

植物学报 ›› 2021, Vol. 56 ›› Issue (6): 761-773.DOI: 10.11983/CBB21054 cstr: 32102.14.CBB21054
• 专题论坛 • 上一篇
收稿日期:2021-03-25
接受日期:2021-06-18
出版日期:2021-11-01
发布日期:2021-11-12
通讯作者:
王沛
作者简介:* E-mail: wangpei@swun.edu.cn基金资助:
Xin Liu, Pei Wang( ), Qingping Zhou
), Qingping Zhou
Received:2021-03-25
Accepted:2021-06-18
Online:2021-11-01
Published:2021-11-12
Contact:
Pei Wang
摘要: 根是植物吸收水分和矿质营养以维持生命活动的重要器官。根系的构型和超微结构具有物种特异性, 对水分和矿质营养的吸收有不同程度的影响。其中, 内、外皮层的木栓层和凯氏带是2种重要的质外体屏障, 可非定向地阻断水分和离子运输, 在植物生长发育及响应逆境胁迫中发挥重要作用。尽管如此, 植物根系质外体屏障的结构、化学组成、生理功能、生物合成及其调控仅在模式植物拟南芥(Arabidopsis thaliana)中被广泛研究。近年来, 关于作物大麦(Hordeum vulgare)、水稻(Oryza sativa)以及部分牧草的根系质外体屏障研究报道逐渐增多。该文系统比较了拟南芥、大麦、水稻以及部分牧草根系质外体屏障的异同, 提出今后的研究方向, 以期为深入探索禾本科作物和牧草根系质外体屏障在生长发育和逆境适应中的作用奠定理论基础, 并为作物和牧草育种工作提供新思路。
刘鑫, 王沛, 周青平. 植物根系质外体屏障研究进展. 植物学报, 2021, 56(6): 761-773.
Xin Liu, Pei Wang, Qingping Zhou. Research Progress on Apoplast Barriers of Plant Roots. Chinese Bulletin of Botany, 2021, 56(6): 761-773.
| 物种 | 含量单位 | 脂肪酸 | 醇 | ω-羟基酸 | α,ω-二酸 | 脂肪族 | 芳香族 | 总木栓质 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 拟南芥 | µg∙cm-2 | 0.18 | 0.14 | 0.65 | 0.32 | 1.30 | - | - | Ranathunge and Schreiber, |
| µg∙mg-1 DR | 2.04 | 0.81 | 3.68 | 1.46 | 7.99 | 0.23 | 8.22 | Wang et al., | |
| µg∙mg-1 DW | 2.66 | 1.86 | 11.70 | 5.59 | 21.81 | 0.40 | 22.21 | Baxter et al., | |
| 大麦 | µg∙cm-2 | 1.90 | 0.30 | 3.20 | 1.15 | 6.55 | 9.55 | 16.10 | Ranathunge et al., |
| 水稻(IR64) | µg∙cm-2 | 6.12 | 1.23 | 6.69 | 2.89 | 17.82 | 154.29 | 172.11 | Kreszies et al., Schreiber et al., |
| µg∙mg-1 DW | 3.30 | 1.25 | 7.20 | 1.55 | 14.19 | 110.00 | 124.19 | ||
| 小花碱茅 | µg∙mg-1 DR | 0.22 | 0.06 | 2.14 | 0.29 | 2.71 | 7.47 | 10.18 | 杨海莉, |
表1 不同植物中木栓质化学组分和含量对比
Table 1 Comparison of chemical constituents and contents of suberin in different plant species
| 物种 | 含量单位 | 脂肪酸 | 醇 | ω-羟基酸 | α,ω-二酸 | 脂肪族 | 芳香族 | 总木栓质 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 拟南芥 | µg∙cm-2 | 0.18 | 0.14 | 0.65 | 0.32 | 1.30 | - | - | Ranathunge and Schreiber, |
| µg∙mg-1 DR | 2.04 | 0.81 | 3.68 | 1.46 | 7.99 | 0.23 | 8.22 | Wang et al., | |
| µg∙mg-1 DW | 2.66 | 1.86 | 11.70 | 5.59 | 21.81 | 0.40 | 22.21 | Baxter et al., | |
| 大麦 | µg∙cm-2 | 1.90 | 0.30 | 3.20 | 1.15 | 6.55 | 9.55 | 16.10 | Ranathunge et al., |
| 水稻(IR64) | µg∙cm-2 | 6.12 | 1.23 | 6.69 | 2.89 | 17.82 | 154.29 | 172.11 | Kreszies et al., Schreiber et al., |
| µg∙mg-1 DW | 3.30 | 1.25 | 7.20 | 1.55 | 14.19 | 110.00 | 124.19 | ||
| 小花碱茅 | µg∙mg-1 DR | 0.22 | 0.06 | 2.14 | 0.29 | 2.71 | 7.47 | 10.18 | 杨海莉, |
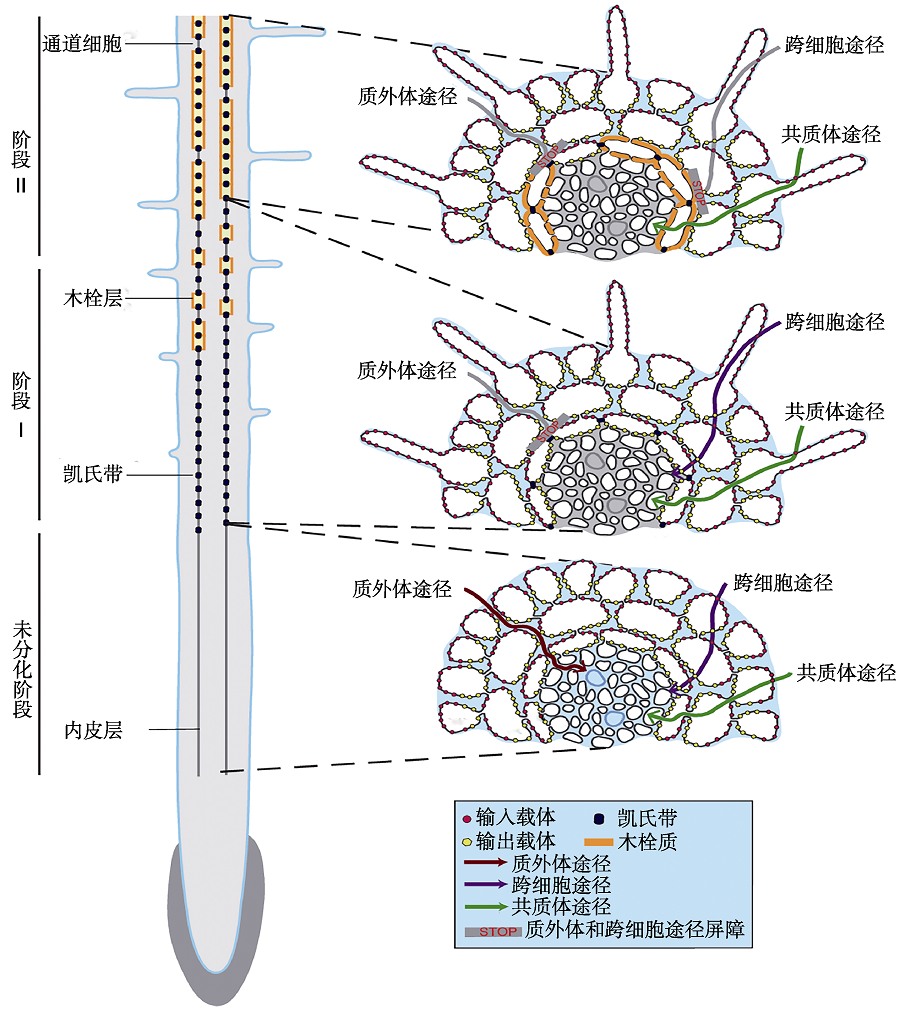
图1 根系质外体屏障的形成及其对根系养分径向运输途径的影响示意图(改自Andersen et al., 2015) 在内皮层未分化阶段, 3种径向运输途径(质外体途径、共质体途径和跨细胞途径)都存在。当发育到阶段I时, 凯氏带形成阻断养分的质外体屏障。当发育到阶段II时, 在次生细胞壁上会沉积木栓质, 凯氏带和木栓层形成阻断养分的质外体屏障和跨细胞屏障。
Figure 1 Schematic view of the formation of root apoplastic barriers and the effects of the radial transport of nutrients in roots (modified from Andersen et al., 2015) In the undifferentiated endodermis, three radial transport pathways (apoplastic, symplastic and coupled trans-cellular) were observed. In stage I, the formation of the Casparian strip blocks the apoplastic pathway of nutrients. In stage II, suberin lamellae was deposited on the secondary cell wall, and the formation of Casparian strip and suberin lamellae blocks the apoplastic pathway and coupled trans-cellular pathway of nutrients.
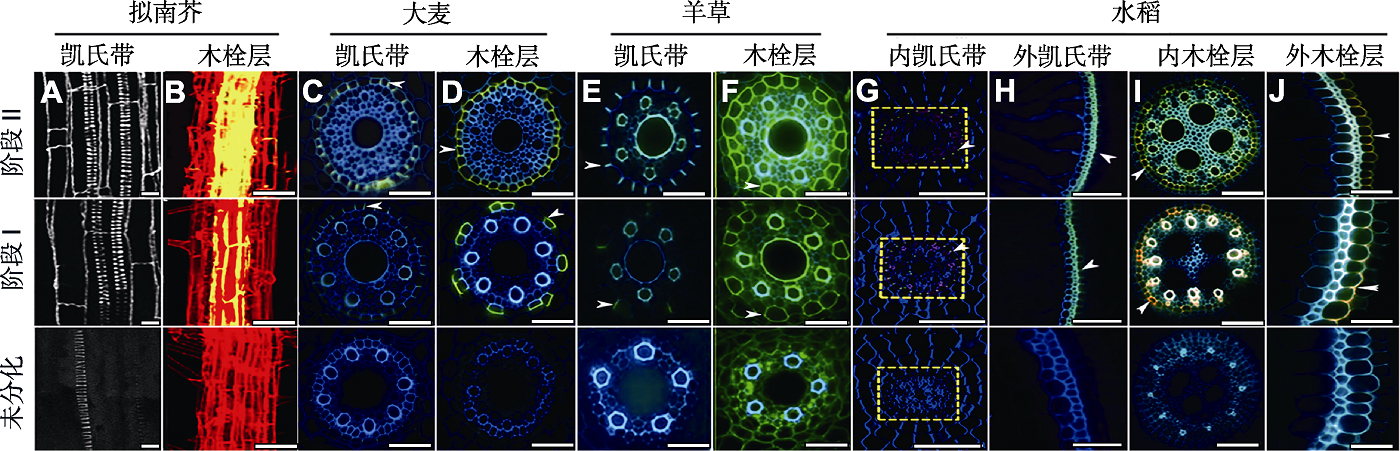
图2 不同植物根系质外体屏障的超微结构(Ranathunge et al., 2003, 2011a; Lee et al., 2013; Kreszies et al., 2019; Wang et al., 2019; Li et al., 2020; Cohen et al., 2020) (A), (B) 拟南芥初生根的悬置荧光及染色 (A) 拟南芥根凯氏带, 在不同发育阶段, 凯氏带网状结构的自发荧光; 图示从表面到内部纵向的多层共聚焦图像投影, 显示凯氏带的网状结构, 明亮的荧光螺旋结构是木质部导管; (B) 激光共聚焦扫描显微图像, 显示拟南芥根轴上木栓层的积累模式(Z投影), 根细胞壁用碘化丙啶(PI, 红色)突出显示, 而木栓质(黄色)用荧光黄(FY)染色并进行组织化学检测; (C) 大麦根凯氏带, 用小檗碱-苯胺蓝染色, 在阶段II出现完整的绿色荧光, 标志凯氏带完整形成(箭头); (D) FY088染色在阶段II出现完整的黄色环, 表明大麦木栓层完整形成(箭头); (E), (F) 羊草根系凯氏带和木栓层的形成 (E) 用半硫酸小檗碱染色的凯氏带(亮黄蓝色信号, 箭头); (F) FY088染色的木栓层沉积(强烈的黄色信号, 箭头); (G)-(J) 水稻内、外皮层凯氏带及内、外皮层木栓层的形成 (G) 用抗绿色荧光蛋白(GFP)抗体(1:1 000稀释)染色, 绿色荧光显示内皮层凯氏带的形成(箭头); (H) 用小檗碱-苯胺蓝染色, 黄绿色荧光示外皮层凯氏带完整形成(箭头); (I), (J) FY088染色, 黄绿色荧光示木栓层的形成(箭头)。(A), (B) Bars=10 μm; (C)-(F), (H)-(J) Bars=50 μm; (G) Bars=100 μm
Figure 2 Ultrastructure of the root apoplast barriers in different plants (Ranathunge et al., 2003, 2011a; Lee et al., 2013; Kreszies et al., 2019; Wang et al., 2019; Li et al., 2020; Cohen et al., 2020) (A), (B) Suspension fluorescence and staining of primary roots of Arabidopsis thaliana (A) In Arabidopsis thaliana roots, the reticulum of the Casparian strip shows autofluorescence at different developmental stages; the image is a longitudinal, confocal image projection from the surface to the center that visualizes the network structure of the Casparian strip, the bright fluorescent spiral structure of the xylem conduit; (B) Confocal laser scanning micrograph shows the accumulation pattern of suberin lamellae on the root axis of Arabidopsis thaliana (Z projection), the root cell wall is high-lighted with propidium iodide (PI, red), the suberin (yellow) was detected by histochemistry with fluorescence yellow (FY) staining; (C) Barley root Casparian strip, dyeing in berberine-aniline blue at stage II appeared a complete green fluorescent Casparian strip complete form (arrows); (D) FY088 dyeing can appear complete yellow ring in stage II, showed that barley suberin lamellae formed complete (arrows); (E), (F) Formation of Casparian strip and suberin lamellae of Leymus chinensis root system (E) Casparian strip stained with berberine semisulfate (bright yellow blue signal, arrows); (F) Suberin lamellae deposits stained with FY088 (strong yellow signal, arrows); (G)-(J) The formation of the endodermis and exodermis Casparian strip, the endodermis and exodermis suberin lamellae of rice (G) Stained with anti-GFP antibody (1:1 000 dilution), and green fluorescence indicated the formation of the endodermis Casparian strip (arrows); (H) Berberine-aniline blue stain, and the yellow-green fluorescence indicates the complete formation of the exodermis Casparian strip (arrows); (I), (J) Stained with FY088, and the yellow-green fluorescence indicates the formation of the suberin lamellae (arrows). (A), (B) Bars=10 μm; (C)-(F), (H)-(J) Bars=50 μm; (G) Bars=100 μm
| [1] | 崔亚宁, 满奕, 宋程威, 张曦, 钱虹萍, 林金星 (2020). 植物凯氏带化学成分、生理功能及相关调控机制的研究进展. 中国科学: 生命科学 50, 102-110. |
| [2] |
付言钊, 杨仁秀, 王桂萍, 王春春, 牛立元, 沈振国 (2010). 大豆初生根凯氏带对铜离子的通透性. 植物学报 45, 198-204.
DOI |
| [3] |
韩雪源, 茅林春 (2017). 木栓质组成成分、组织化学特性及其生物合成研究进展. 植物学报 52, 358-374.
DOI |
| [4] | 陆静梅, 李建东 (1994). 同种不同生态环境植物解剖结构比较研究. 东北师大学报(自然科学版) (3), 100-103. |
| [5] | 罗文巧 (2018). 二穗短柄草根中木栓质脂肪醇合成基因的克隆与功能验证. 硕士论文. 杨凌: 西北农林科技大学. pp. 2-10. |
| [6] | 石英, 韩毅强, 郑殿峰, 冯乃杰, 刘涛 (2015). 赤霉素对拟南芥主根分生区和伸长区的调控. 植物生理学报 51, 21-28. |
| [7] | 王平, 周青平, 王沛 (2019). 植物内皮层的分化及其屏障功能研究进展. 西北植物学报 39, 752-762. |
| [8] | 许智宏, 薛红卫 (2012). 植物激素作用的分子机理. 上海: 上海科学技术出版社. pp. 296-298. |
| [9] | 杨朝东, 李守峰, 姚兰, 艾训儒, 蔡小东, 张霞 (2015). 天胡荽的解剖和屏障结构特征研究. 草业学报 24(7), 139-145. |
| [10] | 杨朝东, 张霞, 刘国锋, 张俊卫, 包满珠, 周志翔 (2013). 植物根中质外体屏障结构和生理功能研究进展. 植物研究 33, 114-119. |
| [11] | 杨海莉 (2019). 小花碱茅对渗透胁迫与等渗透势盐胁迫的生理响应. 硕士论文. 兰州: 兰州大学. pp. 45-50. |
| [12] | 赵金花 (2001). 小麦族根茎型牧草形态、解剖学比较研究. 硕士论文. 呼和浩特: 内蒙古农业大学. pp. 5-13. |
| [13] | 朱宇旌, 张勇, 胡自治, 阎顺国 (2001). 小花碱茅根适应盐胁迫的显微结构研究. 中国草地 23, 37-40. |
| [14] |
Alassimone J, Naseer S, Geldner N (2010). A developmental framework for endodermal differentiation and polarity. Proc Natl Acad Sci USA 107, 5214-5219.
DOI URL |
| [15] |
Alassimone J, Roppolo D, Geldner N, Vermeer JEM (2012). The endodermis-development and differentiation of the plant's inner skin. Protoplasma 249, 433-443.
DOI PMID |
| [16] |
Aloni R, Enstone DE, Peterson CA (1998). Indirect evidence for bulk water flow in root cortical cell walls of three dicotyledonous species. Planta 207, 1-7.
DOI URL |
| [17] |
Andersen TG, Barberon M, Geldner N (2015). Suberization-the second life of an endodermal cell. Curr Opin Plant Biol 28, 9-15.
DOI PMID |
| [18] |
Armstrong W, Cousins D, Armstrong J, Turner DW, Beckett PM (2000). Oxygen distribution in wetland plant roots and permeability barriers to gas-exchange with the rhizosphere: a microelectrode and modelling study with Phragmites australis. Ann Bot 86, 687-703.
DOI URL |
| [19] |
Barberon M, Vermeer JEM, De Bellis D, Wang P, Naseer S, Andersen TG, Humbel BM, Nawrath C, Takano J, Salt DE, Geldner N (2016). Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell 164, 447-459.
DOI PMID |
| [20] | Baxter I, Hosmani PS, Rus A, Lahner B, Borevitz JO, Muthukumar B, Mickelbart MV, Schreiber L, Franke RB, Salt DE (2009). Root suberin forms an extracellular barrier that affects water relations and mineral nutrition in Arabidopsis. PLoS Genet 5, e1000492. |
| [21] |
Bernards MA (2002). Demystifying suberin. Can J Bot 80, 227-240.
DOI URL |
| [22] | Cabane M, Afif D, Hawkins S (2012). Lignins and abiotic stresses. Adv Bot Res 61, 219-262. |
| [23] |
Cai X, Chen T, Zhou QY, Xu L, Qu LQ, Hua XJ, Lin JX (2011). Development of Casparian strip in rice cultivars. Plant Signal Behav 6, 59-65.
DOI URL |
| [24] | Caspary R (1865). Bemerkungen Über die Schutzscheide und die Bildung des Stammes und der Wurzel. Jahrb Wiss Bot 4, 101-124. |
| [25] |
Cohen H, Fedyuk V, Wang CH, Wu S, Aharoni A (2020). SUBERMAN regulates developmental suberization of the Arabidopsis root endodermis. Plant J 102, 431-447.
DOI URL |
| [26] |
Colmer TD, Gibberd MR, Wiengweera A, Tinh TK (1998). The barrier to radial oxygen loss from roots of rice (Oryza sativa L.) is induced by growth in stagnant solution. J Exp Bot 49, 1431-1436.
DOI URL |
| [27] |
Doblas VG, Geldner N, Barberon M (2017). The endodermis, a tightly controlled barrier for nutrients. Curr Opin Plant Biol 39, 136-143.
DOI URL |
| [28] |
Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B (1993). Cellular organisation of the Arabidopsis thaliana root. Development 119, 71-84.
PMID |
| [29] |
Enstone DE, Peterson CA, Ma FS (2002). Root endodermis and exodermis: structure, function, and responses to the environment. J Plant Growth Regul 21, 335-351.
DOI URL |
| [30] |
Fiscus EL (1986). Diurnal changes in volume and solute transport coefficients of Phaseolus roots. Plant Physiol 80, 752-759.
PMID |
| [31] |
Franke R, Schreiber L (2007). Suberin-a biopolyester forming apoplastic plant interfaces. Curr Opin Plant Biol 10, 252-259.
DOI URL |
| [32] |
Geldner N (2013). The endodermis. Annu Rev Plant Biol 64, 531-558.
DOI PMID |
| [33] |
Graça J (2015). Suberin: the biopolyester at the frontier of plants. Front Chem 3, 62
DOI PMID |
| [34] |
Höfer R, Briesen I, Beck M, Pinot F, Schreiber L, Franke R (2008). The Arabidopsis cytochrome P450 CYP86A1 encodes a fatty acid ω-hydroxylase involved in suberin monomer biosynthesis. J Exp Bot 59, 2347-2360.
DOI PMID |
| [35] |
Hose E, Clarkson DT, Steudle E, Schreiber L, Hartung W (2001). The exodermis: a variable apoplastic barrier. J Exp Bot 52, 2245-2264.
PMID |
| [36] |
Huang L, Li WC, Tam NFY, Ye ZH (2019). Effects of root morphology and anatomy on cadmium uptake and translocation in rice (Oryza sativa L.). J Environ Sci 75, 296-306.
DOI URL |
| [37] |
Kim YX, Ranathunge K, Lee S, Lee Y, Lee D, Sung J (2018). Composite transport model and water and solute transport across plant roots: an update. Front Plant Sci 9, 193.
DOI URL |
| [38] |
Knipfer T, Fricke W (2010). Root pressure and a solute reflection coefficient close to unity exclude a purely apoplastic pathway of radial water transport in barley (Hordeum vulgare). New Phytol 187, 159-170.
DOI URL |
| [39] |
Knipfer T, Fricke W (2011). Water uptake by seminal and adventitious roots in relation to whole-plant water flow in barley (Hordeum vulgare L.). J Exp Bot 62, 717-733.
DOI URL |
| [40] |
Kotula L, Ranathunge K, Schreiber L, Steudle E (2009). Functional and chemical comparison of apoplastic barriers to radial oxygen loss in roots of rice (Oryza sativa L.) grown in aerated or deoxygenated solution. J Exp Bot 60, 2155-2167.
DOI URL |
| [41] | Kramer PJ, Boyer JS (1996). Water Relations of Plants and Soils. San Diego: Academic Press. pp. 183-225. |
| [42] |
Kreszies T, Schreiber L, Ranathunge K (2018). Suberized transport barriers in Arabidopsis, barley and rice roots: from the model plant to crop species. J Plant Physiol 227, 75-83.
DOI URL |
| [43] |
Kreszies T, Shellakkutti N, Osthoff A, Yu P, Baldauf JA, Zeisler-Diehl VV, Ranathunge K, Hochholdinger F, Schreiber L (2019). Osmotic stress enhances suberization of apoplastic barriers in barley seminal roots: analysis of chemical, transcriptomic and physiological responses. New Phytol 221, 180-194.
DOI PMID |
| [44] |
Krishnamurthy P, Ranathunge K, Nayak S, Schreiber L, Mathew MK (2011). Root apoplastic barriers block Na+ transport to shoots in rice (Oryza sativa L.). J Exp Bot 62, 4215-4228.
DOI PMID |
| [45] |
Lanoue A, Burlat V, Henkes GJ, Koch I, Schurr U, Röse USR (2010). De novo biosynthesis of defense root exudates in response to Fusarium attack in barley. New Phytol 185, 577-588.
DOI URL |
| [46] |
Lee Y, Rubio MC, Alassimone J, Geldner N (2013). A mechanism for localized lignin deposition in the endodermis. Cell 153, 402-412.
DOI URL |
| [47] |
Li LY, Pan SR, Melzer R, Fricke W (2020). Apoplastic barriers, aquaporin gene expression and root and cell hydraulic conductivity in phosphate-limited sheepgrass plants. Physiol Planta 168, 118-132.
DOI URL |
| [48] |
Liesche J, Martens HJ, Schulz A (2011). Symplasmic transport and phloem loading in gymnosperm leaves. Protoplasma 248, 181-190.
DOI URL |
| [49] |
Lulai EC, Corsini DL (1998). Differential deposition of suberin phenolic and aliphatic domains and their roles in resistance to infection during potato tuber (Solanum tuberosum L.) wound-healing. Physiol Mol Plant Pathol 53, 209-222.
DOI URL |
| [50] |
Lupoi JS, Singh S, Parthasarathi R, Simmons BA, Henry RJ (2015). Recent innovations in analytical methods for the qualitative and quantitative assessment of lignin. Renew Sustain Energy Rev 49, 871-906.
DOI URL |
| [51] |
Ma JF, Yamaji N, Mitani N, Tamai K, Konishi S, Fujiwara T, Katsuhara M, Yano M (2007). An efflux transporter of silicon in rice. Nature 448, 209-212.
DOI URL |
| [52] |
Miyamoto N, Steudle E, Hirasawa T, Lafitte R (2001). Hydraulic conductivity of rice roots. J Exp Bot 52, 1835-1846.
PMID |
| [53] |
Naseer S, Lee Y, Lapierre C, Franke R, Nawrath C, Geldner N (2012). Casparian strip diffusion barrier in Arabidopsis is made of a lignin polymer without suberin. Proc Natl Acad Sci USA 109, 10101-10106.
DOI URL |
| [54] | Nawrath C, Schreiber L, Franke RB, Geldner N, Reina-Pinto J, Kunst L (2013). Apoplastic diffusion barriers in Arabidopsis. Arabidopsis Book 11, e0167. |
| [55] |
Neil E, Robbins II, Trontin C, Duan L, Dinneny JR (2014). Beyond the barrier: communication in the root through the endodermis. Plant Physiol 166, 551-559.
DOI PMID |
| [56] | Nobel PS (2009). Physicochemical and Environmental Plant Physiology. Amsterdam: Elsevier. pp. 325-339. |
| [57] |
Pollard M, Beisson F, Li YH, Ohlrogge JB (2008). Building lipid barriers: biosynthesis of cutin and suberin. Trends Plant Sci 13, 236-246.
DOI PMID |
| [58] | Ranathunge K, Kim YX, Wassmann F, Kreszies T, Zeisler V, Schreiber L (2017). The composite water and solute transport of barley (Hordeum vulgare) roots: effect of suberized barriers. Ann Bot 119, 629-643. |
| [59] |
Ranathunge K, Lin JX, Steudle E, Schreiber L (2011a). Stagnant deoxygenated growth enhances root suberization and lignifications, but differentially affects water and NaCl permeabilities in rice (Oryza sativa L.) roots. Plant Cell Environ 34, 1223-1240.
DOI URL |
| [60] |
Ranathunge K, Schreiber L (2011). Water and solute permeabilities of Arabidopsis roots in relation to the amount and composition of aliphatic suberin. J Exp Bot 62, 1961-1974.
DOI PMID |
| [61] |
Ranathunge K, Schreiber L, Franke R (2011b). Suberin research in the genomics era-new interest for an old polymer. Plant Sci 180, 399-413.
DOI URL |
| [62] |
Ranathunge K, Steudle E, Lafitte R (2003). Control of water uptake by rice (Oryza sativa L.): role of the outer part of the root. Planta 217, 193-205.
PMID |
| [63] |
Ranathunge K, Steudle E, Lafitte R (2005). A new precipitation technique provides evidence for the permeability of Casparian bands to ions in young roots of corn (Zea mays L.) and rice (Oryza sativa L.). Plant Cell Environ 28, 1450-1462.
DOI URL |
| [64] |
Raven JA, Edwards D (2001). Roots: evolutionary origins and biogeochemical significance. J Exp Bot 52, 381-401.
PMID |
| [65] |
Reina JJ, Domínguez E, Heredia A (2001). Water sorption-desorption in conifer cuticles: the role of lignin. Physiol Plant 112, 372-378.
PMID |
| [66] |
Robbins II NE, Trontin C, Duan LN, Dinneny JR (2014). Beyond the barrier: communication in the root through the endodermis. Plant Physiol 166, 551-559.
DOI PMID |
| [67] |
Robert D,Warmbrodt (1985). Studies on the root of Hordeum vulgare L. ultrastructure of the seminal root with special reference to the phloem. Am J Bot 72, 414-432.
DOI URL |
| [68] |
Salas-González I, Reyt G, Flis P, Custódio V, Gopaulchan D, Bakhoum N, Dew TP, Suresh K, Franke RB, Dangl JL, Salt DE, Castrillo G (2021). Coordination between microbiota and root endodermis supports plant mineral nutrient homeostasis. Science 371, eabd0695.
DOI URL |
| [69] | Schaaf G, Honsbein A, Meda AR, Kirchner S, Wipf D, Von Wirén N (2006). AtIREG2 encodes a tonoplast transport protein involved in iron-dependent nickel detoxification in Arabidopsis thaliana roots. J Exp Bot 281, 25532-25540. |
| [70] |
Schreiber L (2010). Transport barriers made of cutin, suberin and associated waxes. Trends Plant Sci 15, 546-553.
DOI PMID |
| [71] |
Schreiber L, Franke R, Hartmann KD, Ranathunge K, Steudle E (2005). The chemical composition of suberin in apoplastic barriers affects radial hydraulic conductivity differently in the roots of rice (Oryza sativa L. cv. ‘IR64') and corn (Zea mays L. cv. ‘Helix'). J Exp Bot 56, 1427-1436.
PMID |
| [72] | Schreiber L, Hartmann K, Skrabs M, Zeier J (1999). Apoplastic barriers in roots: chemical composition of endodermal and hypodermal cell walls. J Exp Bot 50, 1267-1280. |
| [73] |
Soukup A, Votrubová O, Čížková H (2002). Development of anatomical structure of roots of Phragmites australis. New Phytol 153, 277-287.
DOI URL |
| [74] | Steudle E (1993). Pressure probe techniques: basic principles and application to studies of water and solute relations at the cell, tissue and organ level. In: Smith JAC, Grif fiths H, eds. Water Deficits: Plant Responses from Cell to Community. Oxford: BIOS Scientific Publishers. pp. 5-36. |
| [75] |
Steudle E (2000a). Water uptake by plant roots: an integration of views. Plant Soil 226, 45-56.
DOI URL |
| [76] |
Steudle E (2000b). Water uptake by roots: effects of water deficit. J Exp Bot 51, 1531-1542.
DOI URL |
| [77] |
Steudle E, Brinckmann E (1989). The osmometer model of the root: water and solute relations of roots of Phaseolus coccineus. Bot Acta 102, 85-95.
DOI URL |
| [78] |
Steudle E, Jeschke WD (1983). Water transport in barley roots. Measurements of root pressure and hydraulic conductivity of roots in parallel with turgor and hydraulic conductivity of root cells. Planta 158, 237-248.
DOI PMID |
| [79] |
Steudle E, Oren R, Schulze ED (1987). Water transport in maize roots: measurement of hydraulic conductivity, solute permeability, and of reflection coefficients of excised roots using the root pressure probe. Plant Physiol 84, 1220-1232.
PMID |
| [80] | Steudle E, Peterson CA (1998). How does water get through roots? J Exp Bot 49, 775-788. |
| [81] |
Suku S, Knipfer T, Fricke W (2014). Do root hydraulic properties change during the early vegetative stage of plant development in barley (Hordeum vulgare)? Ann Bot 113, 385-402.
DOI URL |
| [82] |
Thomas R, Fang XX, Ranathunge K, Anderson TR, Peterson CA, Bernards MA (2007). Soybean root suberin: anatomical distribution, chemical composition, and relationship to partial resistance to Phytophthora sojae. Plant Physiol 144, 299-311.
DOI URL |
| [83] |
Van Fleet DS (1961). Histochemistry and function of the endodermis. Bot Rev 27, 165-220.
DOI URL |
| [84] |
Vishwanath SJ, Delude C, Domergue F, Rowland O (2015). Suberin: biosynthesis, regulation, and polymer assembly of a protective extracellular barrier. Plant Cell Rep 34, 573-586.
DOI PMID |
| [85] |
Wang P, Wang CM, Gao L, Cui YN, Yang HL, De Silva NDG, Ma Q, Bao AK, Flowers TJ, Rowland O, Wang SM (2020). Aliphatic suberin confers salt tolerance to Arabidopsis by limiting Na+ influx, K+ efflux and water backflow. Plant Soil 448, 603-620.
DOI URL |
| [86] | Wang ZG, Yamaji N, Huang S, Zhang X, Shi MX, Fu S, Yang GZ, Ma JF, Xia JX (2019). OsCASP1 is required for Casparian strip formation endodermal cells of rice roots for selective uptake of mineral elements. Plant Cell 31, 2636-2648. |
| [87] |
Zeier J, Schreiber L (1998). Comparative investigation of primary and tertiary endodermal cell walls isolated from the roots of five monocotyledoneous species: chemical composition in relation to fine structure. Planta 206, 349-361.
DOI URL |
| [88] |
Zimmermann HM, Hartmann K, Schreiber L, Steudle E (2000). Chemical composition of apoplastic transport barriers in relation to radial hydraulic conductivity of corn roots (Zea mays L.). Planta 210, 302-311.
PMID |
| [89] |
Zimmermann HM, Steudle E (1998). Apoplastic transport across young maize roots: effect of the exodermis. Planta 206, 7-19.
DOI URL |
| [1] | 张标, 吴健, 张杨, 董小卫, 韩硕, 高昕, 杜从伍, 李慧英, 种学法, 朱莹莹, 刘海伟. 木栓层在水和溶质运输中的生理功能研究进展[J]. 植物学报, 2023, 58(6): 1008-1018. |
| [2] | 付言钊;杨仁秀;王桂萍;王春春;牛立元;沈振国*. 大豆初生根凯氏带对铜离子的通透性[J]. 植物学报, 2010, 45(02): 198-204. |
| [3] | 吴小琴 朱锦懋 王钦丽 胡玉熹 林金星. 植物凯民带的研究进展[J]. 植物学报, 2002, 19(03): 302-309. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||