

植物学报 ›› 2019, Vol. 54 ›› Issue (3): 396-404.DOI: 10.11983/CBB18099 cstr: 32102.14.CBB18099
• 专题论坛 • 上一篇
单婷婷1,陈晓梅1,*( ),郭顺星1,*(
),郭顺星1,*( ),田丽霞1,严林2,王欣1
),田丽霞1,严林2,王欣1
收稿日期:2018-04-16
接受日期:2018-10-06
出版日期:2019-05-01
发布日期:2019-11-24
通讯作者:
陈晓梅,郭顺星
基金资助:
Tingting Shan1,Xiaomei Chen1,*( ),Shunxing Guo1,*(
),Shunxing Guo1,*( ),Lixia Tian1,Lin Yan2,Xin Wang1
),Lixia Tian1,Lin Yan2,Xin Wang1
Received:2018-04-16
Accepted:2018-10-06
Online:2019-05-01
Published:2019-11-24
Contact:
Xiaomei Chen,Shunxing Guo
摘要: 鞘脂是细胞生物膜结构的重要组分, 鞘脂及其代谢产物参与许多重要的信号转导过程。在植物-真菌互作中, 植物鞘脂的主要作用是诱导细胞发生程序性死亡; 真菌鞘脂既能引起植物死亡, 也能诱导植物产生抗病性。该文总结了植物和真菌鞘脂的结构及代谢特点, 综述了鞘脂参与调控植物-真菌互作的分子机制研究进展, 并展望了植物-真菌共生关系中鞘脂作用的研究方向。
单婷婷,陈晓梅,郭顺星,田丽霞,严林,王欣. 鞘脂在植物-真菌互作中的分子调控机制研究进展. 植物学报, 2019, 54(3): 396-404.
Tingting Shan,Xiaomei Chen,Shunxing Guo,Lixia Tian,Lin Yan,Xin Wang. Advances in Molecular Regulation of Sphingolipids in Plant-fungus Interactions. Chinese Bulletin of Botany, 2019, 54(3): 396-404.
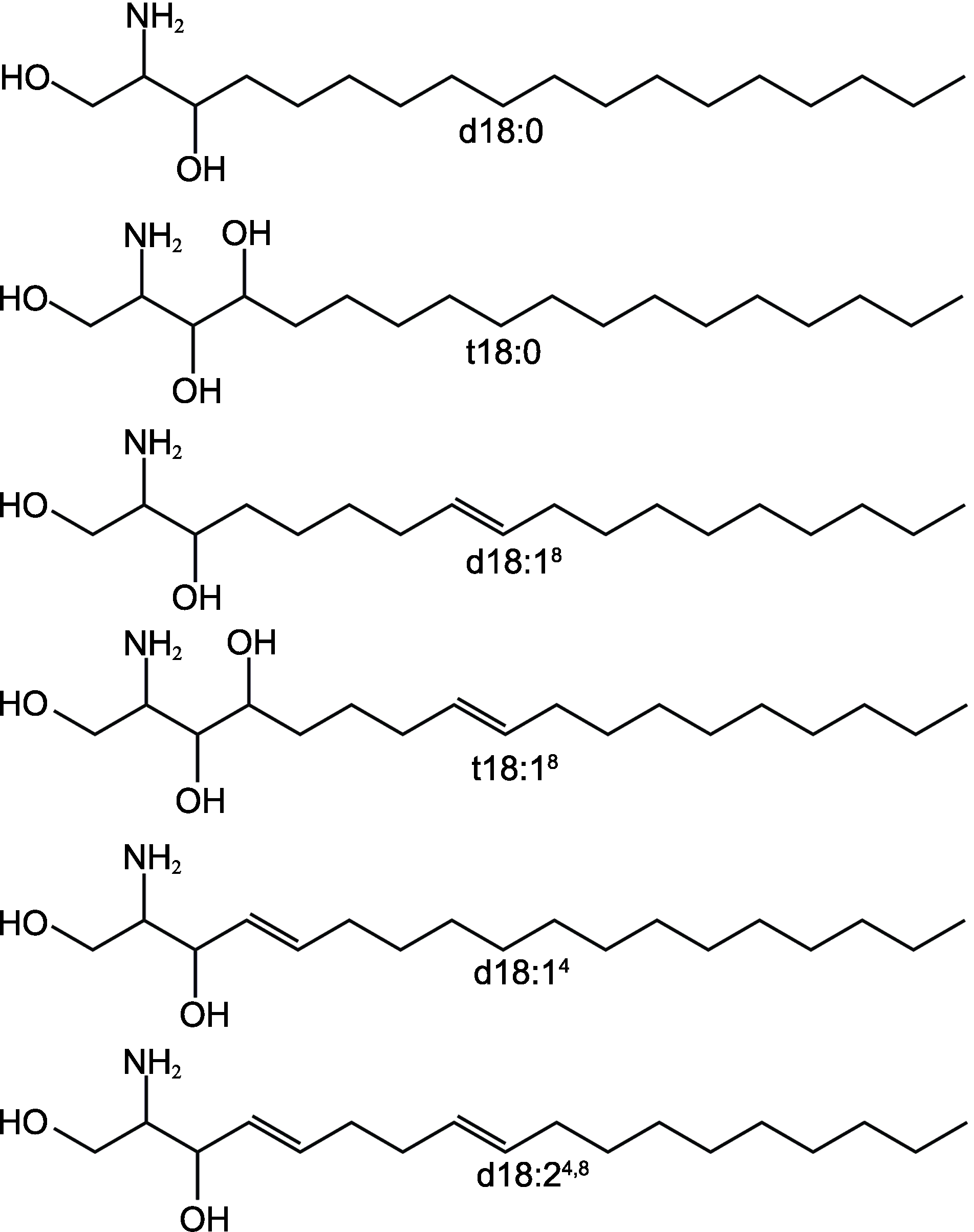
图2 植物鞘脂结构中常见的鞘氨醇改自(Warnecke and Heinz, 2003)d18:0: 二氢鞘氨醇; t18:0: 4-羟基鞘氨醇; d18:18: 2-氨基-8-十八烯-1,3-二醇; t18:18: 2-氨基-8-十八烯-1,3,4-三醇; d18:14: 2-氨基-4-十八烯-1,3-二醇; d18:24,8: 2-氨基-4,8-十八二烯-1,3-二醇
Figure 2 Sphingosines in the structure of plant sphingolipids (modified from Warnecke and Heinz, 2003)d18:0: Dihydrosphingosine; t18:0: Phytosphingosine; d18:18: Sphing-8-enine; t18:18: 4-hydroxysphing-8-enine; d18:14: Sphingosine; d18:24,8: Sphinga-4,8-dienine
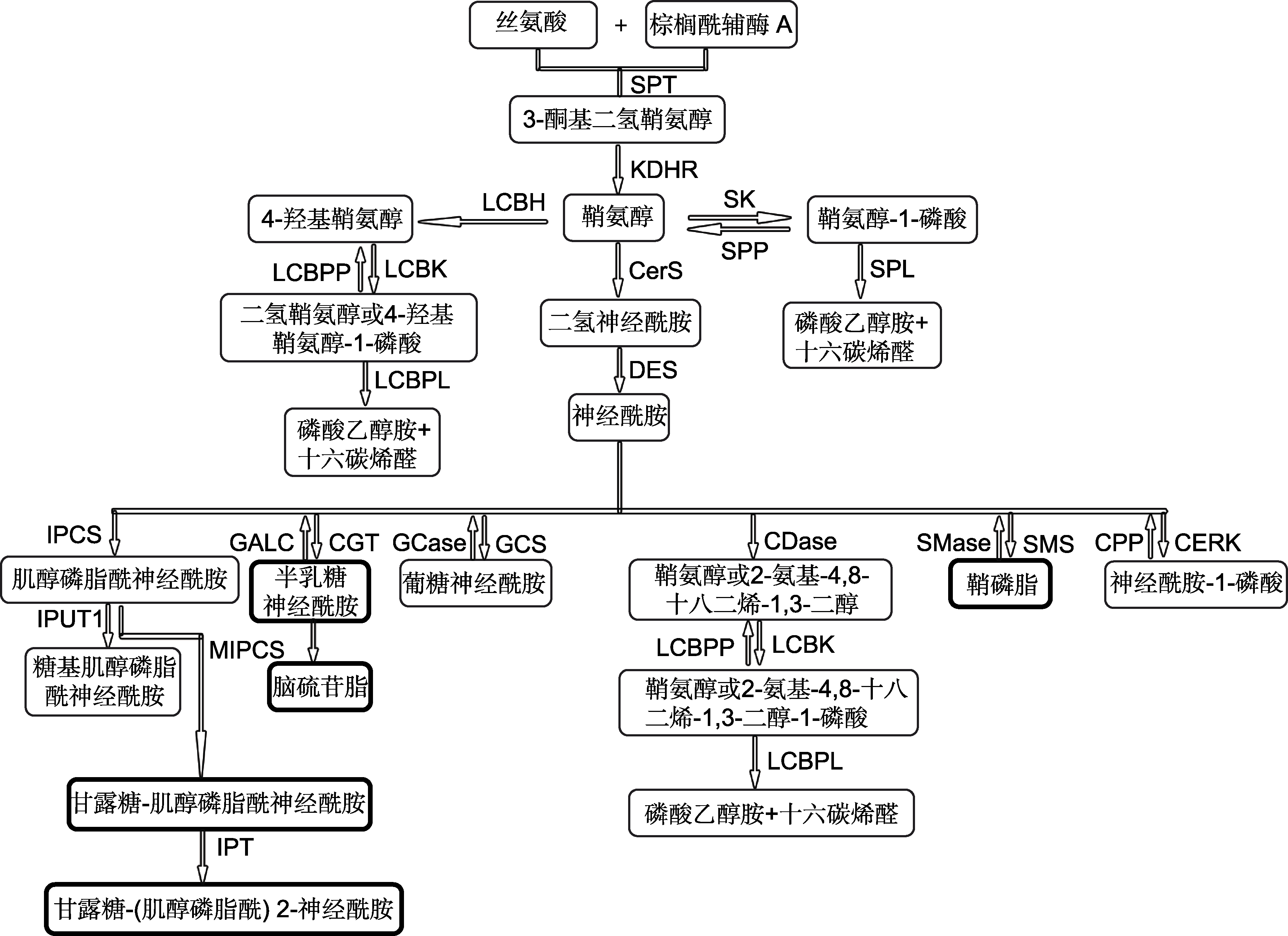
图3 植物和真菌鞘脂代谢途径(改自Michaelson et al., 2016; Rollin-pinheiro et al., 2016) 黑色加粗边框为真菌特有的产物。SPT: 丝氨酸棕榈酰转移酶; KDHR: 3-酮二氢鞘氨醇还原酶; CerS: (二氢)神经酰胺合酶; DES: 二氢神经酰胺去饱和酶; SK: 鞘氨醇激酶; SPP: 鞘氨醇-1-磷酸磷酸酶; SPL: 鞘氨醇-1-磷酸裂合酶; LCBH: 鞘氨醇羟化酶; CERK: 神经酰胺激酶; CPP: 神经酰胺-1-磷酸磷酸酶; SMS: 鞘磷脂合酶; SMase: 鞘磷脂酶; LCBK: LCB激酶; LCBPP: LCB-1-磷酸磷酸酶; LCBPL: LCB-1-磷酸裂合酶; IPCS: 肌醇磷脂酰神经酰胺合酶; IPUT1: 葡萄糖肌醇磷脂酰神经酰胺醛酸基转移酶1; MIPCS: 甘露糖肌醇磷脂酰神经酰胺合酶; IPT: 肌醇磷酸转移酶; CGT: 神经酰胺半乳糖基转移酶; GALC: 半乳糖神经酰胺酶; GCS: 葡糖基神经酰胺合酶; GCase: 葡糖基神经酰胺酶; CDase: 神经鞘氨醇酶
Figure 3 Sphingolipid biosynthesis in plant and fungi (modified from Michaelson et al., 2016; Rollin-pinheiro et al., 2016) The bold border boxes are unique products of fungi. SPT: Serine palmitoyltransferase; KDHR: 3-ketodihydrosphingosine reductase; CerS: (dihydro) Ceramide synthase; DES: Dihydroceramide desaturase; SK: Sphingosine kinase; SPP: higoine- 1-phosphate phosphatases; SPL: Sphingosine-1-phosphate lyase; LCBH: LCB hydroxylase; CERK: Ceramide kinase; CPP: Ceramide-1-phosphate phosphatases; SMS: Sphingomyelin synthase; SMase: Sphingomyelinases; LCBK: LCB kinase; LCBPP: LCB-1-phosphate phosphatases; LCBPL: LCB-1-phosphate lyase; IPCS: Inositol phosphoryl ceramide synthase; IPUT1: Inositol phosphorylceramide glucuronosyl transferases 1; MIPCS: Mannosylinositol phosphorylceramide synthase; IPT: Inositol phosphoryl transferase; CGT: Galactosyltransferase; GALC: Galactosylceramidase; GCS: Glucosylceramide synthase; GCase: Glucosylceramidase; CDase: Ceramidase
| [1] | 郭顺星 ( 2016). 药用植物内生真菌生物学. 北京: 科学出版社. pp. 190-191. |
| [2] | 林久生, 王根轩 ( 2001). 活性氧与植物细胞编程性死亡. 植物生理学通讯 37, 551-555. |
| [3] |
刘润华, 江文波, 余迪求 ( 2009). 植物鞘脂的结构、代谢途径及其功能. 植物学报 44, 619-628.
DOI URL |
| [4] |
田磊, 李元敬, 何兴元, 田春杰 ( 2016). 丛枝菌根真菌脂类代谢对共生信号调控的响应和反馈. 微生物学报 56, 26-34.
DOI URL |
| [5] | 张海涵 ( 2011). 黄土高原枸杞根际微生态特征及其共生真菌调控宿主生长与耐旱响应机制. 博士论文. 杨凌: 西北农林科技大学. pp. 38-40. |
| [6] |
Aerts AM, François IEJA, Bammens L, Cammue BPA, Smets B, Winderickx J, Accardo S, De Vos DE, Thevissen K ( 2006). Level of M(IP)2C sphingolipid affects plant defensin sensitivity, oxidative stress resistance and chronological life-span in yeast. FEBS Lett 580, 1903-1907.
DOI PMID |
| [7] |
Arora P, Porcelli SA ( 2008). A glycan shield for bacterial sphingolipids. Chem Biol 15, 642-644.
DOI URL PMID |
| [8] |
Barreto-Bergter E, Sassaki GL, De Souza LM ( 2011). Structural analysis of fungal cerebrosides. Front Microbiol 2, 239.
DOI URL PMID |
| [9] |
Bi FC, Liu Z, Wu JX, Liang H, Xi XL, Fang C, Sun TJ, Yin J, Dai GY, Rong C, Greenberg JT, Su WW, Yao N ( 2014). Loss of ceramide kinase in Arabidopsis impairs defenses and promotes ceramide accumulation and mito- chondrial H2O2 bursts. Plant Cell 26, 3449-3467.
DOI URL PMID |
| [10] |
Brandwagt BF, Kneppers TJA, Nijkamp HJJ, Hille J ( 2002). Overexpression of the tomato ASC-1 gene mediates high insensitivity to AAL toxins and fumonisin B1 in tomato hairy roots and confers resistance to Alternaria alternata f. sp. lycopersici in Nicotiana umbratica plants. Mol Plant Microbe Interact 15, 35-42.
DOI URL PMID |
| [11] |
Chen M, Markham JE, Cahoon EB ( 2012). Sphingolipid Δ8 unsaturation is important for glucosylceramide biosynthesis and low-temperature performance in Arabidopsis. Plant J 69, 769-781.
DOI URL PMID |
| [12] |
De Zélicourt A, Montiel G, Pouvreau JB, Thoiron S, Delgrange S, Simier P, Delavault P ( 2009). Susceptibility of Phelipanche and Orobanche species to AAL-toxin. Planta 230, 1047-1055.
DOI URL PMID |
| [13] |
Deepak SA, Raj SN, Umemura K, Kono T, Shetty HS ( 2003). Cerebroside as an elicitor for induced resistance against the downy mildew pathogen in pearl millet. Ann Appl Biol 143, 169-173.
DOI URL |
| [14] |
Healey KR, Challa KK, Edlind TD, Katiyar SK ( 2015). Sphingolipids mediate differential echinocandin susceptibility in Candida albicans and Aspergillus nidulans. Anti microb Agents Chemother 59, 3377-3384.
DOI URL PMID |
| [15] |
Hechtberger P, Zinser E, Saf R, Hummel K, Paltauf F, Daum G ( 1994). Characterization, quantification and subcellular localization of inositol-containing sphingolipids of the yeast, Saccharomyces cerevisiae. Eur J Biochem 225, 641-649.
DOI URL PMID |
| [16] |
Lachaud C, Silva DD, Cotelle V, Thuleau P, Xiong TC, Jauneau A, Brière C, Graziana A, Bellec Y, Faure JD, Ranjeva R, Mazars C ( 2010). Nuclear calcium controls the apoptotic-like cell death induced by D-erythro-sphinganine in tobacco cells. Cell Calcium 47, 92-100.
DOI URL PMID |
| [17] |
Lindahl L, Santos AXS, Olsson H, Olsson L, Bettiga M ( 2017). Membrane engineering of S. cerevisiae targeting sphingolipid metabolism. Sci Rep 7, 41868.
DOI URL PMID |
| [18] |
Magnin-Robert M, Le Bourse D, Markham J, Dorey S, Clément C, Baillieul F, Dhondt-Cordelier S ( 2015). Modifications of sphingolipid content affect tolerance to hemibiotrophic and necrotrophic pathogens by modulating plant defense responses in Arabidopsis. Plant Physiol 169, 2255-2274.
DOI URL PMID |
| [19] |
Maillet F, Poinsot V, André O, Puech-Pagès V, Haouy A, Gueunier M, Cromer L, Giraudet D, Formey D, Niebel A, Martinez EA, Driguez H, Bécard G, Dénarié J ( 2011). Fungal lipochitooligosaccharide symbiotic signals in arbu- scular mycorrhiza. Nature 469, 58-63.
DOI URL PMID |
| [20] |
Markham JE, Lynch DV, Napier JA, Dunn TM, Cahoon EB ( 2013). Plant sphingolipids: function follows form. Curr Opin Plant Biol 16, 350-357.
DOI URL PMID |
| [21] |
Michaelson LV, Napier JA, Molino D, Faure JD ( 2016). Plant sphingolipids: their importance in cellular organization and adaption. Biochim Biophys Acta 1861, 1329-1335.
DOI URL PMID |
| [22] |
Mortimer JC, Yu X, Albrecht S, Sicilia F, Huichalaf M, Ampuero D, Michaelson LV, Murphy AM, Matsunaga T, Kurz S, Stephens E, Baldwin TC, Ishii T, Napier JA, Weber APM, Handford MG, Dupree P ( 2013). Abnormal glycosphingolipid mannosylation triggers salicylic acid mediated responses in Arabidopsis. Plant Cell 25, 1881-1894.
DOI URL PMID |
| [23] |
Oguro Y, Yamazaki H, Takagi M, Takaku H ( 2014). Antifungal activity of plant defensin AFP1 in Brassica juncea involves the recognition of the methyl residue in glucosylceramide of target pathogen Candida albicans. Curr Genet 60, 89-97.
DOI URL PMID |
| [24] |
Olsen I, Jantzen E ( 2001). Sphingolipids in bacteria and fungi. Anaerobe 7, 103-112.
DOI URL |
| [25] |
Peer M, Stegmann M, Mueller MJ, Waller F ( 2010). Pseudomonas syringae infection triggers de novo synthesis of phytosphingosine from sphinganine in Arabidopsis thaliana. FEBS Lett 584, 4053-4056.
DOI URL PMID |
| [26] |
Ramamoorthy V, Cahoon EB, Li J, Thokala M, Minto RE, Shah DM ( 2007). Glucosylceramide synthase is essential for alfalfa defensin-mediated growth inhibition but not for pathogenicity of Fusarium graminearum. Mol Microbiol 66, 771-786.
DOI URL PMID |
| [27] |
Ramamoorthy V, Cahoon EB, Thokala M, Kaur J, Li J, Shah DM ( 2009). Sphingolipid C-9 methyltransferases are important for growth and virulence but not for sensitivity to antifungal plant defensins in Fusarium graminearum. Eukaryot Cell 8, 217-229.
DOI URL PMID |
| [28] |
Rittershaus PC, Kechichian TB, Allegood JC, Merrill AH, Hennig M, Luberto C, Del Poeta M ( 2006). Glucosylceramide synthase is an essential regulator of pathogenicity of Cryptococcus neoformans. J Clin Invest 116, 1651-1659.
DOI URL PMID |
| [29] |
Rollin-Pinheiro R, Singh A, Barreto-Bergter E, Poeta MD ( 2016). Sphingolipids as targets for treatment of fungal infections. Future Med Chem 8, 1469-1484.
DOI URL PMID |
| [30] |
Saucedo-García M, Guevara-García A, González-Solís A, Cruz-García F, Vázquez-Santana S, Markham JE, Guadalupe Lozano-Rosas M, Dietrich CR, Ramos-Vega M, Cahoon EB, Gavilanes-Ruíz M ( 2011). MPK6, sphinganine and the LCB2a gene from serine palmitoyltransferase are required in the signaling pathway that mediates cell death induced by long chain bases in Arabidopsis. New Phytol 191, 943-957.
DOI URL PMID |
| [31] |
Sharma L, Prakash H ( 2017). Sphingolipids are dual specific drug targets for the management of pulmonary infections: perspective. Front Immunol 8, 378.
DOI URL PMID |
| [32] |
Shi LH, Bielawski J, Mu JY, Dong HL, Teng C, Zhang J, Yang XH, Tomishige N, Hanada K, Hannun YA, Zuo J ( 2007). Involvement of sphingoid bases in mediating reactive oxygen intermediate production and programmed cell death in Arabidopsis. Cell Res 17, 1030-1040.
DOI URL PMID |
| [33] |
Siebers M, Brands M, Wewer V, Duan YJ, Hölzl G, Dörmann P ( 2016). Lipids in plant-microbe interactions. Biochim Biophys Acta 1861, 1379-1395.
DOI URL PMID |
| [34] |
Spassieva SD, Markham JE, Hille J ( 2002). The plant disease resistance gene Asc-1 prevents disruption of sphingolipid metabolism during AAL-toxin-induced programmed cell death. Plant J 32, 561-572.
DOI URL PMID |
| [35] |
Tani Y, Amaishi Y, Funatsu T, Ito M, Itonor S, Hata Y, Ashida H, Yamamoto K ( 2014). Structural analysis of cerebrosides from Aspergillus fungi: the existence of galactosylceramide in A. oryzae. Biotechnol Lett 36, 2507-2513.
DOI URL PMID |
| [36] |
Tartaglio V, Rennie EA, Cahoon R, Wang G, Baidoo E, Mortimer JC, Cahoon EB, Scheller HV ( 2017). Glycosylation of inositol phosphorylceramide sphingolipids is required for normal growth and reproduction in Arabidosis. Plant J 89, 278-290.
DOI URL PMID |
| [37] |
Thevissen K, François IEJA, Takemoto JY, Ferket KKA, Meert EMK, Cammue BPA ( 2003). DmAMP1, an antifungal plant defensin from dahlia (Dahlia merckii), interacts with sphingolipids from Saccharomyces cerevisiae. FEMS Microbiol Lett 226, 169-173.
DOI URL PMID |
| [38] |
Thevissen K, Warnecke DC, François IEJA, Leipelt M, Heinz E, Ott C, Zähringer U, Thomma BPHJ, Ferket KKA, Cammue BPA ( 2004). Defensins from insects and plants interact with fungal glucosylceramides. J Biol Chem 279, 3900-3905.
DOI URL PMID |
| [39] |
Townley HE, McDonald K, Jenkins GI, Knight MR, Leaver CJ ( 2005). Ceramides induce programmed cell death in Arabidopsis cells in a calcium-dependent manner. Biol Chem 386, 161-166.
DOI URL PMID |
| [40] |
Ueda N ( 2017). Sphingolipids in genetic and acquired forms of chronic kidney diseases. Curr Med Chem 24, 1238-1275.
DOI URL PMID |
| [41] |
Umemura K, Ogawa N, Koga J, Iwata M, Usami H ( 2002). Elicitor activity of cerebroside, a sphingolipid elicitor, in cell suspension cultures of rice. Plant Cell Physiol 43, 778-784.
DOI URL PMID |
| [42] |
Umemura K, Ogawa N, Yamauchi T, Iwata M, Shimura M, Koga J ( 2000). Cerebroside elicitors found in diverse phytopathogens activate defense responses in rice plants. Plant Cell Physiol 41, 676-683.
DOI URL PMID |
| [43] | Virolainen E, Blokhina O, Fagerstedt K ( 2002). Ca 2+-in- duced high amplitude swelling and cytochrome c release from wheat (Triticum aestivum L.) mitochondria under anoxic stress . Ann Bot 90, 509-516. |
| [44] |
Wang WM, Yang XH, Tangchaiburana S, Ndeh R, Markham JE, Tsegaye Y, Dun TM, Wang GL, Bellizzi M, Parsons JF, Morrissey D, Bravo JE, Lynch DV, Xiao SY ( 2008). An inositolphosphorylceramide synthase is involved in regulation of plant programmed cell death associated with defense in Arabidopsis. Plant Cell 20, 3163-3179.
DOI URL |
| [45] |
Warnecke D, Heinz E ( 2003). Recently discovered functions of glucosylceramides in plants and fungi. Cell Mol Life Sci 60, 919-941.
DOI URL PMID |
| [46] |
Yanagawa D, Ishikawa T, Imai H ( 2017). Synthesis and degradation of long-chain base phosphates affect fumonisin B1-induced cell death in Arabidopsis thaliana. J Plant Res 130, 571-585.
DOI URL PMID |
| [47] |
Zhu CY, Wang MS, Wang WL, Ruan RJ, Ma HJ, Mao CG, Li HY ( 2014). Glucosylceramides are required for mycelial growth and full virulence in Penicillium digitatum. Biochem Biophys Res Commun 455, 165-171.
DOI URL PMID |
| [1] | 陈自宏, 张翼飞, 陈凯, 陈见影, 徐玲. 高黎贡山南段昆虫病原真菌物种多样性及影响因素[J]. 生物多样性, 2025, 33(1): 24228-. |
| [2] | 刘向, 刘木, 肖瑶. 叶片病原真菌对植物物种共存的影响: 进展与挑战[J]. 生物多样性, 2023, 31(2): 22525-. |
| [3] | 戚海迪, 张定海, 单立山, 陈国鹏, 张勃. 昆虫病原真菌感染昆虫宿主的机制和宿主昆虫的防御策略研究进展[J]. 生物多样性, 2023, 31(11): 23273-. |
| [4] | 支添添, 周舟, 韩成云, 任春梅. PAD4突变加速拟南芥酪氨酸降解缺陷突变体sscd1的程序性细胞死亡[J]. 植物学报, 2022, 57(3): 288-298. |
| [5] | 石新建, 张靖歆, 秦天姿, 刘金铭, 高玉葆, 任安芝. 内生真菌感染对宿主羽茅及邻生植物抗病性的影响[J]. 植物生态学报, 2021, 45(8): 860-869. |
| [6] | 袁海生, 魏玉莲, 周丽伟, 秦问敏, 崔宝凯, 何双辉. 东北4种林木干基腐朽病原真菌潜在分布范围预测及其生态位分析[J]. 生物多样性, 2019, 27(8): 873-879. |
| [7] | 何光明, 邓兴旺. 死亡信号传递: 叶绿体与线粒体间信号交流调控植物程序性细胞死亡[J]. 植物学报, 2018, 53(4): 441-444. |
| [8] | 赵曦娟, 钱礼超, 刘玉乐. 中国科学家在植物程序性细胞死亡领域取得重要成果[J]. 植物学报, 2018, 53(4): 447-450. |
| [9] | 张宪省. 我国科学家在程序性细胞死亡机制研究领域取得重大突破[J]. 植物学报, 2018, 53(4): 445-446. |
| [10] | 牛毅, 高远, 李隔萍, 任安芝, 高玉葆. 内生真菌对羽茅抗病性的影响[J]. 植物生态学报, 2016, 40(9): 925-932. |
| [11] | 黄晓, 李发强. 细胞自噬在植物细胞程序性死亡中的作用[J]. 植物学报, 2016, 51(6): 859-862. |
| [12] | 景红娟, 周广舟, 谭晓荣, 平康康, 任雪建. 活性氧对植物自噬调控的研究进展[J]. 植物学报, 2012, 47(5): 534-542. |
| [13] | 马聪, 孔维文. 植物Metacaspase研究进展[J]. 植物学报, 2012, 47(5): 543-549. |
| [14] | 程红焱 宋松泉. 植物一氧化氮生物学的研究进展[J]. 植物学报, 2005, 22(06): 723-737. |
| [15] | 吴光旭 何庭玉 刘爱嫒 陈维信. 植物中抗病原真菌的活性物质[J]. 植物学报, 2004, 21(03): 367-375. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||