

植物学报 ›› 2016, Vol. 51 ›› Issue (2): 194-201.DOI: 10.11983/CBB15081 cstr: 32102.14.CBB15081
李林, 谭康, 唐秀光, 晁晓婷, 汶晨曦, 白壮东, 丰华玲, 刘文哲*( ), 苏慧*(
), 苏慧*( )
)
收稿日期:2015-05-09
接受日期:2015-07-05
出版日期:2016-03-01
发布日期:2016-03-31
通讯作者:
E-mail: 基金资助:
Lin Li, Kang Tan, Xiuguang Tang, Xiaoting Chao, Chenxi Wen, Zhuangdong Bai, Hualing Feng, Wenzhe Liu*( ), Hui Su*(
), Hui Su*( )
)
Received:2015-05-09
Accepted:2015-07-05
Online:2016-03-01
Published:2016-03-31
Contact:
E-mail: 摘要: 根毛是植物体吸收养分的重要器官, 自然条件下根毛的寿命很短, 仅能存活2-3周, 随即脱落死亡。以模式植物拟南芥(Arabidopsis thaliana)根毛为材料, 对根毛死亡的细胞学特征进行了报道。 结果发现,根毛衰老死亡后细胞内的原生质体发生了收缩, 并在胞质中观察到凝集物的出现; 通过原位末端标记(TUNEL)检测, 发现幼根上的根毛细胞核DNA发生了片段化。上述结果表明, 拟南芥根毛的衰老死亡很可能是植物体自主调控的程序性细胞死亡(PCD)。另外, 当根毛衰老死亡后, 细胞核大多会迁移到靠近根毛基部的位置, 且正常的长管状根毛发生旋转扭曲。
李林, 谭康, 唐秀光, 晁晓婷, 汶晨曦, 白壮东, 丰华玲, 刘文哲, 苏慧. 拟南芥根毛衰老死亡过程的PCD检测. 植物学报, 2016, 51(2): 194-201.
Lin Li, Kang Tan, Xiuguang Tang, Xiaoting Chao, Chenxi Wen, Zhuangdong Bai, Hualing Feng, Wenzhe Liu, Hui Su. Characterization of Programmed Cell Death During the Senescence of Root Hairs in Arabidopsis. Chinese Bulletin of Botany, 2016, 51(2): 194-201.
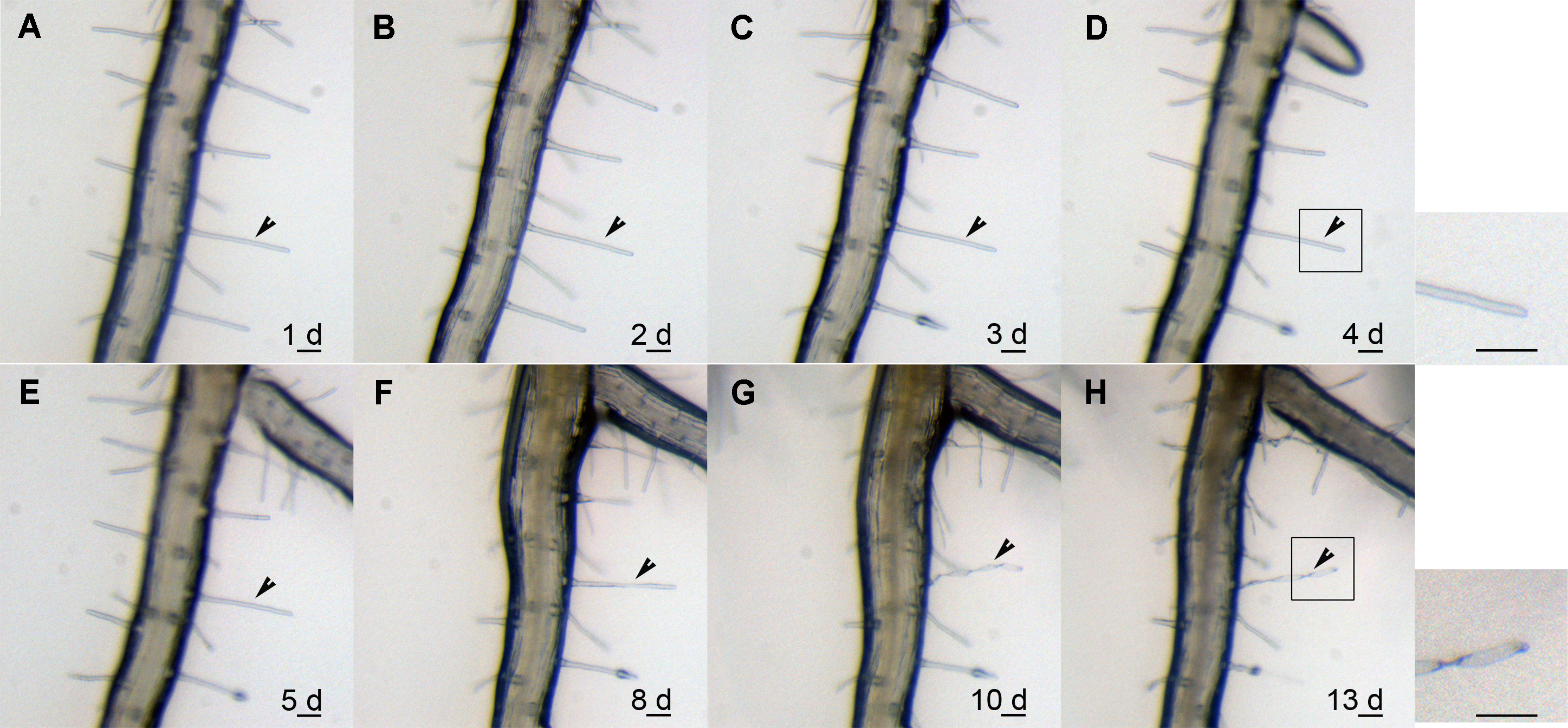
图1 模式植物拟南芥根毛的死亡过程 (A)-(E) 连续跟踪观察1、2、3、4和5天的根毛(箭头所示); (F) 在观察的第8天, 根毛形态发生变化(箭头所示); (G) 在观察的第10天, 根毛发生明显的扭曲(箭头所示), 此时已经死亡 ; (H) 观察第13天的扭曲的根毛(箭头所示)。Bar=10 μm
Figure 1 Time-lapse of root hairs in Arabidopsis (A)-(E) The morphology of the root hair indicated by the arrow head was observed for 1, 2, 3, 4 and 5 d, respectively; (F) The vary of the root hair was observed in the 8th day (indicating by the arrow head) ; (G) The root hair obviously twisted and curved in the 10th day (indicating by the arrow head), and at that time, root hair has been died); (H) Twisted and curved root hair in the 13th day (indicating by the arrow head). Bar=10 μm
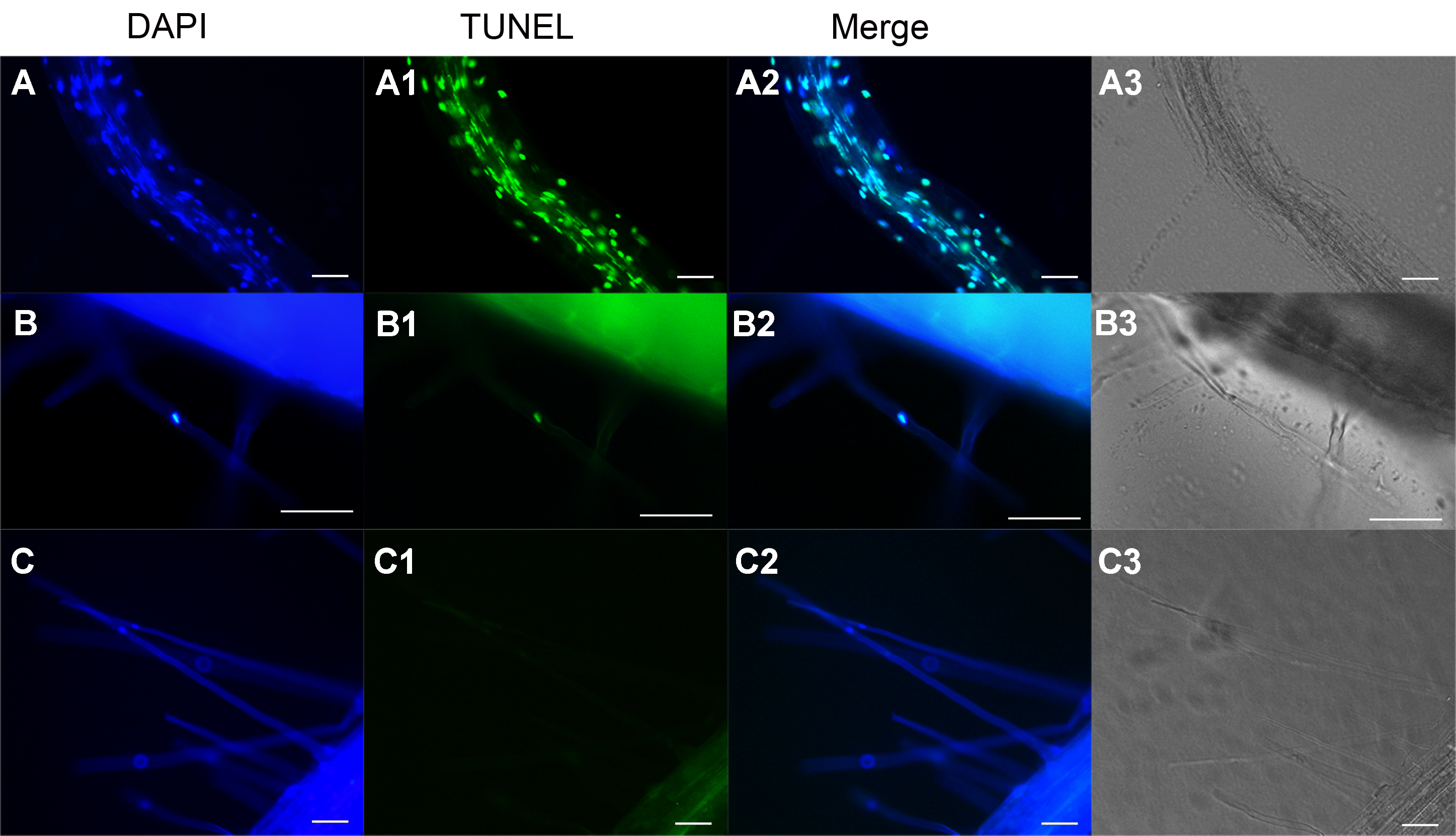
图2 拟南芥根毛细胞的TUNEL原位检测 (A)-(A3) 阳性对照。DNA酶I处理后, 根毛及主根细胞的细胞核均为TUNEL阳性。(B)-(B3) 发生PCD的根毛细胞。图中所示的根毛细胞TUNEL原位检测为阳性。(C)-(C3) 阴性对照。图中所示的根毛细胞TUNEL原位检测为阴性。Bar=50 μm
Figure 2 The TUNEL assay in roots and root hairs of Arabidopsis (A)-(A3) DNase I treated seedlings were fixed and subjected to TUNEL assay. The root and root hairs were TUNEL-positive; (B)-(B3) PCD root hair. A representative image shows TUNEL-positive root hair. (C)-(C3) Negative control. A representative image shows TUNEL-negative root hair. Bar=50 μm
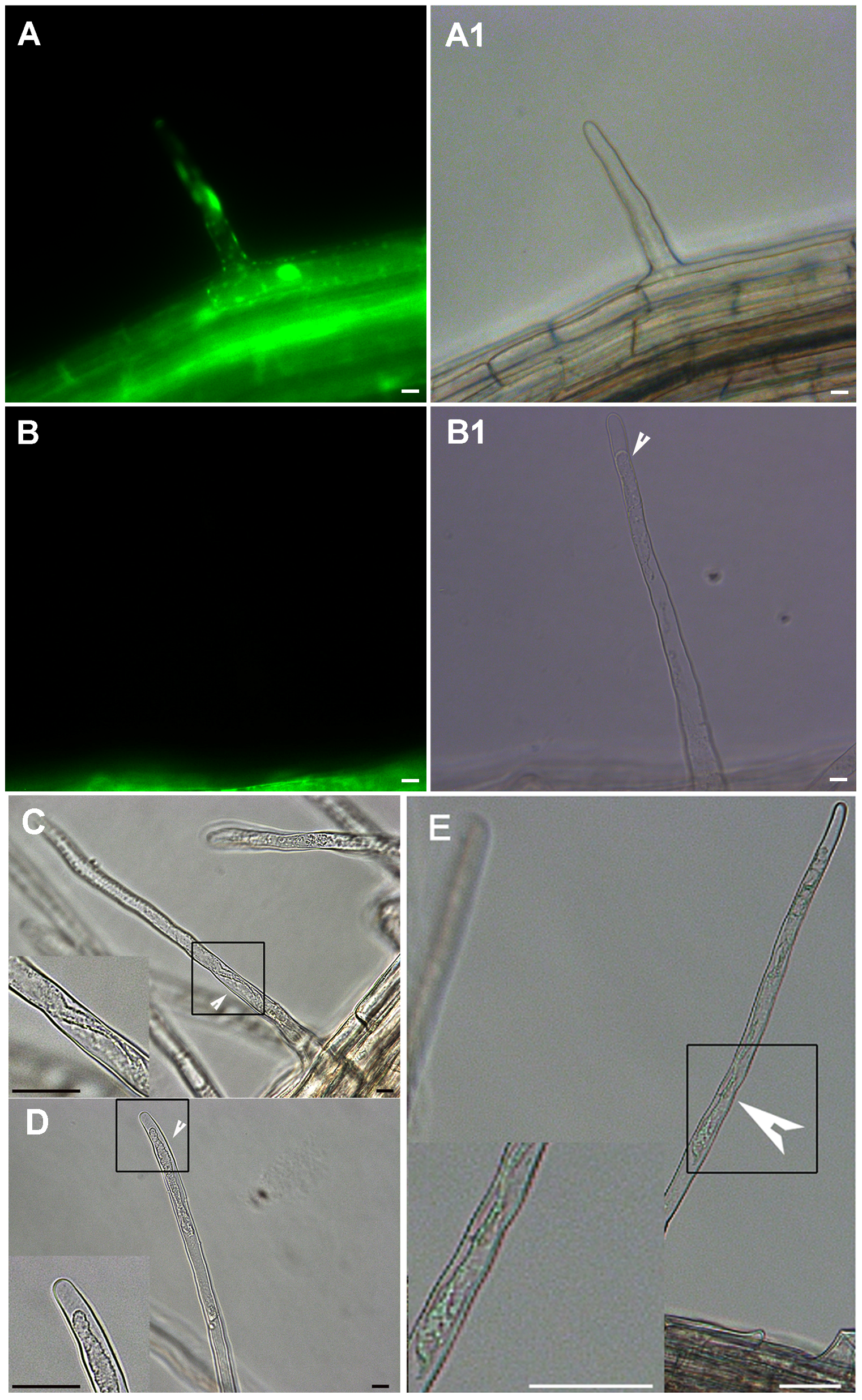
图3 自然死亡的拟南芥根毛细胞发生质壁分离 (A)-(A1) 正常的根毛细胞。FDA染色可以观察到明显的绿色荧光(A); 明场下细胞形态正常(A1)。(B)-(B1) 自然死亡的根毛细胞。FDA染色显示根毛已死亡(B); 明场下可以观察到明显的质壁分离(B1)。(C)和(D) 自然死亡的根毛细胞基部发生了质壁分离; 死亡后期的根毛细胞内部原生质体断裂。(E) 自然生长1周的拟南芥幼苗的热激处理(阳性对照)。Bar=10 μm
Figure 3 The plasmolysis of cell in Arabidopsis naturally dead root hair (A)-(A1) Normal root hairs stained with FDA and viewed under white (A1) or fluorescent light (A); (B)-(B1) Naturally dead root hair. The root hairs with no fluorescence after FDA staining shows obvious retraction of the cytoplasm; (C) and (D) The extent of protoplast shrinking varies depending on the sequential process of programmed cell death. (E) Root hair of Arabidopsis thaliana 6 h after a 10 minutes heat shock at 55°C. The root hair shows retraction of the cytoplasm indicating it has undergone AL-PCD (the positive control). Bar=10 μm
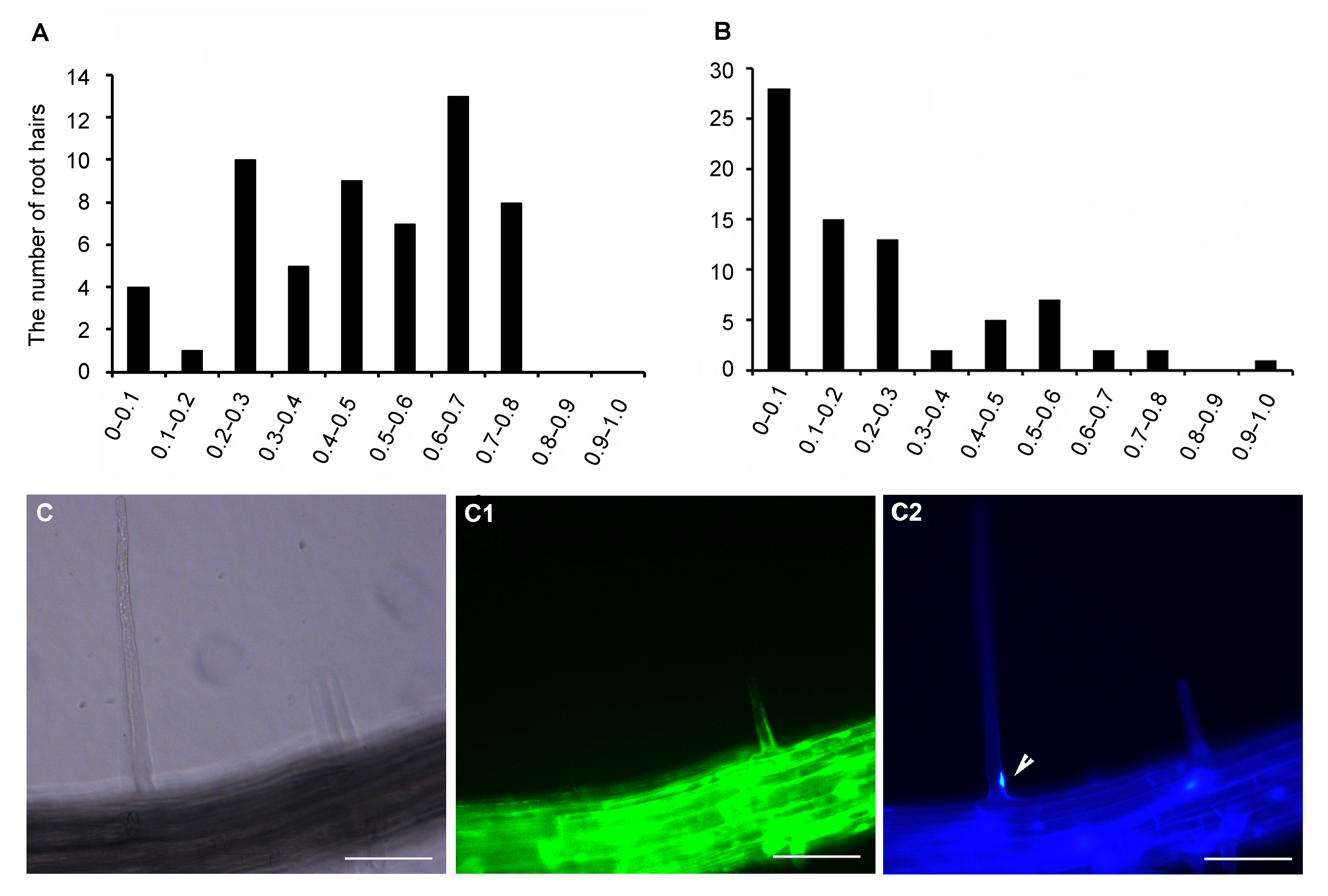
图5 自然死亡的根毛细胞的细胞核大多接近根毛基部 (A) 活着的根毛细胞核中心距根毛基部的距离与根毛全长比率的统计。横坐标为比值, 纵坐标为根毛数目(n=57)。(B) 自然死亡的根毛细胞核中心距根毛基部的距离与根毛全长比率的统计。横坐标为比值, 纵坐标为根毛数目(n=75)。(C)-(C2) FDA (C1)和DAPI (C2)染色表明死亡的根毛细胞核的位置靠近根毛基部。C: 明场。箭头指示细胞核的位置。Bar=50 μm
Figure 5 The position of nuclei in natural death root hairs is close to the basement of root hairs (A) Quantitative analysis of the position of nuclei in living root hairs; Abscissa represents ratio between the distance of the nucleus center from the basement of the root hair and root hair length; Ordinate represents the number of root hairs (n=57); (B) Quantitative analysis of the position of nuclei in dead root hairs; Abscissa represents ratio; Ordinate represents the number of root hairs (n=75); (C)-(C2) A representative image stained by FDA and DAPI shows that the nucleus in the dead root hair can be colored by DAPI (indicating by the arrow head) and the location of the nucleus is near the basement of the root hair. C: Bright field. Arrow head: Nucleus. Bar=50 μm
| [1] |
贺新强, 吴鸿 (2013). 植物发育性细胞程序死亡的发生机制. 植物学报 48, 357-370.
DOI |
| [2] | 李云霞, 程晓霞, 代小梅, 曾会明, 韩烈宝 (2009). 植物在逆境胁迫中的细胞程序性死亡. 生物技术通报 4, 711. |
| [3] |
Delorme VG, McCabe PF, Kim DJ, Leaver CJ (2000). A matrix metalloproteinase gene is expressed at the boun- dary of senescence and programmed cell death in cucumber. Plant Physiol 123, 917-928.
DOI PMID |
| [4] |
Doyle SM, Diamond M, McCabe PF (2010). Chloroplast and reactive oxygen species involvement in apoptotic-like programmed cell death in Arabidopsis suspension cultures. J Exp Bot 61, 473-482.
DOI PMID |
| [5] | Filonova LH, Bozhkov PV, Brukhin VB, Daniel G, Zhivo- tovsky B, Von Arnold S (2000). Two waves of program- med cell death occur during formation and development of somatic embryos in the gymnosperm, Norway spruce. J Cell Sci 113, 4399-4411. |
| [6] |
Filonova LH, Von Arnold S, Daniel G, Bozhkov PV (2002). Programmed cell death eliminates all but one embryo in a polyembryonic plant seed. Cell Death Differ 9, 1057-1062.
PMID |
| [7] | Fukuda H (1997). Tracheary element differentiation. Plant Cell 9, 1147. |
| [8] | Grierson C, Schiefelbein J (2002). Root Hairs. Rockville, MD: American Society of Plant Biologists. pp. 1-22 |
| [9] |
Groover A, Jones AM (1999). Tracheary element differentiation uses a novel mechanism coordinating programmed cell death and secondary cell wall synthesis. Plant Physiol 119, 375-384.
DOI PMID |
| [10] |
Gunawardena AH (2008). Programmed cell death and tissue remodelling in plants. J Exp Bot 59, 445-451.
DOI PMID |
| [11] |
Gunawardena AH, Greenwood JS, Dengler NG (2004). Programmed cell death remodels lace plant leaf shape during development. Plant Cell 16, 60-73.
DOI PMID |
| [12] |
Gunawardena AH, Pearce DM, Jackson MB, Hawes CR, Evans DE (2001). Characterisation of programmed cell death during aerenchyma formation induced by ethylene or hypoxia in roots of maize (Zea mays L.). Planta 212, 205-214.
DOI PMID |
| [13] | Hogg BV, Kacprzyk J, Molony EM, O’Reilly C, Gallagher TF, Gallois P, McCabe PF (2011). An in vivo root hair assay for determining rates of apoptotic-like programmed cell death in plants. Plant Methods 7: 45. |
| [14] |
Ketelaar T, Faivre-Moskalenko C, Esseling JJ, de Ruijter NC, Grierson CS, Dogterom M, Emons AMC (2002). Positioning of nuclei in Arabidopsis root hairs: an actin-regulated process of tip growth. Plant Cell 14, 2941-2955.
DOI PMID |
| [15] | Li D, Yang X, Cui K, Li Z (2003). Morphological changes in nucellar cells undergoing programmed cell death (PCD) during pollen chamber formation in Ginkgo biloba. Acta Botanica Sinica 45, 53-63. |
| [16] |
McCabe PF, Leaver CJ (2000). Programmed cell death in cell cultures. Plant Mol Biol 44, 359-368.
PMID |
| [17] | Miller DD, De Ruijter NC, Emons AMC (1997). From signal to form: aspects of the cytoskeleton-plasma membrane-cell wall continuum in root hair tips. J Exp Bot 48, 1881-1896. |
| [18] |
Mittler R, Lam E (1995). In situ detection of nDNA fragmentation during the differentiation of tracheary elements in higher plants. Plant Physiol 108, 489-493.
DOI PMID |
| [19] | Peterson RL, Farquhar ML (1996). Root hairs: specialized tubular cells extending root surface. Bot Rev 62, 1-40. |
| [20] |
Reape TJ, McCabe PF (2010). Apoptotic-like regulation of programmed cell death in plants. Apoptosis 15, 249-256.
DOI PMID |
| [21] |
Shishkova S, Dubrovsky JG (2005). Developmental programmed cell death in primary roots of Sonoran desert Cactaceae. Am J Bot 92, 1590-1594.
DOI PMID |
| [22] |
Van Doorn WG (2011). Classes of programmed cell death in plants, compared to those in animals. J Exp Bot 62, 4749-4761.
DOI PMID |
| [23] |
Van Doorn WG, Woltering EJ (2005). Many ways to exit? Cell death categories in plants. Trends Plant Sci 10, 117-122.
DOI PMID |
| [24] | Wang H, Li J, Bostock RM, Gilchrist DG (1996). Apoptosis: a functional paradigm for programmed plant cell death induced by a host-selective phytotoxin and invoked during development. Plant Cell 8, 375-391. |
| [25] |
Zuppini A, Navazio L, Mariani P (2004). Endoplasmic reticulum stress-induced programmed cell death in soybean cells. J Cell Sci 117, 2591-2598.
DOI PMID |
| [1] | 张慧, 曾文静, 龚新桃, 马泽清. 亚热带典型树种根毛特征及其与共生真菌的关系[J]. 植物生态学报, 2023, 47(1): 88-100. |
| [2] | 姚玉婷,马家琦,冯晓莉,潘建伟,王超. 磷酸肌醇激酶FAB1调控拟南芥根毛伸长[J]. 植物学报, 2020, 55(2): 126-136. |
| [3] | 丁沃娜, 童艳丽, 宁永强, 朱世华. 水稻短根毛突变体Ossrh2的表型分析与基因定位[J]. 植物学报, 2011, 46(6): 625-631. |
| [4] | 马怀宇, 吕德国, 杨洪强. NaCl胁迫下平邑甜茶根系线粒体特性和细胞死亡特征[J]. 植物生态学报, 2010, 34(12): 1448-1453. |
| [5] | 李荣峰, 蔡妙珍, 刘鹏, 徐根娣, 陈敏燕, 梁和. Al3+对大豆根边缘细胞程序性死亡诱导的生理生态作用[J]. 植物生态学报, 2008, 32(3): 690-697. |
| [6] | 陈宇亮;张飞雄;张贵友*. 植物细胞程序性死亡中的类caspases 蛋白酶[J]. 植物学报, 2008, 25(05): 616-623. |
| [7] | 孔妤;王忠*;顾蕴洁;汪月霞. 植物根内通气组织形成机理的研究进展[J]. 植物学报, 2008, 25(02): 248-253. |
| [8] | 李杰 朱碧岩 张铭光. 植物发育过程中的细胞程序性死亡[J]. 植物学报, 2005, 22(增刊): 22-28. |
| [9] | 王立德 廖红 王秀荣 严小龙. 植物根毛的发生、发育及养分吸收[J]. 植物学报, 2004, 21(06): 649-659. |
| [10] | 于惠敏. 植物中的细胞程序性死亡[J]. 植物学报, 1998, 15(06): 30-37. |
| [11] | 邢树平 李兴国 张宪省 杨玉芳. Ca2 +对小麦种根及其根毛生长发育的影响[J]. 植物学报, 1998, 15(02): 41-45. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||