 其内部复杂的组织结构、相互作用及动力学过程等决定着整个生物体的生命形态和生命过程。开展活体状态下细胞结构和功能研究, 对于探索和掌握生命的本质具有重要意义。随着科学技术的不断进步, 一系列新的活细胞标记技术被不断革新。近年来, Halo-tag标记技术逐渐发展成为一种广泛应用的新型分子标记技术, 在活细胞成像和追踪研究领域取得了极大进展, 并逐渐在植物细胞成像中得到应用。该文首先系统介绍了Halo-tag标记技术的定义、分类和发展过程, 然后详细介绍了Halo-tag标记技术发生脱卤有机反应的机制及其荧光配基的种类, 最后从观察分子的精细定位、追踪分子的实时运动和检测分子间的相互作用3方面着重阐述了该技术在植物活细胞成像中的最新进展, 以期为后续Halo-tag标记技术在植物中的潜在应用奠定理论基础并提供技术支持。
钱虹萍, 罗鹏云, 刘帅, 徐昌文, 殷金环, 崔亚宁, 林金星. Halo-tag标记技术及其在植物细胞成像中的应用. 植物学报, 2023, 58(3): 475-485.
Hongping Qian, Pengyun Luo, Shuai Liu, Changwen Xu, Jinhuan Yin, Yaning Cui, Jinxing Lin. Halo-tag Labeling Technology and It’s Application in Plant Living Cell Imaging. Chinese Bulletin of Botany, 2023, 58(3): 475-485.
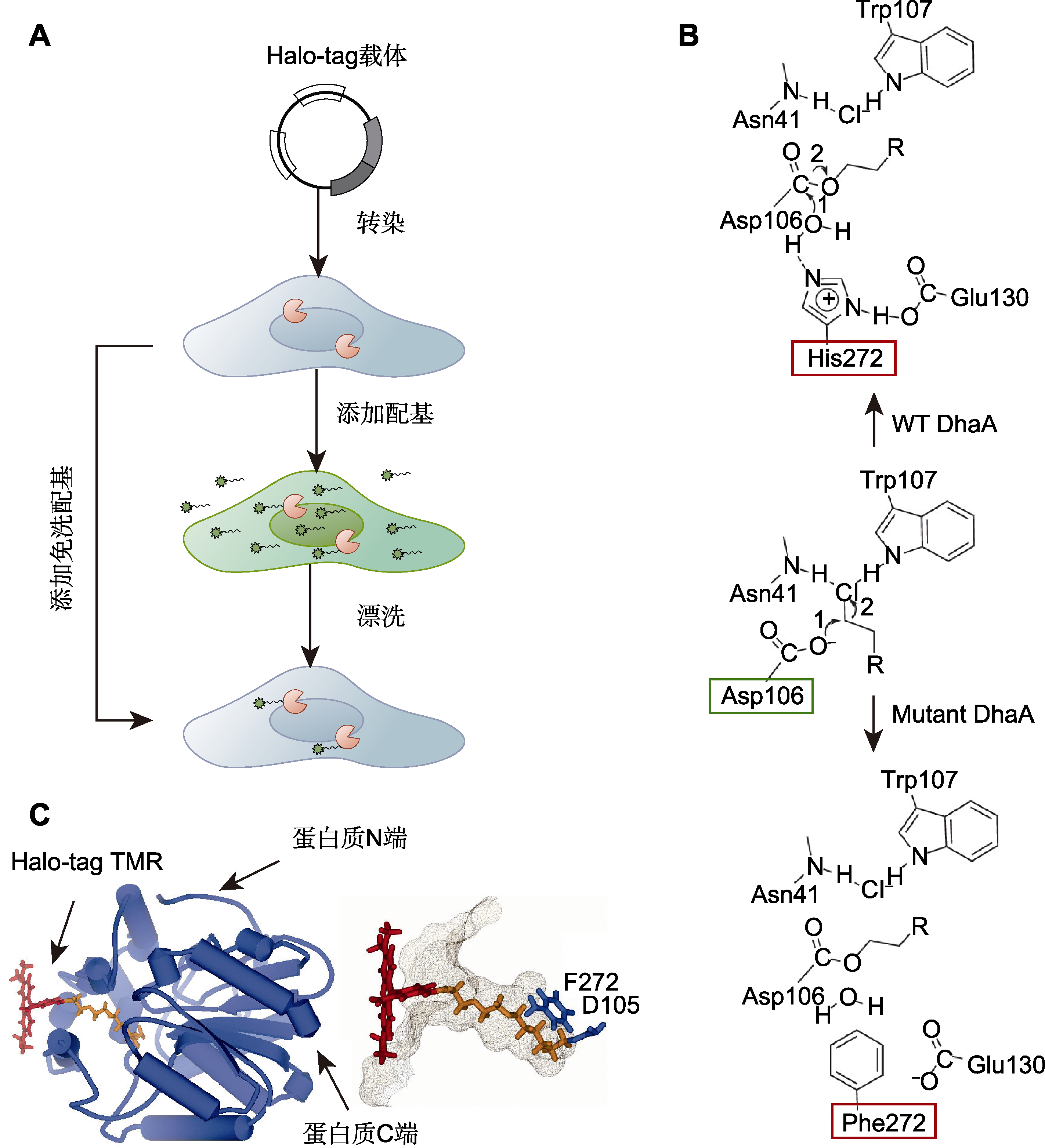
图1 Halo-tag标记技术 (A) 活体细胞Halo-tag标记流程图; (B) 野生型(WT)和突变型脱卤酶的化学结构式(绿框表示脱卤酶的第106位天冬氨酸(Asp), 是指发生脱卤并形成中间产物酯的位点; 红框表示野生型与突变型脱卤酶的区别, 其中野生型脱卤酶第272位是组氨酸(His), 而在突变型脱卤酶第272位突变成苯丙氨酸(Phe)); (C) Halo-tag蛋白与TMR配基共价结合的分子模型(改自England et al., 2015)。
Figure 1 Halo-tag technology (A) Schematic showing Halo-tag labeling in living cells; (B) Chemical structural formulae of wild-type (WT) and mutant dehalogenases (green box indicates that aspartic acid (Asp) at position 106 of dehalogenase, which is the site where dehalogenation occurs and the intermediate ester is formed; red box indicates the difference between the wild-type dehalogenase and the mutant dehalogenase, where the wild-type dehalogenase is histidine (His) at position 272, while the mutant dehalogenase mutates to phenylalanine (Phe) at position 272); (C) Molecular model of the Halo-tag protein with a covalently bound TMR ligand (modified from England et al., 2015).
| 配基类别 | 膜渗透性 | 激发/发射波长的峰值(nm) | 是否需要清洗 | 化学结构式 |
|---|---|---|---|---|
| TMR | 细胞渗透性 | 555/585 | 是 | 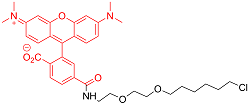 |
| diAcFAM | 细胞渗透性 | 494/526 | 是 | 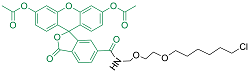 |
| Oregon Green | 细胞渗透性 | 496/516 | 是 | 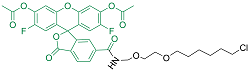 |
| Coumarin | 细胞渗透性 | 353/434 | 是 | 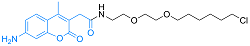 |
| Alexa Fluor? 488 | 非细胞渗透性 | 494/517 | 是 | 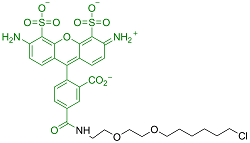 |
| Alexa Fluor? 660 | 非细胞渗透性 | 663/690 | 是 | 结构图无法获得 |
| TMRDirect? | 细胞渗透性 | 555/585 | 否 | 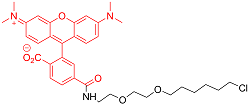 |
| R110Direct? | 细胞渗透性 | 502/527 | 否 | 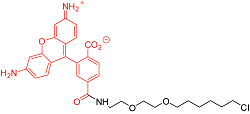 |
表1 用于活细胞成像的Halo-tag配基种类(改自Benink and Urh, 2015)
Table 1 Types of Halo-tag ligands for living cell imaging (modified from Benink and Urh, 2015)
| 配基类别 | 膜渗透性 | 激发/发射波长的峰值(nm) | 是否需要清洗 | 化学结构式 |
|---|---|---|---|---|
| TMR | 细胞渗透性 | 555/585 | 是 |  |
| diAcFAM | 细胞渗透性 | 494/526 | 是 |  |
| Oregon Green | 细胞渗透性 | 496/516 | 是 |  |
| Coumarin | 细胞渗透性 | 353/434 | 是 | 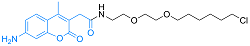 |
| Alexa Fluor? 488 | 非细胞渗透性 | 494/517 | 是 |  |
| Alexa Fluor? 660 | 非细胞渗透性 | 663/690 | 是 | 结构图无法获得 |
| TMRDirect? | 细胞渗透性 | 555/585 | 否 |  |
| R110Direct? | 细胞渗透性 | 502/527 | 否 |  |
| [1] |
邝嘉怡, 李洪清, 沈文锦, 高彩吉 (2021). 基于TurboID的植物蛋白邻近标记实验方法. 植物学报 56, 584-593.
DOI |
| [2] | 李晓娟, 王钦丽, 苏月, 林金星 (2009). 量子点标记及其在植物细胞生物学中的应用. 电子显微学报 28, 495-504. |
| [3] |
钱虹萍, 陈博, 林金星, 崔亚宁 (2021). RNA聚合酶II动态调控及其成像技术的研究进展. 生物技术通报 37, 293-302.
DOI |
| [4] |
钱前, 漆小泉, 林荣呈, 杨淑华, 董爱武, 左建儒, 陈凡, 萧浪涛, 顾红雅, 陈之端, 白永飞, 王小菁, 王雷, 姜里文, 种康, 王台 (2019). 2018年中国植物科学若干领域重要研究进展. 植物学报 54, 405-440.
DOI |
| [5] | 王锋, 高婧, 王宏达 (2014). Halotag多功能标记系统及其在生物学中的应用. 电子显微学报 33, 147-155. |
| [6] |
吴凡, 沈锦波, 胡帅 (2022). 植物ESCRT复合体的功能研究进展. 植物学报 57, 697-712.
DOI |
| [7] | 夏鹏, 窦震, 姚雪彪 (2015). 超高分辨率显微技术研究进展. 生命的化学 35, 430-437. |
| [8] | 张娇, 何勤, 武泽凯, 于斌, 屈军乐, 林丹樱 (2021). 超分辨显微成像技术在活细胞成像中的应用与发展. 生物化学与生物物理进展 48, 1301-1315. |
| [9] |
张原, 张雪萍, 张月倩, 李晓娟 (2022). 单分子荧光检测技术的发展及其在植物生物学研究中的应用. 生物技术通报 38, 33-43.
DOI |
| [10] |
Ai X, Fischer P, Palyha OC, Wisniewski D, Hubbard B, Akinsanya K, Strack AM, Ehrhardt AG (2012). Utilizing HaloTag technology to track the fate of PCSK9 from intracellular vs. extracellular sources. Curr Chem Genomics 6, 38-47.
DOI PMID |
| [11] |
Banaz N, Mäkelä J, Uphoff S (2019). Choosing the right label for single-molecule tracking in live bacteria: side-by- side comparison of photoactivatable fluorescent protein and Halo tag dyes. J Phys D Appl Phys 52, 064002.
DOI URL |
| [12] | Benink HA, Urh M (2015). HaloTag technology for specific and covalent labeling of fusion proteins. In: Gautier A, Hinner MJ, eds. Site-Specific Protein Labeling: Methods and Protocols. New York: Humana. pp. 119-128. |
| [13] |
Blackstock D, Chen W (2014). Halo-tag mediated self- labeling of fluorescent proteins to molecular beacons for nucleic acid detection. Chem Commun 50, 13735-13738.
DOI URL |
| [14] |
Bruchez M Jr, Moronne M, Gin P, Weiss S, Alivisatos AP (1998). Semiconductor nanocrystals as fluorescent biological labels. Science 281, 2013-2016.
PMID |
| [15] |
Cao Y, Dong HF, Pu ST, Zhang XJ (2018). Photoluminescent two-dimensional SiC quantum dots for cellular imaging and transport. Nano Res 11, 4074-4081.
DOI |
| [16] | Charubin K, Streett H, Papoutsakis ET (2020). Development of strong anaerobic fluorescent reporters for Clostridium acetobutylicum and Clostridium ljungdahlii using HaloTag and SNAP-tag proteins. Appl Environ Microbiol 86, e01271-20. |
| [17] |
Chen H, Zhou Z, Li ZY, He XJ, Shen JL (2021). Highly sensitive fluorescent sensor based on coumarin organic dye for pyrophosphate ion turn-on biosensing in synovial fluid. Spectrochim Acta A Mol Biomol Spectrosc 257, 119792.
DOI URL |
| [18] |
Cho WK, Spille JH, Hecht M, Lee C, Li C, Grube V, Cisse II (2018). Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science 361, 412-415.
DOI URL |
| [19] |
Cui YN, Zhang X, Yu M, Zhu YF, Xing JJ, Lin JX (2019). Techniques for detecting protein-protein interactions in living cells: principles, limitations, and recent progress. Sci China Life Sci 62, 619-632.
DOI PMID |
| [20] |
Deprey K, Kritzer JA (2021). HaloTag forms an intramolecular disulfide. Bioconjugate Chem 32, 964-970.
DOI PMID |
| [21] |
England CG, Luo HM, Cai WB (2015). HaloTag technology: a versatile platform for biomedical applications. Bioconjugate Chem 26, 975-986.
DOI PMID |
| [22] |
Erdmann RS, Baguley SW, Richens JH, Wissner RF, Xi ZQ, Allgeyer ES, Zhong S, Thompson AD, Lowe N, Butler R, Bewersdorf J, Rothman JE, St Johnston D, Schepartz A, Toomre D (2019). Labeling strategies matter for super-resolution microscopy: a comparison between HaloTags and SNAP-tags. Cell Chem Biol 26, 584-592.
DOI PMID |
| [23] |
Feng SY, Sekine S, Pessino V, Li H, Leonetti MD, Huang B (2017). Improved split fluorescent proteins for endogenous protein labeling. Nat Commun 8, 370.
DOI PMID |
| [24] |
Fischer S, Ward TR, Liang AD (2021). Engineering a metathesis-catalyzing artificial metalloenzyme based on HaloTag. ACS Catal 11, 6343-6347.
DOI PMID |
| [25] | Grimm JB, Brown TA, English BP, Lionnet T, Lavis LD (2017a). Synthesis of Janelia Fluor HaloTag and SNAP- tag ligands and their use in cellular imaging experiments. In: Erfle H, ed. Super-Resolution Microscopy: Methods and Protocols. New York: Humana. pp. 179-188. |
| [26] |
Grimm JB, English BP, Chen JJ, Slaughter JP, Zhang ZJ, Revyakin A, Patel R, Macklin JJ, Normanno D, Singer RH, Lionnet T, Lavis LD (2015). A general method to improve fluorophores for live-cell and single-molecule microscopy. Nat Methods 12, 244-250.
DOI PMID |
| [27] |
Grimm JB, English BP, Choi H, Muthusamy AK, Mehl BP, Dong P, Brown TA, Lippincott-Schwartz J, Liu Z, Lionnet T, Lavis LD (2016). Bright photoactivatable fluorophores for single-molecule imaging. Nat Methods 13, 985-988.
DOI PMID |
| [28] | Grimm JB, Muthusamy AK, Liang YJ, Brown TA, Lemon WC, Patel R, Lu RW, Macklin JJ, Keller PJ, Ji N, Lavis LD (2017b). A general method to fine-tune fluorophores for live-cell and in vivo imaging. Nat Methods 14, 987-994. |
| [29] |
Guo AY, Zhang YM, Wang L, Bai D, Xu YP, Wu WQ (2021). Single-molecule imaging in living plant cells: a methodological review. Int J Mol Sci 22, 5071.
DOI URL |
| [30] | Halstead JM, Wilbertz JH, Wippich F, Lionnet T, Ephrussi A, Chao JA (2016). TRICK: a single-molecule method for imaging the first round of translation in living cells and animals. Methods Enzymol 572, 123-157. |
| [31] |
Hoelzel CA, Zhang X (2020). Visualizing and manipulating biological processes by using HaloTag and SNAP-Tag technologies. ChemBioChem 21, 1935-1946.
DOI PMID |
| [32] |
Jing YY, Zhou LL, Chen JL, Xu HJ, Sun JY, Cai MJ, Jiang JG, Gao J, Wang HD (2020). Quantitatively mapping the assembly pattern of EpCAM on cell membranes with peptide probes. Anal Chem 92, 1865-1873.
DOI PMID |
| [33] |
Kashapov RR, Bekmukhametova AM, Petrov KA, Nizameev IR, Kadirov MK, Zakharova LY (2018). Supramolecular strategy to construct quantum dot-based sensors for detection of paraoxon. Sens Actuators B Chem 273, 592-599.
DOI URL |
| [34] |
Knight SC, Xie LQ, Deng WL, Guglielmi B, Witkowsky LB, Bosanac L, Zhang ET, El Beheiry M, Masson JB, Dahan M, Liu Z, Doudna JA, Tjian R (2015). Dynamics of CRISPR-Cas9 genome interrogation in living cells. Science 350, 823-826.
DOI PMID |
| [35] |
Lang C, Schulze J, Mendel RR, Hänsch R (2006). HalotagTM: a new versatile reporter gene system in plant cells. J Exp Bot 57, 2985-2992.
DOI URL |
| [36] |
Li CG, Tebo AG, Gautier A (2017a). Fluorogenic labeling strategies for biological imaging. Int J Mol Sci 18, 1473.
DOI URL |
| [37] |
Li K, Hou JT, Yang J, Yu XQ (2017b). A tumor-specific and mitochondria-targeted fluorescent probe for real-time sensing of hypochlorite in living cells. Chem Commun (Camb) 53, 5539-5541.
DOI URL |
| [38] |
Liu H, Dong P, Ioannou MS, Li L, Shea J, Pasolli HA, Grimm JB, Rivlin PK, Lavis LD, Koyama M, Liu Z (2018). Visualizing long-term single-molecule dynamics in vivo by stochastic protein labeling. Proc Natl Acad Sci USA 115, 343-348.
DOI URL |
| [39] |
Liu J, Cui ZQ (2020). Fluorescent labeling of proteins of interest in live cells: beyond fluorescent proteins. Bioconjugate Chem 31, 1587-1595.
DOI PMID |
| [40] |
Liu Y, Miao K, Dunham NP, Liu HB, Fares M, Boal AK, Li XS, Zhang X (2017). The cation-π interaction enables a Halo-tag fluorogenic probe for fast no-wash live cell imaging and gel-free protein quantification. Biochemistry 56, 1585-1595.
DOI PMID |
| [41] | Liu Z, Legant WR, Chen BC, Li L, Grimm JB, Lavis LD, Betzig E, Tjian R (2014). 3D imaging of Sox2 enhancer clusters in embryonic stem cells. eLife 3, e04236. |
| [42] |
Locatelli-Hoops S, Sheen FC, Zoubak L, Gawrisch K, Yeliseev AA (2013). Application of HaloTag technology to expression and purification of cannabinoid receptor CB2. Protein Expr Purif 89, 62-72.
DOI URL |
| [43] |
Los GV, Encell LP, McDougall MG, Hartzell DD, Karassina N, Zimprich C, Wood MG, Learish R, Ohana RF, Urh M, Simpson D, Mendez J, Zimmerman K, Otto P, Vidugiris G, Zhu J, Darzins A, Klaubert DH, Bulleit RF, Wood KV (2008). HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem Biol 3, 373-382.
DOI PMID |
| [44] |
Minner-Meinen R, Weber JN, Albrecht A, Matis R, Behnecke M, Tietge C, Frank S, Schulze J, Buschmann H, Walla PJ, Mendel RR, Hänsch R, Kaufholdt D (2021). Split-HaloTag imaging assay for sophisticated microscopy of protein-protein interactions in planta. Plant Commun 2, 100212.
DOI URL |
| [45] |
Ovečka M, Sojka J, Tichá M, Komis G, Basheer J, Marchetti C, Šamajová O, Kuběnová L, Šamaj J (2022). Imaging plant cells and organs with light-sheet and super-resolution microscopy. Plant Physiol 188, 683-702.
DOI PMID |
| [46] |
Pulsipher A, Griffin ME, Stone SE, Hsieh-Wilson LC (2015). Long-lived engineering of glycans to direct stem cell fate. Angew Chem Int Ed 54, 1466-1470.
DOI PMID |
| [47] |
Rasheed T, Li CL, Zhang YL, Nabeel F, Peng JX, Qi J, Gong LD, Yu CY (2018). Rhodamine-based multianalyte colorimetric probe with potentialities as on-site assay kit and in biological systems. Sens Actuators B Chem 258, 115-124.
DOI URL |
| [48] | Rodermund L, Coker H, Oldenkamp R, Wei GF, Bowness J, Rajkumar B, Nesterova T, Susano Pinto DM, Schermelleh L, Brockdorff N (2021). Time-resolved structured illumination microscopy reveals key principles of Xist RNA spreading. Science 372, eabe7500. |
| [49] |
Shao SP, Xue BX, Sun YJ (2018). Intranucleus single- molecule imaging in living cells. Biophys J 115, 181-189.
DOI URL |
| [50] |
Shao SP, Zhang HC, Zeng Y, Li YL, Sun CY, Sun YJ (2021). TagBiFC technique allows long-term single-molecule tracking of protein-protein interactions in living cells. Commun Biol 4, 378.
DOI PMID |
| [51] | Shao SP, Zhang WW, Hu H, Xue BX, Qin JS, Sun CY, Sun YA, Wei WS, Sun YJ (2016). Long-term dual-color tracking of genomic loci by modified sgRNAs of the CRISPR/Cas9 system. Nucleic Acids Res 44, e86. |
| [52] |
Shen WW, Ma LY, Zhang X, Li XX, Zhao YY, Jing YP, Feng Y, Tan XK, Sun F, Lin JX (2020). Three-dimensional reconstruction of Picea wilsonii Mast. pollen grains using automated electron microscopy. Sci China Life Sci 63, 171-179.
DOI |
| [53] |
Simpson LM, Macartney TJ, Nardin A, Fulcher LJ, Röth S, Testa A, Maniaci C, Ciulli A, Ganley IG, Sapkota GP (2020). Inducible degradation of target proteins through a tractable affinity-directed protein missile system. Cell Chem Biol 27, 1164-1180.
DOI PMID |
| [54] | Teng IT, Bu XN, Chung I (2019). Conjugation of fab' fragments with fluorescent dyes for single-molecule tracking on live cells. Bio Protoc 9, e3375. |
| [55] |
Verschueren KHG, Seljée F, Rozeboom HJ, Kalk KH, Dijkstra BW (1993). Crystallographic analysis of the catalytic mechanism of haloalkane dehalogenase. Nature 363, 693-698.
DOI |
| [56] |
Wang ZM, Detomasi TC, Chang CJ (2021). A dual-fluorophore sensor approach for ratiometric fluorescence imaging of potassium in living cells. Chem Sci 12, 1720-1729.
DOI URL |
| [57] |
Wilhelm J, Kühn S, Tarnawski M, Gotthard G, Tünnermann J, Tänzer T, Karpenko J, Mertes N, Xue L, Uhrig U, Reinstein J, Hiblot J, Johnsson K (2021). Kinetic and structural characterization of the self-labeling protein tags HaloTag7, SNAP-tag, and CLIP-tag. Biochemistry 60, 2560-2575.
DOI PMID |
| [58] |
Yagi N, Yoshinari A, Iwatate RJ, Isoda R, Frommer WB, Nakamura M (2021). Advances in synthetic fluorescent probe labeling for live-cell imaging in plants. Plant Cell Physiol 62, 1259-1268.
DOI URL |
| [59] |
Yan Q, Bruchez MP (2015). Advances in chemical labeling of proteins in living cells. Cell Tissue Res 360, 179-194.
DOI PMID |
| [60] | Yazaki J, Kawashima Y, Ogawa T, Kobayashi A, Okoshi M, Watanabe T, Yoshida S, Kii I, Egami S, Amagai M, Hosoya T, Shiroguchi K, Ohara O (2020). HaloTag- based conjugation of proteins to barcoding-oligonucleotides. Nucleic Acids Res 48, e8. |
| [61] |
Zhang X, Man Y, Zhuang XH, Shen JB, Zhang Y, Cui YN, Yu M, Xing JJ, Wang GC, Lian N, Hu ZJ, Ma LY, Shen WW, Yang SY, Xu HM, Bian JH, Jing YP, Li XJ, Li RL, Mao TL, Jiao YL, Sodmergen, Ren HY, Lin JX (2021). Plant multiscale networks: charting plant connectivity by multi-level analysis and imaging techniques. Sci China Life Sci 64, 1392-1422.
DOI PMID |
| [62] |
Zhang Y, Lu YQ, El Sayyed H, Bian JH, Lin JX, Li XJ (2022). Transcription factor dynamics in plants: insights and technologies for in vivo imaging. Plant Physiol 189, 23-36.
DOI PMID |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||