

Chinese Bulletin of Botany ›› 2018, Vol. 53 ›› Issue (6): 773-781.DOI: 10.11983/CBB17195
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Sun Jianfei, Zhai Jianyun, Ma Yuandan*( ), , Fu Lucheng, Bu Keli, Wang Keyang, Gao Yan, Zhang Rumin
), , Fu Lucheng, Bu Keli, Wang Keyang, Gao Yan, Zhang Rumin
Received:2017-10-22
Online:2018-11-01
Published:2018-12-05
Contact:
Ma Yuandan
Sun Jianfei, Zhai Jianyun, Ma Yuandan, , Fu Lucheng, Bu Keli, Wang Keyang, Gao Yan, Zhang Rumin. Differences in Photosynthetic Pigments and Photosynthetic Enzyme Activities in Different Internodes of Phyllostachys edulis During Rapid Growth Stage[J]. Chinese Bulletin of Botany, 2018, 53(6): 773-781.
| Internode number | Chlorophyll a (μg·g -1 FW) | Chlorophyll b (μg·g -1 FW) | Total chlorophyll (μg·g -1 FW) | Carotenoids (μg·g -1 FW) | Chlorophyll a/b |
|---|---|---|---|---|---|
| 1 | 15.19±0.28 a | 5.07±0.04 a | 20.26±0.29 a | 6.37±0.27 a | 3.00±0.24 c |
| 4 | 16.22±0.27 a | 5.27±0.40 a | 21.49±0.39 a | 7.00±0.28 a | 3.10±0.27 c |
| 7 | 15.80±1.09 a | 5.21±0.20 a | 21.01±1.28 a | 6.66±0.48 a | 3.03±0.10 c |
| 10 | 13.98±0.42 b | 3.91±0.11 b | 17.89±0.53 b | 6.27±0.32 a | 3.58±0.02 c |
| 13 | 9.51±0.63 c | 2.71±0.03 c | 12.22±0.61 c | 4.27±0.19 b | 3.50±0.25 c |
| 16 | 6.49±0.29 d | 1.70±0.09 d | 8.19±0.23 d | 2.89±0.17 c | 3.83±0.35 bc |
| 19 | 2.43±0.07 e | 0.53±0.02 e | 2.96±0.09 e | 1.23±0.09 d | 4.59±0.1 ab |
| 22 | 1.83±0.15 e | 0.38±0.03 e | 2.22±0.16 e | 0.97±0.05 d | 4.82±0.55 a |
| 25 | 1.25±0.01 e | 0.25±0.01 e | 1.50±0.01 e | 0.63±0.09 d | 5.07±0.23 a |
Table 1 Differences of pigment contents in the Phyllostachys edulis stems of different internodes (means±SD)
| Internode number | Chlorophyll a (μg·g -1 FW) | Chlorophyll b (μg·g -1 FW) | Total chlorophyll (μg·g -1 FW) | Carotenoids (μg·g -1 FW) | Chlorophyll a/b |
|---|---|---|---|---|---|
| 1 | 15.19±0.28 a | 5.07±0.04 a | 20.26±0.29 a | 6.37±0.27 a | 3.00±0.24 c |
| 4 | 16.22±0.27 a | 5.27±0.40 a | 21.49±0.39 a | 7.00±0.28 a | 3.10±0.27 c |
| 7 | 15.80±1.09 a | 5.21±0.20 a | 21.01±1.28 a | 6.66±0.48 a | 3.03±0.10 c |
| 10 | 13.98±0.42 b | 3.91±0.11 b | 17.89±0.53 b | 6.27±0.32 a | 3.58±0.02 c |
| 13 | 9.51±0.63 c | 2.71±0.03 c | 12.22±0.61 c | 4.27±0.19 b | 3.50±0.25 c |
| 16 | 6.49±0.29 d | 1.70±0.09 d | 8.19±0.23 d | 2.89±0.17 c | 3.83±0.35 bc |
| 19 | 2.43±0.07 e | 0.53±0.02 e | 2.96±0.09 e | 1.23±0.09 d | 4.59±0.1 ab |
| 22 | 1.83±0.15 e | 0.38±0.03 e | 2.22±0.16 e | 0.97±0.05 d | 4.82±0.55 a |
| 25 | 1.25±0.01 e | 0.25±0.01 e | 1.50±0.01 e | 0.63±0.09 d | 5.07±0.23 a |
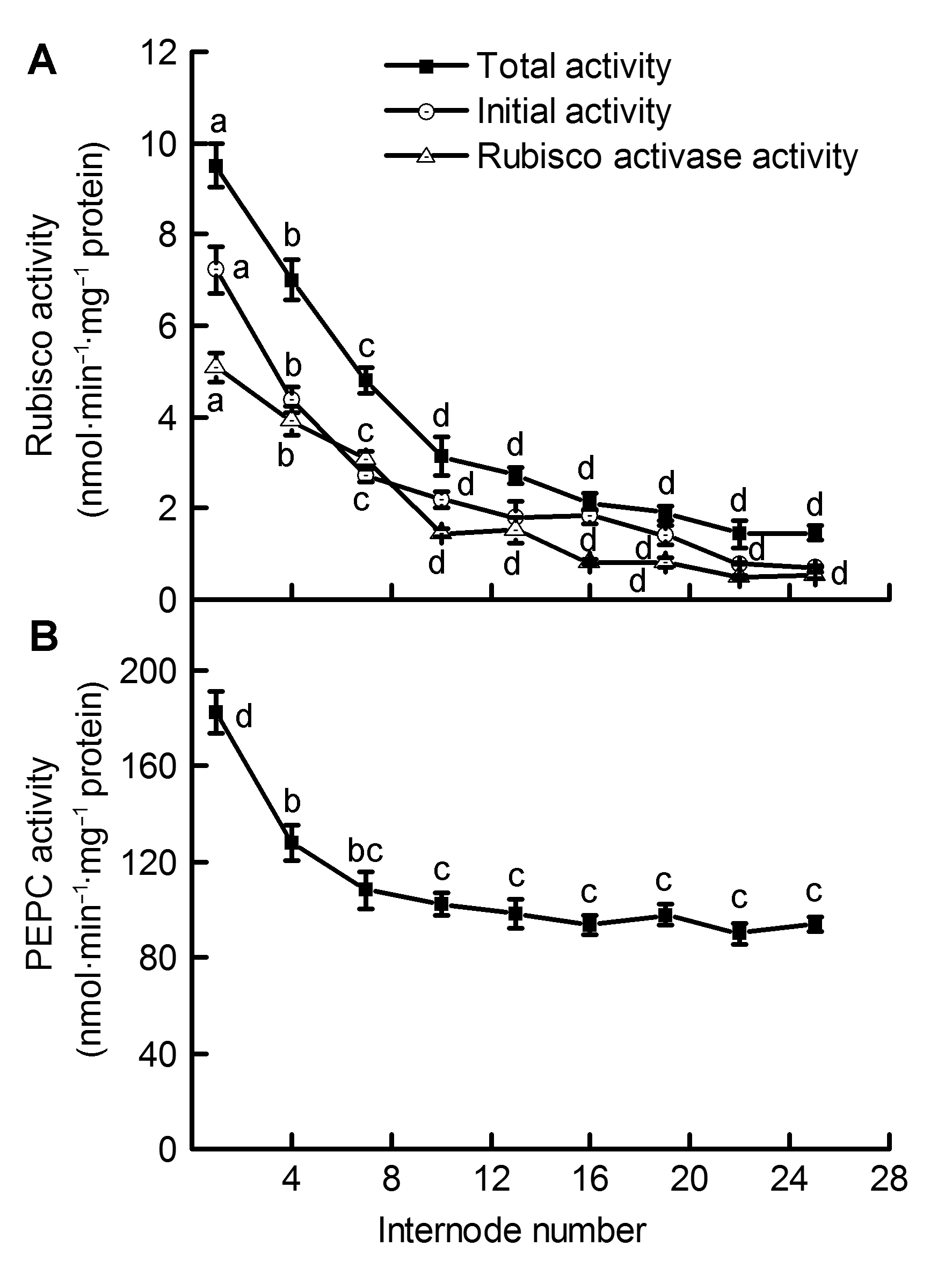
Figure 1 Differences of Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activity (A) and Phosphoenolpyruvate carboxylase (PEPC) activity (B) in the Phyllostachys edulis stems of different internodes (means±SD)
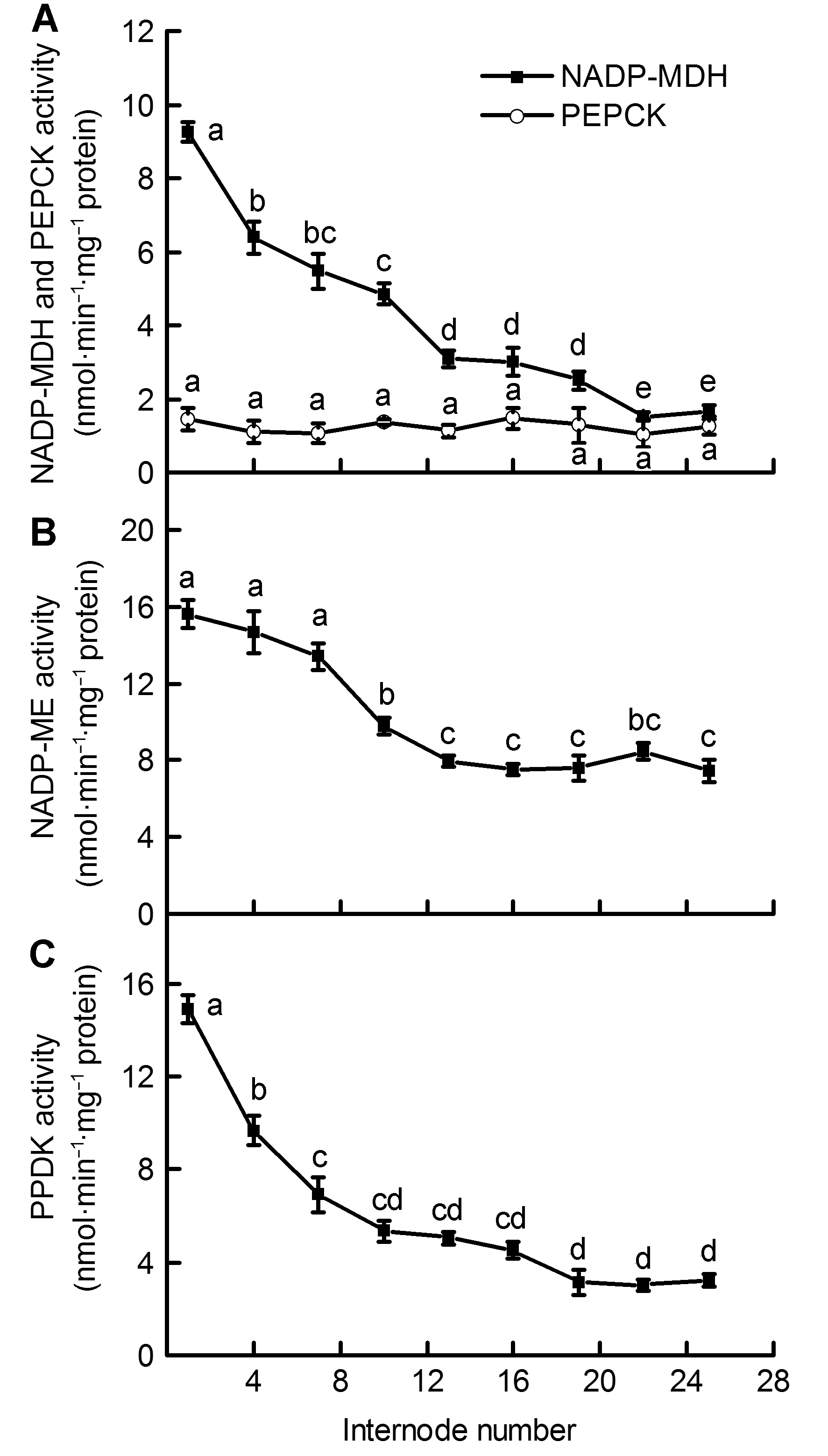
Figure 2 Differences of activities of 4 C4 enzymes in the Phyllostachys edulis stems of different internodes (means± SD) (A) NADP-malate dehydrogenase (NADP-MDH) activity and Phosphoenolpyruvate carboxy-kinase (PEPCK) activity; (B) NADP-malic enzyme (NADP-ME) activity; (C) Pyruvate, orthophosphate dikinase (PPDK) activity
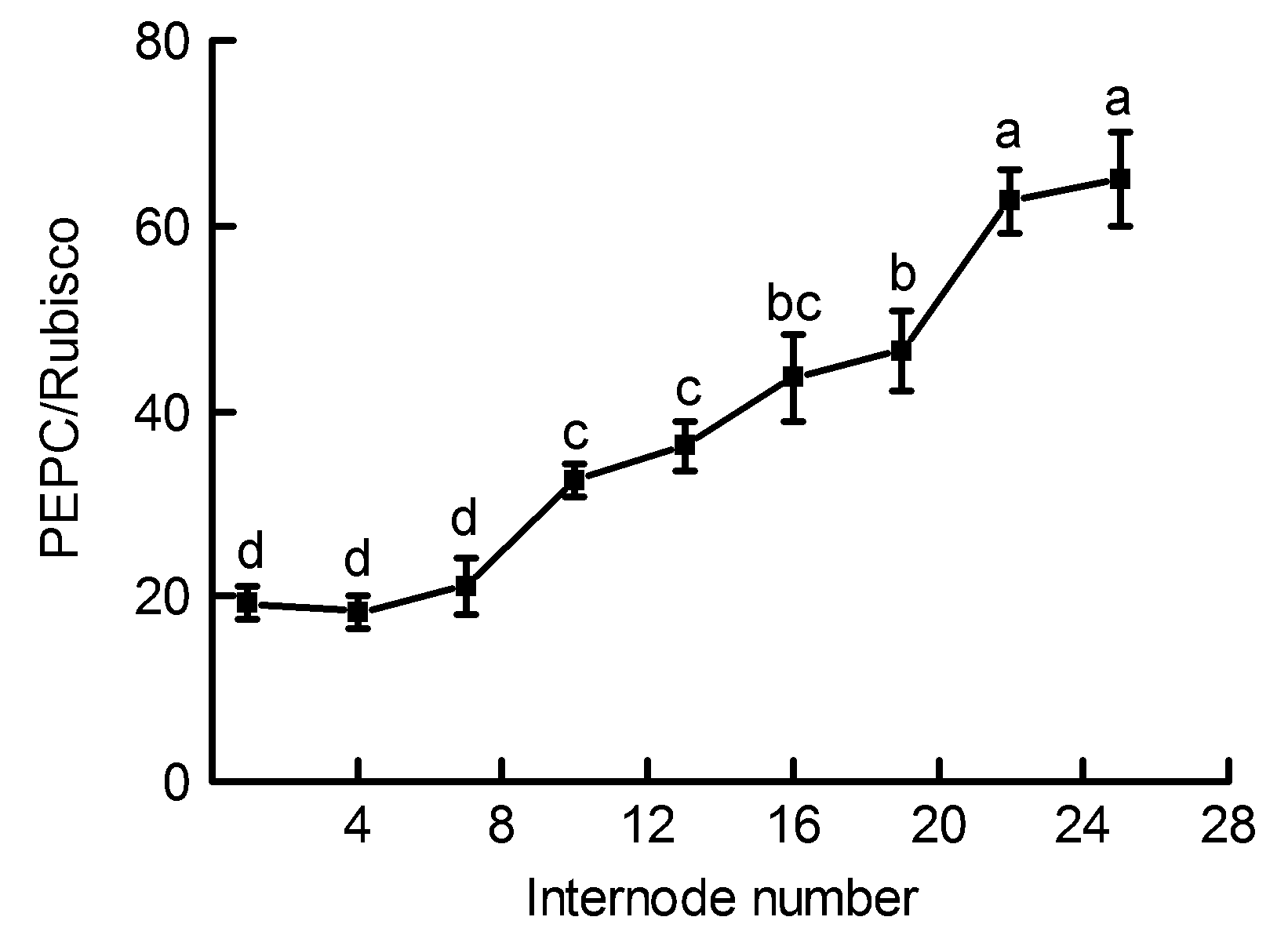
Figure 3 Changes of the ratios of Phosphoenolpyruvate carboxylase/Ribulose-1,5-bisphosphate carboxylase/oxygen- ase (PEPC/Rubisco) in the Phyllostachys edulis stems of different internodes (means±SD)
| [1] |
陈登举, 高培军, 吴兴波, 高岩, 温国胜, 王玉魁, 高荣孚, 张汝民 (2013). 毛竹茎秆叶绿体超微结构及其发射荧光光谱特征. 植物学报 48, 635-642.
DOI URL |
| [2] |
高苏娟, 谢修志, 陈兆平, 黄志刚, 赵琦, 王小菁 (2009). 蓝光调节高粱突变体har1幼苗的去黄化反应. 植物学报 44, 69-78.
DOI URL |
| [3] |
龚春梅, 宁蓬勃, 王根轩, 梁宗锁 (2009). C3和C4植物光合途径的适应性变化和进化. 植物生态学报 33, 206-221.
DOI URL |
| [4] | 郝迺斌, 谭克辉, 那松青, 贾志旺, 戈巧英, 张玉竹, 杜维广 (1991). C3植物绿色器官PEP羧化酶活性的比较研究. 植物学报 33, 692-697. |
| [5] | 姜振升, 孙晓琦, 艾希珍, 王美玲, 毕焕改, 王洪涛 (2010). 低温弱光对黄瓜幼苗Rubisco与Rubisco活化酶的影响. 应用生态学报 21, 2045-2050. |
| [6] |
孙建磊, 王崇启, 肖守华, 高超, 李利斌, 曹齐卫, 王晓, 董玉梅, 焦自高 (2017). 弱光对黄瓜幼苗光合特性及Rubisco酶的影响. 核农学报 31, 1200-1209.
DOI URL |
| [7] | 王文杰, 许慧男, 王莹, 于兴洋, 郑广宇, 祖元刚 (2010). 盐碱地土壤改良对银中杨叶片、树枝和树皮绿色组织色素和C4光合酶的影响. 植物研究 30, 299-304. |
| [8] |
王文杰, 祖元刚, 王慧梅 (2007). 林木非同化器官树枝(干)光合功能研究进展. 生态学报 27, 1583-1595.
DOI URL |
| [9] |
王星星, 刘琳, 张洁, 王玉魁, 温国胜, 高荣孚, 高岩, 张汝民 (2012). 毛竹出笋后快速生长期内茎秆中光合色素和光合酶活性的变化. 植物生态学报 36, 456-462.
DOI URL |
| [10] | 王莹, 王文杰, 许慧男, 郑广宇, 孙伟, 祖元刚 (2011). 3种C3木本植物绿色组织C4酶活性、色素含量及叶绿素荧光参数的比较. 植物研究 31, 461-466. |
| [11] |
徐超华, 李军营, 崔明昆, 马二登, 黄国宾, 龚明 (2013). 延长光照时间对烟草叶片生长发育及光合特性的影响. 西北植物学报 33, 763-770.
DOI URL |
| [12] |
杨春菊, 陈永刚, 汤孟平, 施拥军, 侯建花, 孙燕飞 (2016). 不同管理模式下毛竹幼竹的生长规律. 植物学报 51, 774-781.
DOI URL |
| [13] |
张金尧, 刘俊祥, 巨关升, 韩蕾, 孙振元 (2014). 旱柳非叶光合组织(皮层)叶绿体光合特性. 林业科学 50, 30-35.
DOI URL |
| [14] | 张汝民 (2005). 绿豆幼苗脱黄化初期质体发育生理生化机制的研究. 博士论文. 北京: 北京林业大学. pp. 45-47. |
| [15] |
庄明浩, 李迎春, 郭子武, 杨清平, 顾李俭, 陈双林, 邓宗付 (2013). 大气CO2与O3浓度升高对毛竹叶片膜脂过氧化和抗氧化系统的影响. 西北植物学报 33, 322-328.
DOI URL |
| [16] | 祖元刚, 张衷华, 王文杰, 杨逢建, 贺海升 (2006). 薇甘菊叶和茎的光合特性. 植物生态学报 30, 998-1004. |
| [17] |
Aschan G, Pfanz H (2003). Non-foliar photosynthesis—a strategy of additional carbon acquisition.Flora 198, 81-97.
DOI URL |
| [18] |
Aschan G, Wittmann C, Pfanz H (2001). Age-dependent bark photosynthesis of aspen twigs.Trees 15, 431-437.
DOI URL |
| [19] |
ávila E, Herrera A, Tezara W (2014). Contribution of stem CO2 fixation to whole-plant carbon balance in nonsucculent species.Photosynthetica 52, 3-15.
DOI URL |
| [20] |
Berveiller D, Damesin C (2008). Carbon assimilation by tree stems: potential involvement of phosphoenolpyruvate car- boxylase.Trees 22, 149-157.
DOI URL |
| [21] |
Berveiller D, Kierzkowski D, Damesin C (2007). Interspecific variability of stem photosynthesis among tree species.Tree Physiol 27, 53-61.
DOI URL PMID |
| [22] |
Bloemen J, Vergeynst LL, Overlaet-Michiels L, Steppe K (2016). How important is woody tissue photosynthesis in poplar during drought stress? Trees 30, 63-72.
DOI URL |
| [23] |
Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Anal Biochem 72, 248-254.
DOI URL PMID |
| [24] |
Burnell JN (1986). Purification and properties of phosphoenolpyruvate carboxykinase from C4 plants.Aust J Plant Physiol 13, 577-587.
DOI URL |
| [25] |
Burnell JN, Chastain CJ (2006). Cloning and expression of maize-leaf pyruvate, Pi dikinase regulatory protein gene.Biochem Biophys Res Commun 345, 675-680.
DOI URL PMID |
| [26] |
Carnal NW, Agostino A, Hatch MD (1993). Photosynthesis in phosphoenolpyruvate carboxykinase-type C4 plants: mechanism and regulation of C4 acid decarboxylation in bundle sheath cells.Arch Biochem Biophys 306, 360-367.
DOI URL |
| [27] |
Cen YP, Sage RF (2005). The regulation of rubisco activity in response to variation in temperature and atmospheric CO2 partial pressure in sweet potato.Plant Physiol 139, 979-990.
DOI URL PMID |
| [28] |
Chao Q, Liu XY, Mei YC, Gao ZF, Chen YB, Qian CR, Hao YB, Wan BC (2014). Light-regulated phosphorylation of maize phosphoenolpyruvate carboxykinase plays a vital role in its activity.Plant Mol Biol 85, 95-105.
DOI URL PMID |
| [29] |
Chastain CJ, Failing CJ, Manandhar L, Zimmerman MA, Lakner MM, Nguyen THT (2011). Functional evolution of C4 pyruvate, orthophosphate dikinase.J Exp Bot 62, 3083-3091.
DOI URL PMID |
| [30] |
Chen YB, Lu TC, Wang HX, Shen J, Bu TT, Chao Q, Gao ZF, Zhu XG, Wang YF, Wang BC (2014). Posttranslational modification of maize chloroplast pyruvate orthophosphate dikinase reveals the precise regulatory mechanism of its enzymatic activity.Plant Physiol 165, 534-549.
DOI URL PMID |
| [31] |
Cui K, He CY, Zhang JG, Duan AG, Zeng YF (2012). Temporal and spatial profiling of internode elongation-associated protein expression in rapidly growing culms of bamboo.J Proteome Res 11, 2492-2507.
DOI URL PMID |
| [32] |
Damesin C (2003). Respiration and photosynthesis characteristics of current-year stems of Fagus sylvatica: from the seasonal pattern to an annual balance. New Phytol 158, 465-475.
DOI URL |
| [33] |
Dima E, Manetas Y, Psaras GK (2006). Chlorophyll distribution pattern in inner stem tissues: evidence from epifluorescence microscopy and reflectance measurements in 20 woody species.Trees 20, 515-521.
DOI URL |
| [34] |
Hatch MD, Slack CR (1975). Pyruvate, Pi dikinase from leaves.Methods Enzymol 42, 212-219.
DOI URL |
| [35] |
Hibberd JM, Quick WP (2002). Characteristics of C4 photosynthesis in stems and petioles of C3 flowering plants.Nature 415, 451-454.
DOI URL |
| [36] | Hrstka M, Urban O, Babák L (2012). Seasonal changes of rubisco content and activity in Fagus sylvatica and Picea abies affected by elevated CO2 concentration. Chem Pap 66, 836-841. |
| [37] |
Johnson HS, Hatch MD (1970). Properties and regulation of leaf nicotinamide-adenine dinucleotide phosphate-malate dehydrogenase and ‘malic’ enzyme in plants with the C4-dicarboxylic acid pathway of photosynthesis.Biochem J 119, 273-280.
DOI URL |
| [38] |
Kocurek M, Pilarski J (2011). Activity of C4 enzymes in C3-type herbaceous plants.Photosynthetica 49, 473-477.
DOI URL |
| [39] | Kosvancová M, Urban O, Šprtová M, Hrstka M, Kalina J, Tomáskova I, Špunda V, Marek MV (2009). Photosynthetic induction in broadleaved Fagus sylvatica and conife- rous Picea abies cultivated under ambient and elevated CO2 concentrations. Plant Sci 177, 123-130. |
| [40] |
Leegood RC (2013). Strategies for engineering C4 photosynthesis.J Plant Physiol 170, 378-388.
DOI URL PMID |
| [41] | Leegood RC, Osmond CB (1990). The flux of metabolites in C4 and CAM plants. In: Dennis DT, Turpin DH, eds. Plant Physiology, Biochemistry and Molecular Biology. Essex: Longman Scientific & Technical. pp. 274-298. |
| [42] |
Lichtenthaler HK (1987). Chlorophylls and carotenoids: pigments of photosynthetic biomembranes.Methods Enzymol 148, 350-382.
DOI URL |
| [43] |
Muhaidat R, Sage RF, Dengler NG (2007). Diversity of Kranz anatomy and biochemistry in C4 eudicots.Am J Bot 94, 362-381.
DOI URL PMID |
| [44] |
Ocampo G, Columbus JT (2010). Molecular phylogenetics of suborder cactineae (caryophyllales), including insights into photosynthetic diversification and historical biogeography.Am J Bot 97, 1827-1847.
DOI URL PMID |
| [45] |
Parry MAJ, Andralojc PJ, Scales JC, Salvucci ME, Carmo-Silva AE, Alonso H, Whitney SM (2013). Rubisco activity and regulation as targets for crop improvement.J Exp Bot 64, 717-730.
DOI URL PMID |
| [46] | Peng ZH, Lu Y, Li LB, Zhao Q, Feng Q, Gao ZM, Lu HY, Hu T, Yao N, Liu KY, Li Y, Fan DL, Guo YL, Li WJ, Lu YQ, Weng QJ, Zhou CC, Zhang L, Huang T, Zhao Y, Zhu CR, Liu XE, Yang XW, Wang T, Miao K, Zhuang CY, Cao XL, Tang WL, Liu GS, Liu YL, Chen J, Liu ZJ, Yuan LC, Liu ZH, Huang XH, Lu TT, Fei BH, Ning ZM, Han B, Jiang ZH (2013). The draft genome of the fast-growing non-timber forest species moso bamboo (Phyllostachys heterocycla). Nat Genet 45, 456-461. |
| [47] |
Pfanz H (2008). Bark photosynthesis.Trees 22, 137-138.
DOI URL |
| [48] |
Portis Jr AR (2003). Rubisco activase-Rubisco’s catalytic chaperone.Photosynth Res 75, 11-27.
DOI URL |
| [49] |
Rangan P, Furtado A, Henry RJ (2016). New evidence for grain specific C4 photosynthesis in wheat.Sci Rep 6, 31721.
DOI URL |
| [50] |
Sage RF, Sage TL, Kocacinar F (2012). Photorespiration and the evolution of C4 photosynthesis.Annu Rev Plant Biol 63, 19-47.
DOI URL |
| [51] |
Sage RF, Sage TL, Pearcy RW, Borsch T (2007). The taxonomic distribution of C4 photosynthesis in Amaranthaceae sensu stricto.Am J Bot 94, 1992-2003.
DOI URL |
| [52] |
Saveyn A, Steppe K, Ubierna N, Dawson TE (2010). Woody tissue photosynthesis and its contribution to trunk growth and bud development in young plants.Plant Cell Environ 33, 1949-1958.
DOI URL PMID |
| [53] | Sheth BP, Thaker VS (2014). In silico analyses of Rubisco enzymes from different classes of Algae. Int Res J Biol Sci 3, 11-17. |
| [54] | Sun JL, Sui XL, Wang SH, Wei YX, Huang HY, Hu LP, Zhang ZX (2014). The response of rbcL, rbcS and rca genes in cucumber(Cucumis sativus L.) to growth and induction light intensity. Acta Physiol Plant 36, 2779-2791. |
| [55] |
Suzuki Y, Makino A (2012). Availability of Rubisco small subunit up-regulates the transcript levels of large subunit for stoichiometric assembly of its holoenzyme in rice.Plant Physiol 160, 533-540.
DOI URL PMID |
| [56] |
Teskey RO, Saveyn A, Steppe K, McGuire MA (2008). Origin, fate and significance of CO2 in tree stems.New Phytol 177, 17-32.
DOI URL |
| [57] |
Tikhonov KG, Khristin MS, Klimov VV, Sundireva MA, Kreslavski VD, Sidorov RA, Tsidendambayev VD, Savchenko TV (2017). Structural and functional characteristics of photosynthetic apparatus of chlorophyllcon- taining grape vine tissue.Russian J Plant Physiol 64, 73-82.
DOI URL |
| [58] |
Wang QM, Hou FY, Dong SX, Xie BT, Li AX, Zhang HY, Zhang LM (2014). Effects of shading on the photosynthetic capacity, endogenous hormones and root yield in purple-fleshed sweetpotato ( Ipomoea batatas (L.) Lam). Plant Growth Regul 72, 113-122.
DOI URL |
| [59] |
Wittmann C, Pfanz H (2014). Bark and woody tissue photosynthesis: a means to avoid hypoxia or anoxia in deve- loping stem tissues.Funct Plant Biol 41, 940-953.
DOI URL |
| [1] | LI An-Yan, HUANG Xian-Fei, TIAN Yuan-Bin, DONG Ji-Xing, ZHENG Fei-Fei, XIA Pin-Hua. Chlorophyll a variation and its driving factors during phase shift from macrophyte- to phytoplankton-dominated states in Caohai Lake, Guizhou, China [J]. Chin J Plant Ecol, 2023, 47(8): 1171-1181. |
| [2] | REN Pei-Xin, LI Peng, PENG Chang-Hui, ZHOU Xiao-Lu, YANG Ming-Xia. Temporal and spatial variation of vegetation photosynthetic phenology in Dongting Lake basin and its response to climate change [J]. Chin J Plant Ecol, 2023, 47(3): 319-330. |
| [3] | SHI Sheng-Bo, ZHOU Dang-Wei, LI Tian-Cai, DE Ke-Jia, GAO Xiu-Zhen, MA Jia-Lin, SUN Tao, WANG Fang-Lin. Responses of photosynthetic function of Kobresia pygmaea to simulated nocturnal low temperature on the Qingzang Plateau [J]. Chin J Plant Ecol, 2023, 47(3): 361-373. |
| [4] | Jiayi Jin, Yiting Luo, Huimin Yang, Tao Lu, Hanfei Ye, Jiyi Xie, Kexin Wang, Qianyu Chen, Yuan Fang, Yuexing Wang, Yuchun Rao. QTL Mapping and Expression Analysis on Candidate Genes Related to Chlorophyll Content in Rice [J]. Chinese Bulletin of Botany, 2023, 58(3): 394-403. |
| [5] | YU Yu-Rong, WU Hao, GAO Ya-Fei, ZHAO Yuan-Bo, LI Xiao-Ling, BU Gui-Jun, XUE Dan, LIU Zheng-Xiang, WU Hai-Wen, WU Lin. Effects of simulated nitrogen deposition on physiological and morphological characteristics of Sphagnum in wetland, southwestern Hubei Province, China [J]. Chin J Plant Ecol, 2023, 47(11): 1493-1506. |
| [6] | SHI Sheng-Bo, SHI Rui, ZHOU Dang-Wei, ZHANG Wen. Effects of low temperature on photochemical and non-photochemical energy dissipation of Kobresia pygmaea leaves [J]. Chin J Plant Ecol, 2023, 47(10): 1441-1452. |
| [7] | ZHENG Ning, LI Su-Ying, WANG Xin-Ting, LÜ Shi-Hai, ZHAO Peng-Cheng, ZANG Chen, XU Yu-Long, HE Jing, QIN Wen-Hao, GAO Heng-Rui. Dominance of different plant life forms in the typical steppe evidenced from impacts of environmental factors on chlorophyll [J]. Chin J Plant Ecol, 2022, 46(8): 951-960. |
| [8] | Hao Wang, Ming Wang, Ting Liang, Yuxin Yao, Yuanpeng Du, Zhen Gao. Effects of High Air and Root Zone Temperature on Photosynthetic Fluorescence Characteristics of Grape Leaves [J]. Chinese Bulletin of Botany, 2022, 57(2): 209-216. |
| [9] | XUE Jin-Ru, LÜ Xiao-Liang. Assessment of vegetation productivity under the implementation of ecological programs in the Loess Plateau based on solar-induced chlorophyll fluorescence [J]. Chin J Plant Ecol, 2022, 46(10): 1289-1304. |
| [10] | WU Lin-Sheng, ZHANG Yong-Guang, ZHANG Zhao-Ying, ZHANG Xiao-Kang, WU Yun-Fei. Remote sensing of solar-induced chlorophyll fluorescence and its applications in terrestrial ecosystem monitoring [J]. Chin J Plant Ecol, 2022, 46(10): 1167-1199. |
| [11] | ZHOU Wen, CHI Yong-Gang, ZHOU Lei. Vegetation phenology in the Northern Hemisphere based on the solar-induced chlorophyll fluorescence [J]. Chin J Plant Ecol, 2021, 45(4): 345-354. |
| [12] | DING Jian-Xi, ZHOU Lei, WANG Yong-Lin, ZHUANG Jie, CHEN Ji-Jing, ZHOU Wen, ZHAO Ning, SONG Jun, CHI Yong-Gang. Application prospects for combining active and passive observations of chlorophyll fluorescence [J]. Chin J Plant Ecol, 2021, 45(2): 105-118. |
| [13] | XUN Yan-Han, DI Xue-Ying, JIN Guang-Ze. Vertical variation and economic strategy of leaf trait of major tree species in a typical mixed broadleaved-Korean pine forest [J]. Chin J Plant Ecol, 2020, 44(7): 730-741. |
| [14] | Jianfu Liu, Yucai Chen, Wenjian Wang, Hechuan Wang, Jinfu Cai, Mingyuan Wang, Dandan Li, Bin Zhang, Kun Huang. Effects of Space Treatment on Biological and Growth Characteristics of Camellia sinensis [J]. Chinese Bulletin of Botany, 2020, 55(5): 564-572. |
| [15] | GUO Qing-Hua, HU Tian-Yu, MA Qin, XU Ke-Xin, YANG Qiu-Li, SUN Qian-Hui, LI Yu-Mei, SU Yan-Jun. Advances for the new remote sensing technology in ecosystem ecology research [J]. Chin J Plant Ecol, 2020, 44(4): 418-435. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||