

Chinese Bulletin of Botany ›› 2018, Vol. 53 ›› Issue (4): 509-518.DOI: 10.11983/CBB17115 cstr: 32102.14.CBB17115
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Chen Keyi, Li Zhaona, Cheng Minmin, Zhao Yanghui, Zhou Mingbing, Yang Haiyun*( )
)
Received:2017-06-10
Accepted:2017-10-07
Online:2018-07-01
Published:2018-09-11
Contact:
Yang Haiyun
About author:† These authors contributed equally to this paper
Chen Keyi, Li Zhaona, Cheng Minmin, Zhao Yanghui, Zhou Mingbing, Yang Haiyun. Chloroplast Ultrastructure and Chlorophyll Fluorescence Characteristics of Three Cultivars of Pseudosasa japonica[J]. Chinese Bulletin of Botany, 2018, 53(4): 509-518.
| Photosynthetic pigments | GL | SA | SG | VL |
|---|---|---|---|---|
| Chla (mg·g-1 FW) | 27.19±1.17 a | 0.34±0.17 c | 25.39±2.41 a | 17.09±0.52 b |
| Chlb (mg·g-1 FW) | 8.66±0.33 a | 0.16±0.07 c | 8.27±0.69 a | 5.70±0.17 b |
| Car (mg·g-1 FW) | 4.89±0.28 a | 0.32±0.08 c | 5.52±0.55 a | 4.07±0.13 b |
| Chla/b | 3.14±0.02 a | 1.99±0.09 b | 3.07±0.03 a | 3.00±0.00 a |
| Chla+b (mg·g-1 FW) | 35.86±1.51 a | 0.51±0.25 c | 33.66±3.11 a | 22.79±0.70 b |
Table 1 Photosynthetic pigments content and relative ratio of different cultivars of Pseudosasa japonica leaves (means±SE)
| Photosynthetic pigments | GL | SA | SG | VL |
|---|---|---|---|---|
| Chla (mg·g-1 FW) | 27.19±1.17 a | 0.34±0.17 c | 25.39±2.41 a | 17.09±0.52 b |
| Chlb (mg·g-1 FW) | 8.66±0.33 a | 0.16±0.07 c | 8.27±0.69 a | 5.70±0.17 b |
| Car (mg·g-1 FW) | 4.89±0.28 a | 0.32±0.08 c | 5.52±0.55 a | 4.07±0.13 b |
| Chla/b | 3.14±0.02 a | 1.99±0.09 b | 3.07±0.03 a | 3.00±0.00 a |
| Chla+b (mg·g-1 FW) | 35.86±1.51 a | 0.51±0.25 c | 33.66±3.11 a | 22.79±0.70 b |

Figure 1 Three kinds of Pseudosasa japonica leavesSA+SG: Albino and green sector in leaf of P. japonica f. akebonosuji with strips; VL: Virescent leaf of P. japonica f. akebono. GL: Green leaf of P. japonica
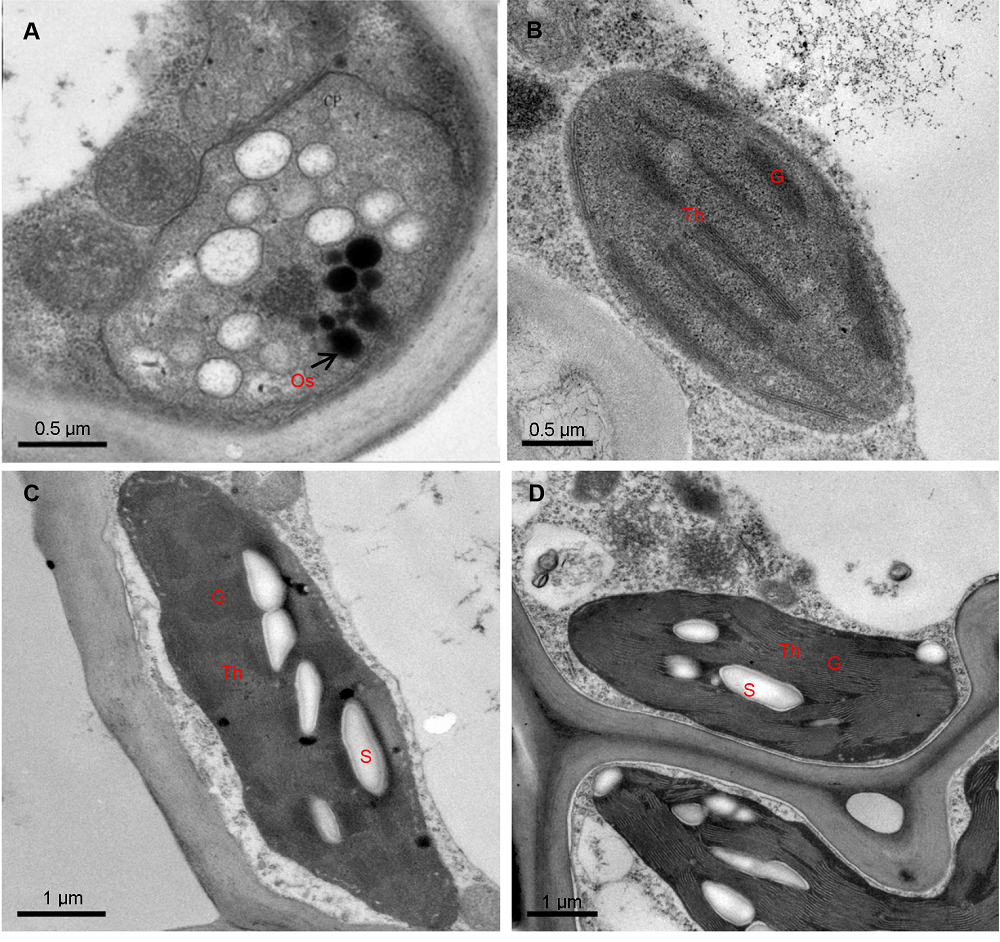
Figure 2 Plate chloroplast ultrastructure of three cultivars of Pseudosasa japonica leaves(A) Mesophyll cells in white zones of zebra leaf of P. japonica f. akebonosuji; (B) Mesophyll cells in green zones of P. japonica f. akebonosuji; (C) Mesophyll cells in the leaf of P. japonica f. akebono; (D) Mesophyll cells in the leaf of P. japonica. G: Granum; Os: Osmiophile globule; S: Starch grain; Th: Thylakoid membranes
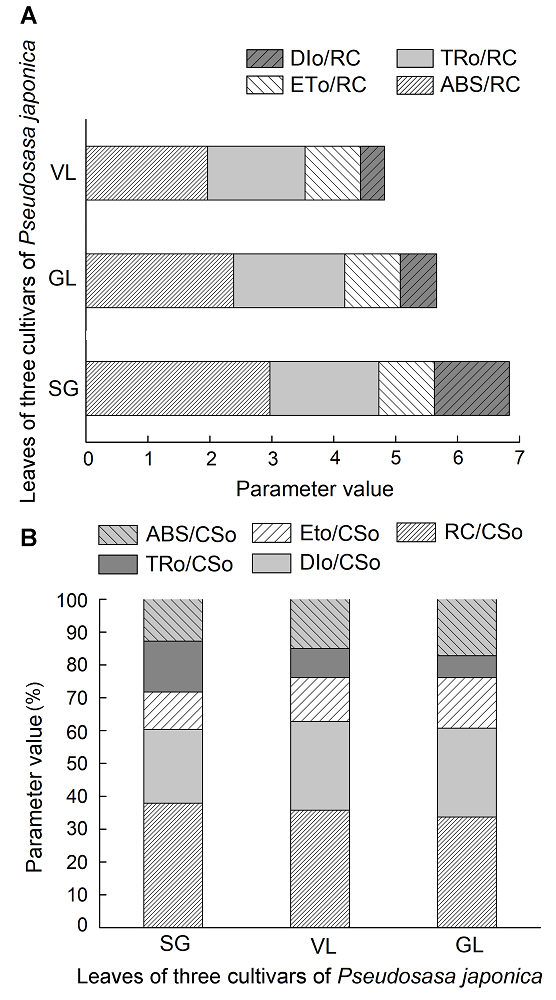
Figure 4 Activity parameters for unit reaction center of different cultivars of Pseudosasa japonica leavesVL, GL and SG see Table 1. ABS/RC: The amount of light absorbed by the unit reaction center; TRo/RC: The large amount of PSII; ETo/RC: The energy of the unit reaction center for electron transfer; DIo/RC: The heat dissipation of the unit reaction center; ABS/CSo: Absorption flux per unit area; TRo/CSo: Trapped energy flux per unit area; ETo/CSo: Electron transport flux per unit area; DIo/CSo: Dissipated energy flux perunit area; RC/CSo: Number of active reaction centers per unit area
| GL | VL | SG | |
|---|---|---|---|
| F0 | 0.40±0.01 b | 0.44±0.03 a | 0.39±0.04 ab |
| Fm | 1.64±0.13 a | 1.35±0.16 a | 1.29±0.17 a |
| Fv/Fm | 0.75±0.02 a | 0.67±0.02 b | 0.63±0.01 b |
| Fv/F0 | 3.11±0.36 a | 2.01±0.19 b | 2.26±0.14 b |
| Y(II) | 0.38±0.04 b | 0.27±0.03 a | 0.31±0.04 a |
| NPQ | 1.27±0.12 b | 1.71±0.21 a | 1.47±0.18 ab |
| qP | 0.77±0.05 a | 0.77±0.03 a | 0.79±0.04 a |
| ETR | 23.00±2.94 a | 16.22±1.64 a | 19.00±2.83 a |
Table 2 Analysis of fluorescence parameters of different cultivars of Pseudosasa japonica leaves
| GL | VL | SG | |
|---|---|---|---|
| F0 | 0.40±0.01 b | 0.44±0.03 a | 0.39±0.04 ab |
| Fm | 1.64±0.13 a | 1.35±0.16 a | 1.29±0.17 a |
| Fv/Fm | 0.75±0.02 a | 0.67±0.02 b | 0.63±0.01 b |
| Fv/F0 | 3.11±0.36 a | 2.01±0.19 b | 2.26±0.14 b |
| Y(II) | 0.38±0.04 b | 0.27±0.03 a | 0.31±0.04 a |
| NPQ | 1.27±0.12 b | 1.71±0.21 a | 1.47±0.18 ab |
| qP | 0.77±0.05 a | 0.77±0.03 a | 0.79±0.04 a |
| ETR | 23.00±2.94 a | 16.22±1.64 a | 19.00±2.83 a |
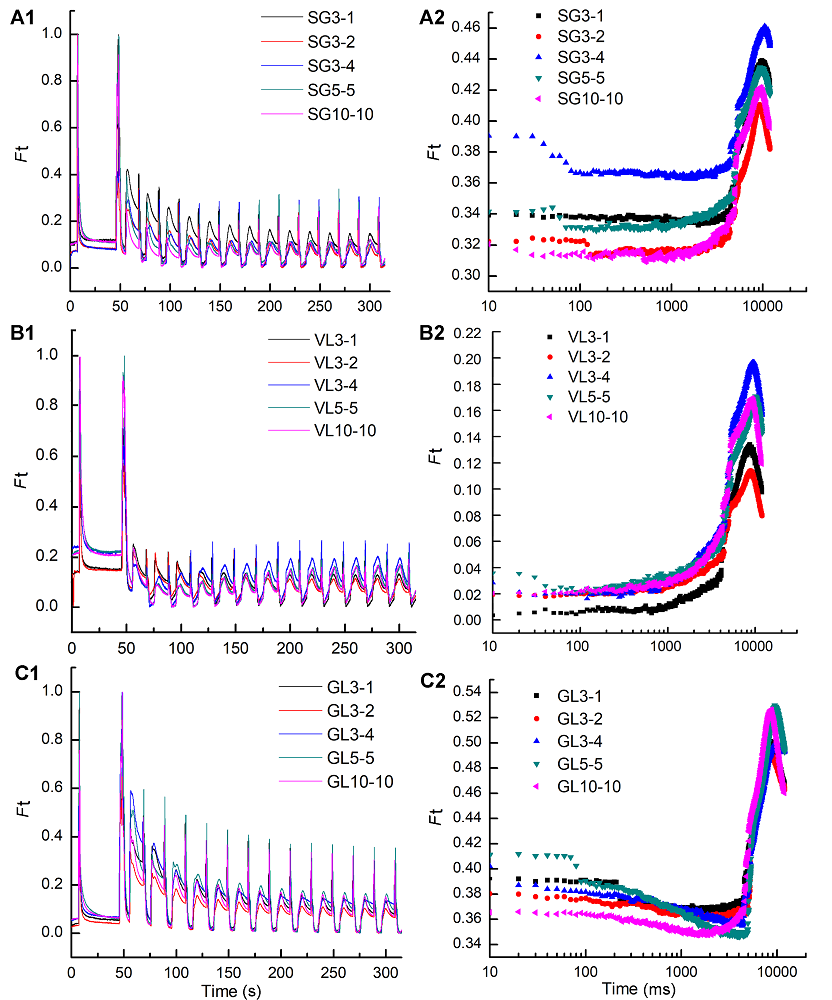
Figure 5 The change of fluorescence transients under different intensity and time of three cultivars of Pseudosasa japonica leaves under far-red light treatments(A1), (A2) The change of fluorescence transients (A1) and steady-state fluorescence (A2) of green sector in leaf with strips of P. japonica f. akebonosuji under different intensity and time of far-red light treatments; (B1), (B2) The change of fluorescence transients (B1) and steady-state fluorescence (B2) of the virescent leaves of P. japonica f. akebono under different intensity and time of far-red light treatments; (C1), (C2) The change of fluorescence transients (C1) and steady-state fluorescence (C2) of the green leaves of P. japonica under different intensity and time of far-red light treatments. SG, VL and GL see Table 1; Ft: Real-time fluorescence curve.
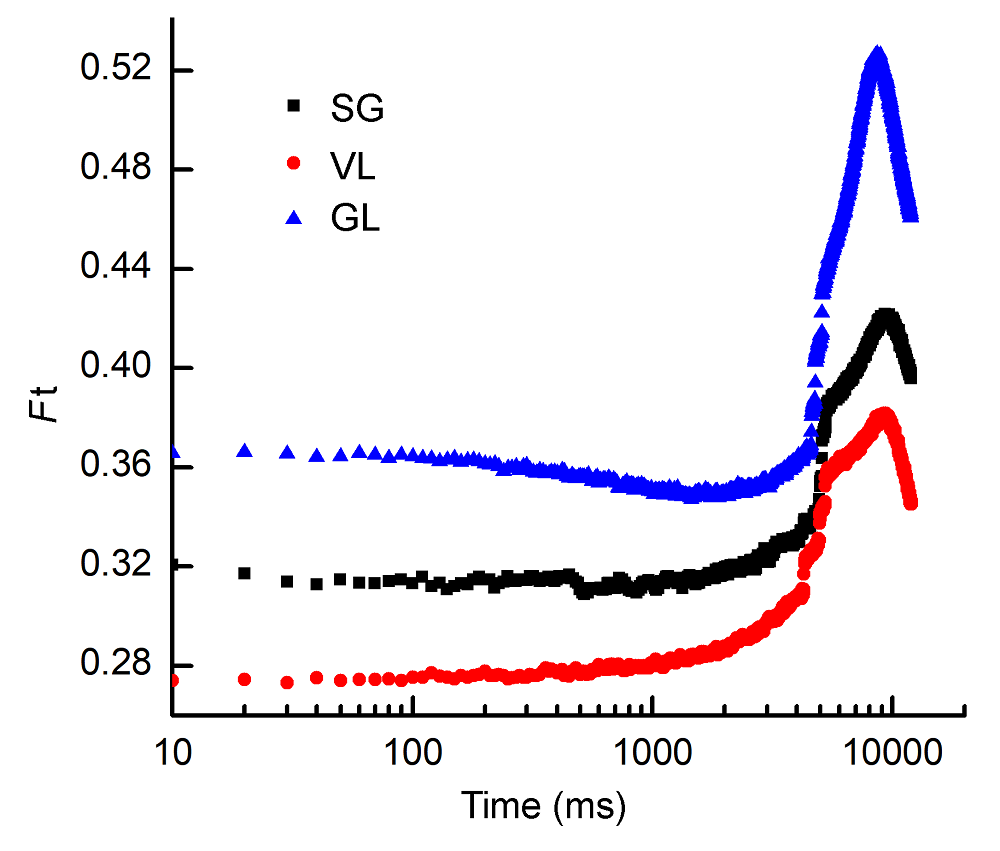
Figure 6 The change of steady-state fluorescence of 10-10 (intensity-time) of three cultivars of Pseudosasa japonica leaves under far-red light treatmentsSG, VL and GL see Table 1; Ft see Figure 5.
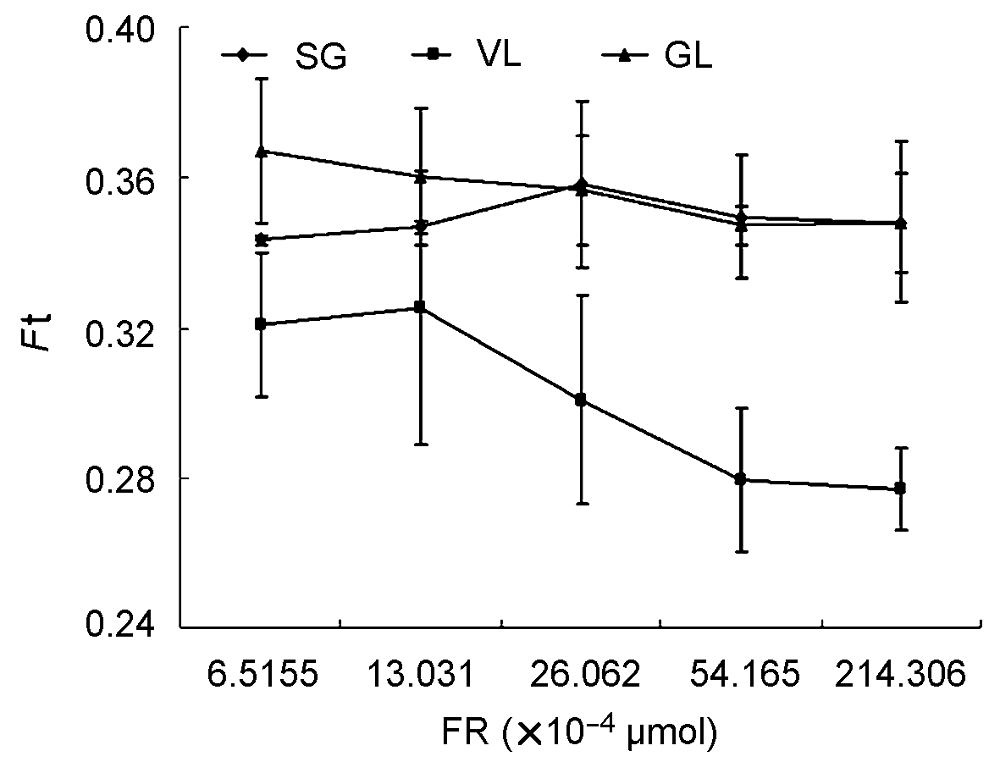
Figure 7 The change of F0′ (minimum fluorescence under the light) of three cultivars of Pseudosasa japonica leaves under different intensity of far-red light treatmentsSG, VL and GL see Table 1; Ft see Figure 5; FR: The intensity of far red light.
| 1 | 耿东梅, 单立山, 李毅, Жигунов Анатолий Васильевич (2014). 土壤水分胁迫对红砂幼苗叶绿素荧光和抗氧化酶活性的影响. 植物学报 49, 282-291. |
| 2 | 桂仁意, 刘亚迪, 郭小勤, 季海宝, 贾月, 余明增, 方伟 (2010). 不同剂量137Cs-γ辐射对毛竹幼苗叶片叶绿素荧光参数的影响. 植物学报 45, 66-72. |
| 3 | 何冰, 刘玲珑, 张文伟, 万建民 (2006). 植物叶色突变体. 植物生理学通讯 1, 1-9. |
| 4 | 李鹏民 (2006). 快速叶绿素荧光诱导动力学在植物逆境生理研究中的应用. 博士论文. 泰安: 山东农业大学. pp. 66-73. |
| 5 | 林世青, 许春辉, 张其德, 徐黎, 毛大璋, 匡廷云 (1992). 叶绿素荧光动力学在植物抗性生理学、生态学和农业现代化中的应用. 植物学通报 9, 1-16. |
| 6 | 欧明明, 蔡伟民 (2005). 铁限制对铜绿微囊藻光系统活性变化的影响. 环境化学 24(6), 22-24. |
| 7 | 孙鲁龙, 耿庆伟, 邢浩, 杜远鹏, 翟衡 (2017). 低温处理葡萄根系对叶片PSII活性的影响. 植物学报 52, 159-166. |
| 8 | 唐茜, 施嘉璠 (1997). 川西茶区主栽品种光合强度与叶片结构相关关系的研究. 四川农业大学学报 15(2), 193-198. |
| 9 | 许大全 (2001). 光合作用效率. 上海: 上海科学技术出版社. pp. 136-150. |
| 10 | 杨莉, 郭蔼光, 关旭 (2003). 小麦突变体返白系返白阶段叶绿体超微结构变化研究. 西北农业学报 12(4), 64-67. |
| 11 | 张阿宏, 齐孟文, 张晔晖 (2008). 调制叶绿素荧光动力学参数及其计量关系的意义和公理化讨论. 核农学报 22, 909-912. |
| 12 | 张宪政 (1986). 植物叶绿素含量测定——丙酮乙醇混合液法. 辽宁农业科学 3, 26-28. |
| 13 | 赵云, 王茂林, 李江, 张义正 (2003). 幼叶黄化油菜(Brassica napus L.)突变体Cr3529叶绿体超微结构观察. 四川大学学报(自然科学版) 5, 974-977. |
| 14 | 郑彩霞, 高荣孚 (1999). 光系统I的异质性及其在类囊体膜上分布的研究进展. 北京林业大学学报 21(5), 79-87. |
| 15 | 钟传飞 (2008). 稳态叶绿素荧光动力学理论构建和常绿阔叶植物越冬光合生理生态研究. 博士论文. 北京: 北京林业大学. pp. 47-49. |
| 16 | Deell JR, Murr DP, Wiley L (2003). 1-Methylcyclopropene (1-MCP) increases CO2 injury in apples.Acta Horticul 600, 277-280. |
| 17 | Gilmore AM, Hazlett TL, Govindjee (1997). Xanthophyll cycle-dependent quenching of photosystem II chlorophyll a fluorescence: formation of a quenching complex with a short fluorescence life time.Proc Natl Acad Sci USA 92, 2273-2277. |
| 18 | Heerden PDRV, Strasser RJ, Krüger GHJ (2010). Reduction of dark chilling stress in N2-fixing soybean by nitrate as indicated by chlorophyll a fluorescence kinetics. Physiol Plantarum 121, 239-249. |
| 19 | Lichtenthaler HK (1987). Chlorophylls and carotenoids: pigments of photosynthetic biomembranes.Methods Enzymol 148, 350-382. |
| 20 | Murchie EH, Lawson T (2013). Chlorophyll fluorescence analysis: a guide to good practice and understanding so- me new applications.J Exp Bot 64, 3983-3998. |
| 21 | Papageorgiou GC, Tsimillimichael M, Stamatakis K (2007). The fast and slow kinetics of chlorophyll a fluorescence induction in plants, algae and cyanobacteria: a viewpoint.Photosynth Res 94, 275-290. |
| 22 | Schreiber U (2004). Pulse-amplitude-modulation (PAM) fluo- rometry and saturation pulse method: an overview. In: Papageorgiou GC, Govindjee, eds. Chlorophyll a Fluorescence. Heidelberg: Springer. pp. 279-319. |
| 23 | Strasser RJ, Tsimilli-michael M, Srivastava A (2004). Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee, eds. Chlorophyll a Fluorescence: A Signature of Photosynthesis. Dordrecht: Springer. pp. 321-362. |
| 24 | Tsimilli-Michael M, Strasser RJ (2008). In vivo assessment of stress impact on Plant’s Vitality: Applications in Detecting and Evaluating the Beneficial Role of Mycorrhization on Host Plants. In: Varma A, eds. Heidelberg: Springer. pp. 679-703. |
| 25 | Wang Q, Sullivan RW, Kight A, Henry RL, Huang JR, Jones AM, Korth KL (2004). Deletion of the chloroplast- localized Thylakoid formation1 gene product in Arabidopsis leads to deficient thylakoid formation and variegated leaves. Plant Physiol 136, 3594-3604. |
| 26 | Waters MT, Langdale JA (2009). The making of a chloroplast.EMBO J 28, 2861-2873. |
| 27 | Wu JX, Zhang ZG, Zhang Q, Han X, Gu XF, Lu TG (2015). The molecular cloning and clarification of a photorespiratory mutant, oscdm1, using enhancer trapping. Front Ge- net 6, 226. |
| 28 | Zhu XG, Govindjee, Baker NR, deSturler E, Ort DR, Long SP (2005). Chlorophyll a fluorescence induction kinetics in leaves predicted from a model describing each discrete step of excitation energy and electron transfer associated with Photosystem II.Planta 223, 114-133. |
| [1] | WANG Bei-Bei, WU Su, WANG Miao-Miao, HU Jin-Tao. Contributions of radiative, structural, and physiological information of solar-induced chlorophyll fluorescence on predicting crop gross primary production across temporal scales [J]. Chin J Plant Ecol, 2025, 49(4): 562-572. |
| [2] | YAN Xiao-Li, LIU Gui-Mei, LI Xiao-Yu, JIANG Yu-Xiang, QUAN Xiao-Qiang, WANG Yan-Ru, QU Lu-Ping, TANG Xing-Hao. Photosynthetic characteristics and chlorophyll fluorescence parameters in Schima superba seedlings under different level of nitrogen addition and NH4+-N to NO3--N ratio [J]. Chin J Plant Ecol, 2025, 49(4): 624-637. |
| [3] | LIU Ke-Yan, HAN Lu, SONG Wu-Ye, ZHANG Chu-Rui, HU Xu, XU Hang, CHEN Li-Xin. Detection of drought effects on photosynthetic stability of vegetation on the Loess Plateau based on solar-induced chlorophyll fluorescence [J]. Chin J Plant Ecol, 2025, 49(3): 415-431. |
| [4] | QUAN Xiao-Qiang, WANG Yan-Ru, LI Xiao-Yu, LIANG Hai-Yan, WANG Li-Dong, YAN Xiao-Li. Effects of nitrogen addition level and NH4+-N to NO3--N ratio on photosynthetic characteristics and chlorophyll fluorescence parameters in Cunninghamia lanceolata seedling [J]. Chin J Plant Ecol, 2024, 48(8): 1050-1064. |
| [5] | WANG Ni, LI Zhao-Na, ZHENG Xu-Li, JIANG Si-Cheng, YANG Hai-Yun. Pigment synthesis and photosynthetic characteristics of leaves in Pseudosasa japonica f. akebonosuji [J]. Chin J Plant Ecol, 2024, 48(11): 1536-1546. |
| [6] | SHI Sheng-Bo, ZHOU Dang-Wei, LI Tian-Cai, DE Ke-Jia, GAO Xiu-Zhen, MA Jia-Lin, SUN Tao, WANG Fang-Lin. Responses of photosynthetic function of Kobresia pygmaea to simulated nocturnal low temperature on the Qingzang Plateau [J]. Chin J Plant Ecol, 2023, 47(3): 361-373. |
| [7] | REN Pei-Xin, LI Peng, PENG Chang-Hui, ZHOU Xiao-Lu, YANG Ming-Xia. Temporal and spatial variation of vegetation photosynthetic phenology in Dongting Lake basin and its response to climate change [J]. Chin J Plant Ecol, 2023, 47(3): 319-330. |
| [8] | SHI Sheng-Bo, SHI Rui, ZHOU Dang-Wei, ZHANG Wen. Effects of low temperature on photochemical and non-photochemical energy dissipation of Kobresia pygmaea leaves [J]. Chin J Plant Ecol, 2023, 47(10): 1441-1452. |
| [9] | Li Chen, Liu Jianting, Fan Yongxin, Zhao Xuehui, Xiao Wei, Chen Xiude, Fu Xiling, Li Ling, Li Dongmei. Effects of UV-B on Photosynthetic Function and Chloroplast Ultrastructure of Peach Leaves Grown in Greenhouse [J]. Chinese Bulletin of Botany, 2022, 57(4): 434-443. |
| [10] | Hao Wang, Ming Wang, Ting Liang, Yuxin Yao, Yuanpeng Du, Zhen Gao. Effects of High Air and Root Zone Temperature on Photosynthetic Fluorescence Characteristics of Grape Leaves [J]. Chinese Bulletin of Botany, 2022, 57(2): 209-216. |
| [11] | XUE Jin-Ru, LÜ Xiao-Liang. Assessment of vegetation productivity under the implementation of ecological programs in the Loess Plateau based on solar-induced chlorophyll fluorescence [J]. Chin J Plant Ecol, 2022, 46(10): 1289-1304. |
| [12] | WU Lin-Sheng, ZHANG Yong-Guang, ZHANG Zhao-Ying, ZHANG Xiao-Kang, WU Yun-Fei. Remote sensing of solar-induced chlorophyll fluorescence and its applications in terrestrial ecosystem monitoring [J]. Chin J Plant Ecol, 2022, 46(10): 1167-1199. |
| [13] | ZHOU Wen, CHI Yong-Gang, ZHOU Lei. Vegetation phenology in the Northern Hemisphere based on the solar-induced chlorophyll fluorescence [J]. Chin J Plant Ecol, 2021, 45(4): 345-354. |
| [14] | DING Jian-Xi, ZHOU Lei, WANG Yong-Lin, ZHUANG Jie, CHEN Ji-Jing, ZHOU Wen, ZHAO Ning, SONG Jun, CHI Yong-Gang. Application prospects for combining active and passive observations of chlorophyll fluorescence [J]. Chin J Plant Ecol, 2021, 45(2): 105-118. |
| [15] | Jianfu Liu, Yucai Chen, Wenjian Wang, Hechuan Wang, Jinfu Cai, Mingyuan Wang, Dandan Li, Bin Zhang, Kun Huang. Effects of Space Treatment on Biological and Growth Characteristics of Camellia sinensis [J]. Chinese Bulletin of Botany, 2020, 55(5): 564-572. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||