

植物学报 ›› 2018, Vol. 53 ›› Issue (1): 72-81.DOI: 10.11983/CBB17004 cstr: 32102.14.CBB17004
许馨露, 李丹丹, 马元丹*( ), 翟建云, 孙建飞, 高岩, 张汝民
), 翟建云, 孙建飞, 高岩, 张汝民
收稿日期:2017-01-06
接受日期:2017-05-04
出版日期:2018-01-01
发布日期:2018-08-10
通讯作者:
马元丹
基金资助:
Xinlu Xu, Dandan Li, Yuandan Ma*( ), Jianyun Zhai, Jianfei Sun, Yan Gao, Rumin Zhang
), Jianyun Zhai, Jianfei Sun, Yan Gao, Rumin Zhang
Received:2017-01-06
Accepted:2017-05-04
Online:2018-01-01
Published:2018-08-10
Contact:
Yuandan Ma
摘要: 以天香台阁四季桂(Osmanthus fragrans cv. ‘Tian Xiang TaiGe’)为材料, 研究干旱(轻度、中度和重度)、高温(40°C)及干旱高温协同胁迫对四季桂叶片抗氧化防御系统的影响。结果显示, 干旱胁迫下, 四季桂活性氧(ROS)逐渐积累, 膜脂过氧化程度加深; 轻度和中度干旱胁迫下, 抗氧化酶活性显著升高; 重度干旱胁迫下, 抗坏血酸(AsA)及其还原力(AsA/DHA)显著降低, 谷胱甘肽(GSH)及其还原力(GSH/GSSG)以及抗坏血酸-谷胱甘肽(AsA-GSH)循环相关酶活性呈先上升后下降的趋势, 在中度干旱胁迫时达到峰值。高温胁迫显著增强ROS积累、抗氧化酶活性、抗氧化剂含量及AsA-GSH循环效率。干旱高温协同胁迫下, 四季桂所受伤害大于单一胁迫, ROS在抗氧化酶的作用下增幅减缓; 随着胁迫强度的加剧, AsA-GSH循环效率呈先增加后下降的趋势, 重度协同胁迫时显著降低, 无法维持氧化还原平衡。四季桂在干旱高温胁迫下能快速启动体内抗氧化防御系统, 清除体内过量的ROS, 增加机体还原力, 以减缓胁迫带来的伤害。
许馨露, 李丹丹, 马元丹, 翟建云, 孙建飞, 高岩, 张汝民. 四季桂抗氧化防御系统对干旱、高温及协同胁迫的响应. 植物学报, 2018, 53(1): 72-81.
Xinlu Xu, Dandan Li, Yuandan Ma, Jianyun Zhai, Jianfei Sun, Yan Gao, Rumin Zhang. Responses of the Antioxidant Defense System of Osmanthus fragrans cv. ‘Tian Xiang TaiGe’ to Drought, Heat and the Synergistic Stress. Chinese Bulletin of Botany, 2018, 53(1): 72-81.
| Temperature | Treatment intensity | O2-· (nmol·g-1 FW) | H2O2 (μmol·g-1 FW) | MDA (μmol·g-1 FW) |
|---|---|---|---|---|
| 28°C | CK | 7.56±0.77 C | 20.11±1.01 D | 4.09±0.64 C |
| Light drought | 14.04±0.44 B | 33.08±2.33 C | 7.42±1.32 B | |
| Moderate drought | 15.49±0.45 B | 40.60±2.30 B | 14.29±2.55 A | |
| Heavy drought | 18.84±2.05 A | 49.24±1.74 A | 15.39±1.69 A | |
| Sum of squares | Between groups (d.f.1=3) | 402.69 | 2743.44 | 532.18 |
| Within groups (d.f.2=20) | 25.94 | 73.89 | 57.58 | |
| 40°C | CK | 10.92±0.81 b | 26.52±0.51 c | 9.14±0.67 b |
| Light drought | 12.79±0.78 a | 37.52±3.73 b | 14.11±1.51 a | |
| Moderate drought | 11.92±0.64 ab | 45.60±0.92 a | 16.36±2.29 a | |
| Heavy drought | 11.30±1.69 ab | 34.64±4.18 b | 8.48±1.74 b | |
| Sum of squares | Between groups (d.f.1=3) | 11.98 | 1117.58 | 264.48 |
| Within groups (d.f.2=20) | 22.59 | 162.38 | 55.13 | |
| P: Ft | ** | ns | ** | |
| P: Fd | ** | ** | * | |
| P: Ft×Fd | ** | ** | ** |
表1 干旱高温胁迫对天香台阁四季桂活性氧和丙二醛含量的影响
Table 1 Effect of drought and heat stress on reactive oxygen species and malondialdehyde (MDA) content in Osmanthus fragrans cv. ‘Tian Xiang TaiGe’
| Temperature | Treatment intensity | O2-· (nmol·g-1 FW) | H2O2 (μmol·g-1 FW) | MDA (μmol·g-1 FW) |
|---|---|---|---|---|
| 28°C | CK | 7.56±0.77 C | 20.11±1.01 D | 4.09±0.64 C |
| Light drought | 14.04±0.44 B | 33.08±2.33 C | 7.42±1.32 B | |
| Moderate drought | 15.49±0.45 B | 40.60±2.30 B | 14.29±2.55 A | |
| Heavy drought | 18.84±2.05 A | 49.24±1.74 A | 15.39±1.69 A | |
| Sum of squares | Between groups (d.f.1=3) | 402.69 | 2743.44 | 532.18 |
| Within groups (d.f.2=20) | 25.94 | 73.89 | 57.58 | |
| 40°C | CK | 10.92±0.81 b | 26.52±0.51 c | 9.14±0.67 b |
| Light drought | 12.79±0.78 a | 37.52±3.73 b | 14.11±1.51 a | |
| Moderate drought | 11.92±0.64 ab | 45.60±0.92 a | 16.36±2.29 a | |
| Heavy drought | 11.30±1.69 ab | 34.64±4.18 b | 8.48±1.74 b | |
| Sum of squares | Between groups (d.f.1=3) | 11.98 | 1117.58 | 264.48 |
| Within groups (d.f.2=20) | 22.59 | 162.38 | 55.13 | |
| P: Ft | ** | ns | ** | |
| P: Fd | ** | ** | * | |
| P: Ft×Fd | ** | ** | ** |

图1 干旱高温胁迫对天香台阁四季桂抗氧化酶活性的影响(A) 超氧化物歧化酶(SOD)活性; (B) 过氧化物酶(POD)活性; (C) 过氧化氢酶(CAT)活性。Ft: 不同温度的影响; Fd: 不同处理强度的影响; Ft×Fd: 植物组织对干旱高温胁迫的不同响应。每个数值为平均值±标准误(n=6)。不同大写字母表示不同干旱处理间差异显著, 不同小写字母表示不同高温处理间差异显著。根据Tukey多重比较, * P<0.05; ** P<0.01; ns: 不显著
Figure 1 The effect of drought and heat stress on the activity of antioxidant enzymes in Osmanthus fragrans cv. ‘Tian Xiang TaiGe’(A) Superoxide dismutase (SOD) activity; (B) Peroxidase (POD) activity; (C) Catalase (CAT) activity. Ft: Effect of different temperature; Fd: Effect of different drought treatment intensity; Ft×Fd: Different responses of plant tissues to drought and heat stress. Each value is the mean±SE (n=6). Different capital letters indicate statistically significant differences of drought stress, different lowercase letters indicate statistically significant differences of heat stress. According to Tukey test, * P<0.05; ** P<0.01; ns: Non-significant
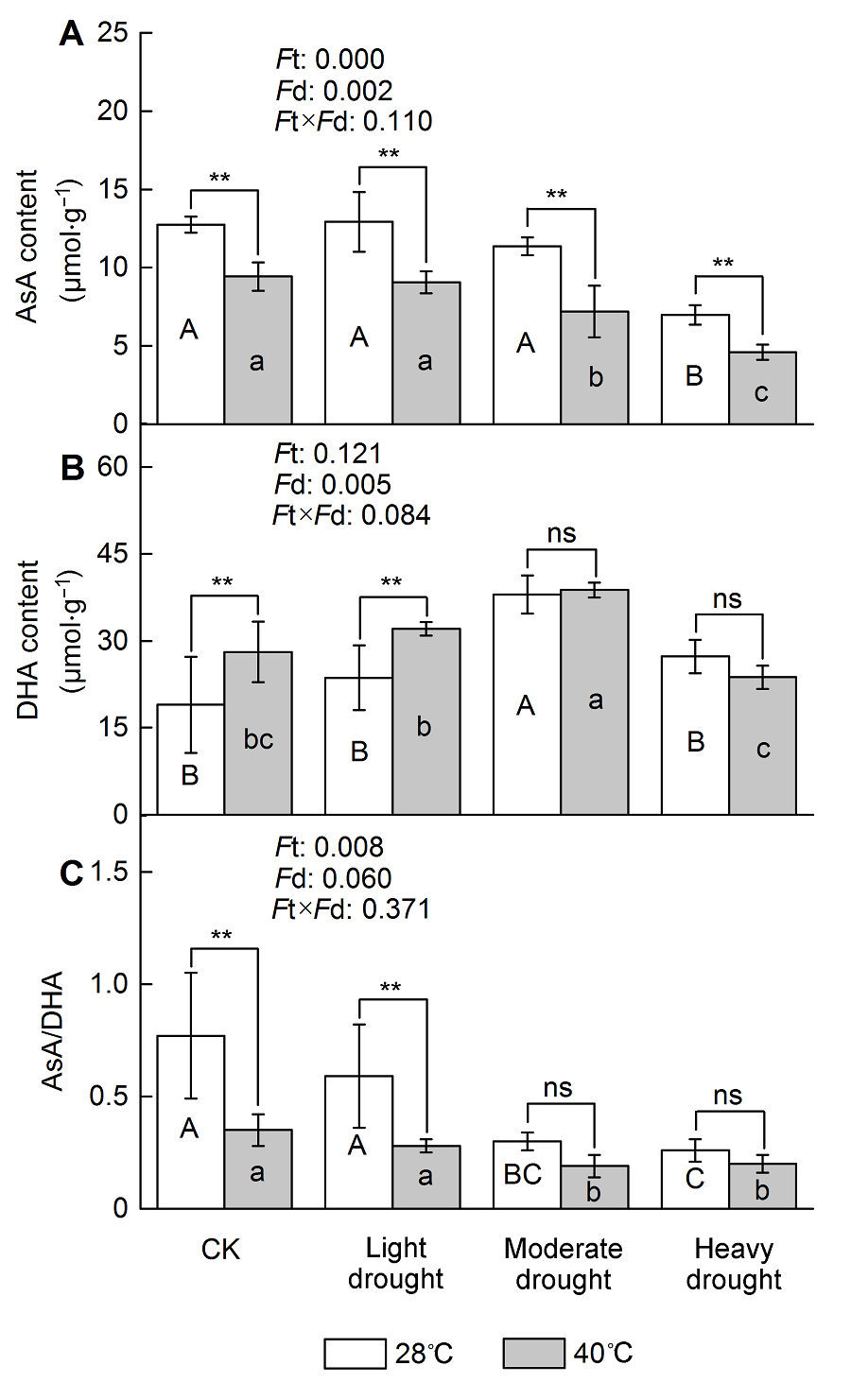
图2 干旱高温胁迫对天香台阁四季桂抗坏血酸含量的影响(A) 抗坏血酸(AsA)含量; (B) 脱氢抗坏血酸(DHA)含量; (C) AsA/DHA。Ft: 不同温度的影响; Fd: 不同处理强度的影响; Ft×Fd: 植物组织对干旱高温胁迫的不同响应。每个数值为平均值±标准误(n=6)。不同大写字母表示不同干旱处理间差异显著, 不同小写字母表示不同高温处理间差异显著。根据Tukey多重比较, * P<0.05; ** P<0.01; ns: 不显著
Figure 2 The effect of drought and heat stress on the AsA content in Osmanthus fragrans cv. ‘Tian Xiang TaiGe’ (A) Ascorbic acid (AsA) content; (B) Dehydroascorbate (DHA) content; (C) AsA/DHA. Ft: Effect of different temperature; Fd: Effect of different drought treatment intensity; Ft×Fd: Different responses of plant tissues to drought and heat stress. Each value is the mean±SE (n=6). Different capital letters indicate statistically significant differences of drought stress, different lowercase letters indicate statistically significant differences of heat stress. According to Tukey test, * P<0.05; ** P<0.01; ns: Non-significant
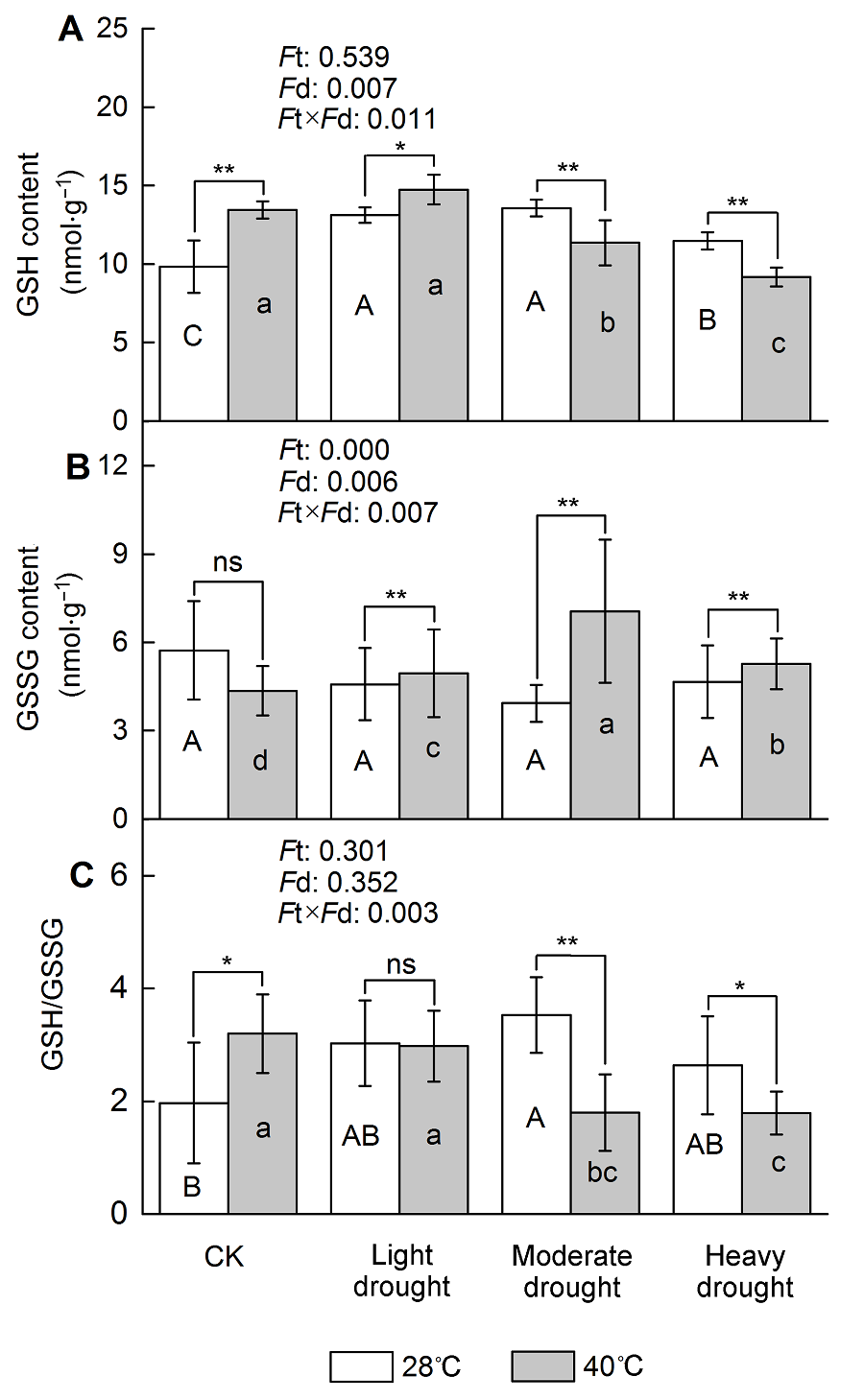
图3 干旱高温胁迫对天香台阁四季桂谷胱甘肽含量的影响(A) 谷胱甘肽(GSH)含量; (B) 氧化型谷胱甘肽(GSSG)含量; (C) GSH/GSSG。Ft: 不同温度的影响; Fd: 不同处理强度的影响; Ft×Fd: 植物组织对干旱高温胁迫的不同响应。每个数值为平均值±标准误(n=6)。不同大写字母表示不同干旱处理间差异显著, 不同小写字母表示不同高温处理间差异显著。根据Tukey多重比较, * P<0.05; ** P<0.01; ns: 不显著
Figure 3 The effect of drought and heat stress on the GSH content in Osmanthus fragrans cv. ‘Tian Xiang TaiGe’ (A) Glutathione (GSH) content; (B) Oxidized glutathione (GSSG) content; (C) GSH/GSSG. Ft: Effect of different temperature; Fd: Effect of different drought treatment intensity; Ft×Fd: Different responses of plant tissues to drought and heat stress. Each value is the mean±SE (n=6). Different capi- tal letters indicate statistically significant differences of drought stress, different lowercase letters indicate statistically significant differences of heat stress. According to Tukey test, * P<0.05; ** P<0.01; ns: Non-significant
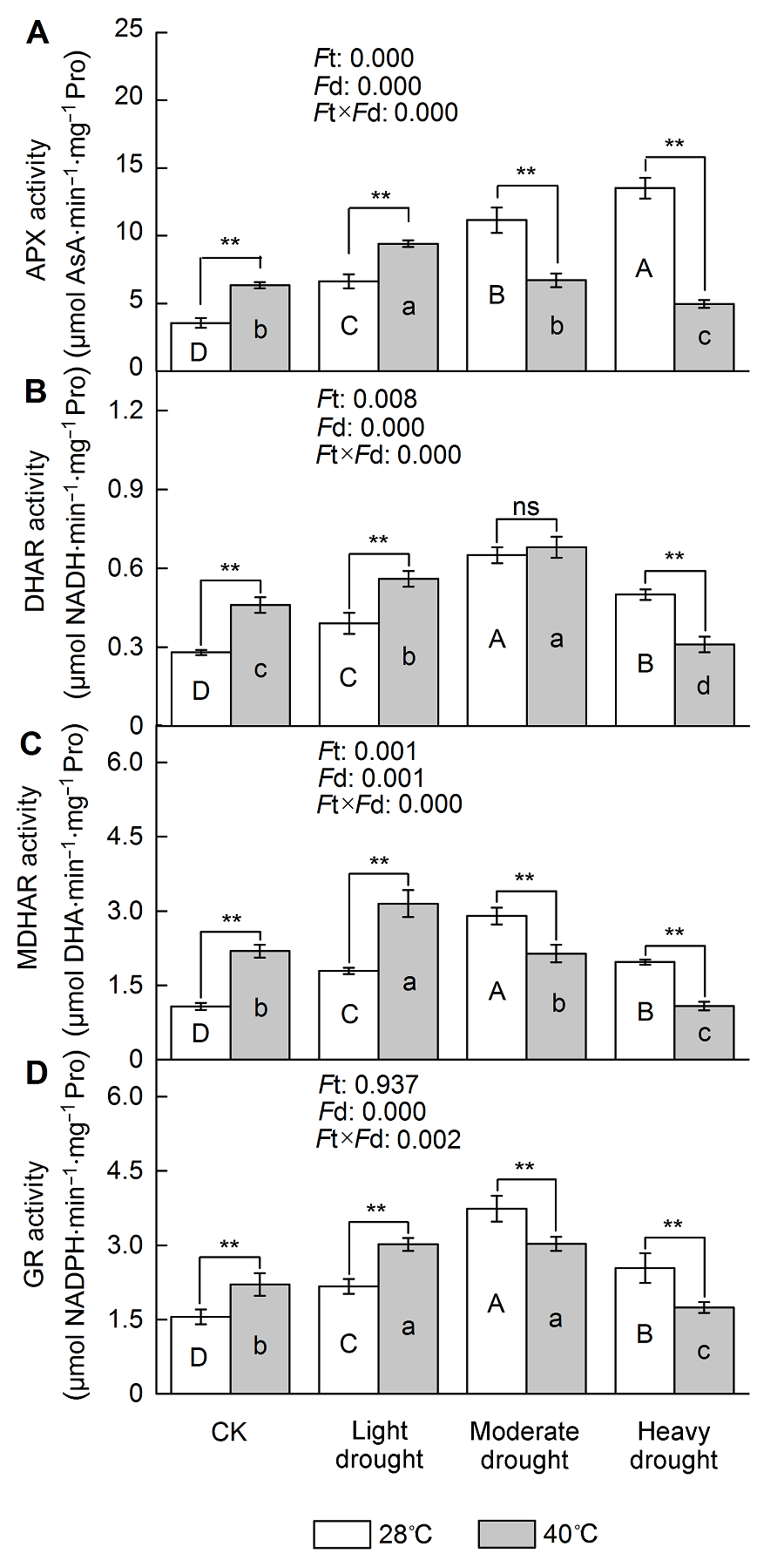
图4 干旱高温胁迫对天香台阁四季桂AsA-GSH循环相关酶活性的影响(A) 抗坏血酸过氧化物酶(APX)活性; (B) 脱氢抗坏血酸还原酶(DHAR)活性; (C) 单脱氢抗坏血酸还原酶(MDHAR)活性; (D) 谷胱甘肽还原酶(GR)活性。Ft: 不同温度的影响; Fd: 不同处理强度的影响; Ft×Fd: 植物组织对干旱高温胁迫的不同响应。每个数值为平均值±标准误(n=6)。不同大写字母表示不同干旱处理间差异显著, 不同小写字母表示不同高温处理间差异显著。根据Tukey多重比较, * P<0.05; ** P<0.01; ns: 不显著
Figure 4 The effect of drought and heat stress on the enzymes activity of AsA-GSH cycle in Osmanthus fragrans cv. ‘Tian Xiang TaiGe’ (A) Ascorbate peroxidase (APX) activity; (B) Dehydroascorbate reductase (DHAR) activity; (C) Monodehydroascobate reductase (MDHAR) activity; (D) Glutathione reductase (GR) activity. Ft: Effect of different temperature; Fd: Effect of different drought treatment intensity; Ft×Fd: Different responses of plant tissues to drought and heat stress. Each value is the mean±SE (n=6). Different capital letters indicate statistically significant differences of drought stress, different lowercase letters indicate statistically significant differences of heat stress. According to Tukey test, * P<0.05; ** P<0.01; ns: Non- significant
| [1] | 陈晓峰, 江洪, 牛晓栋, 张金梦, 刘玉莉, 方成圆 (2016). 季节性高温和干旱对亚热带毛竹林碳通量的影响. 应用生态学报 27, 335-344. |
| [2] | 李忠光, 龚明 (2005). 植物中超氧阴离子自由基测定方法的改进. 云南植物研究 27, 211-216. |
| [3] | 吴永波, 叶波 (2016). 高温干旱复合胁迫对构树幼苗抗氧化酶活性和活性氧代谢的影响. 生态学报 36, 403-410. |
| [4] | 谢华英, 马均, 代邹, 李玥, 孙加威, 赵建红, 徐徽, 孙永健 (2016). 抽穗期高温干旱胁迫对杂交水稻产量及生理特性的影响. 杂交水稻 31, 62-69. |
| [5] | Arab L, Kreuzwieser J, Kruse J, Zimmer I, Ache P, Alfarraj S, Al-rasheid KAS, Schnitzler JP, Hedrich R, Rennenberg H (2016). Acclimation to heat and drought- lessons to learn from the date palm ( Phoenix dactylifera). Environ Exp Bot 125, 20-30. |
| [6] | Doulis AG, Debian N, Kingston-Smith AH, Foyer CH (1997). Differential localization of antioxidants in maize leaves.Plant Physiol 114, 1031-1037. |
| [7] | Foyer CH, Noctor G (2011). Ascorbate and glutathione: the heart of the redox hub.Plant Physiol 155, 2-18. |
| [8] | Giannopolitis CN, Ries SK (1977). Superoxide dismutases: I. Occurrence in higher plants.Plant Physiol 59, 309-314. |
| [9] | Hijioka Y, Lin E, Pereira J, Corlett R, Cui X, Insarov G, Lasco R, Lindgren E, Surjan A (2014).Climate Change 2014: Impacts, Adaptation, and Vulnerability, Part B: Regional Aspects, Contribution of Working Group II, Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press. pp.1327-1370. |
| [10] | Hodges DM, Delong JM, Forney CF, Prange RK (1999). Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds.Pl- anta 207, 604-611. |
| [11] | Hossain MA, Nakano Y, Asada K (1984). Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide.Plant Cell Physiol 25, 385-395. |
| [12] | Imahori Y, Takemura M, Bai JH (2008). Chilling-induced oxidative stress and antioxidant responses in mume ( Pru- nus mume) fruit during low temperature storage. Postharvest Biol Technol 49, 54-60. |
| [13] | Kumari GJ, Reddy AM, Naik ST, Kumar SG, Prasanthi J, Sriranganayakulu G, Reddy PC, Sudhakar C (2006). Jasmonic acid induced changes in protein pattern, antioxidative enzyme activities and peroxidase isozymes in peanut seedlings.Biol Plant 50, 219-226. |
| [14] | Lei P, Xu ZQ, Ding Y, Tang B, Zhang YX, Li HS, Feng XH, Xu H (2015). Effect of poly (γ-glutamic acid) on the physiological responses and calcium signaling of rape seedlings ( Brassica napus L.) under cold stress. J Agric Food Chem 63, 10399-10406. |
| [15] | Lei P, Xu ZQ, Liang JF, Luo XH, Zhang YX, Feng XH, Xu H (2016). Poly (γ-glutamic acid) enhanced tolerance to salt stress by promoting proline accumulation in Brassica napus L. Plant Growth Regul 78, 233-241. |
| [16] | Li H, Xu HL, Zhang PJ, Gao MQ, Wang D, Zhao HJ (2017). High temperature effects on D1 protein turnover in three wheat varieties with different heat susceptibility.Plant Grow- th Regul 78, 1-9. |
| [17] | Liu CC, Liu YG, Guo K, Fan DY, Li GQ, Zheng YR, Yu LF, Yang R (2011). Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China.Environ Exp Bot 71, 174-183. |
| [18] | Ma YH, Ma FW, Wang YH, Zhang JK (2011). The responses of the enzymes related with ascorbate-gluta- thione cycle during drought stress in apple leaves.Acta Physiol Plant 33, 173-180. |
| [19] | Nahar K, Hasanuzzaman M, Alam MM, Fujita M (2015). Exogenous glutathione confers high temperature stress tolerance in mung bean ( Vigna radiata L.) by modulating antioxidant defense and methylglyoxal detoxification system. Environ Exp Bot 112, 44-54. |
| [20] | Nakano Y, Asada K (1981). Hydrogen peroxide is scaven- ged by ascorbate-specific peroxidase in spinach chloroplasts.Plant Cell Physiol 22, 867-880. |
| [21] | Rai AC, Singh M, Shah K (2012). Effect of water withdrawal on formation of free radical, proline accumulation and activities of antioxidant enzymes in ZAT12-transformed transgenic tomato plants. Plant Physiol Biochem 61, 108-114. |
| [22] | Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA (2003). Fingerprints of global warming on wild animals and plants.Nature 421, 57-60. |
| [23] | Schaedle M, Bassham JA (1977). Chloroplast glutathione reductase.Plant Physiol 59, 1011-1012. |
| [24] | Sharma P, Jha AB, Dubey RS, Pessarakli M (2012). Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions.J Bot 2012, 217037. |
| [25] | Silva EN, Ferreira-Silva SL, Fontenele ADV, Ribeiro RV, Viégasc RA, Silveira JAG (2010). Photosynthetic chan- ges and protective mechanisms against oxidative damage subjected to isolated and combined drought and heat stresses in Jatropha curcas plants. J Plant Physiol 167, 1157-1164. |
| [26] | Suzuki N, Koussevitzky S, Mittler R, Miller G (2012). ROS and redox signaling in the response of plants to abiotic stress.Plant Cell Environ 35, 259-270. |
| [27] | Wang SC, Liang D, Li C, Hao Yl, Ma FW, Shu HR (2012). Influence of drought stress on the cellular ultrastructure and antioxidant systemin leaves of drought-tolerant and drought-sensitive apple rootstocks.Plant Physiol Biochem 51, 81-89. |
| [28] | Wu XX, He J, Ding HD, Zhu ZW, Chen JL, Xu S, Zha DS (2015). Modulation of zinc-induced oxidative damage in Solanum melongena by 6-benzylaminopurine involves ascorbate-glutathione cyclemetabolism. Environ Exp Bot 116, 1-11. |
| [29] | Zou MQ, Yuan LY, Zhu SD, Liu S, Ge JT, Wang CG (2016). Response of osmotic adjustment and ascorbate-glutathione cycle to heat stress in a heat-sensitive and a heat-tolerant genotype of wucai ( Brassica campestris L.). Sci Hortic 211, 87-94. |
| [1] | 马富龙, 王雨晴, 郝瑜, 段继超, 刘霏霏, 席琳乔, 韩路. 海拔梯度对昆仑山北坡中部草原植物与土壤微生物群落结构与多样性的影响[J]. 植物生态学报, 2025, 49(5): 732-747. |
| [2] | 王秀媛, 申磊, 刘婷婷, 尉雯雯, 张帅, 张伟. ‘塞外红’苹果-大豆复合系统根系时空分布与种间竞争策略[J]. 植物生态学报, 2025, 49(5): 748-759. |
| [3] | 赵洪贤, 刘鹏, 史曼英, 徐铭泽, 贾昕, 田赟, 查天山. 毛乌素沙地典型固沙植物黑沙蒿和赖草叶片氮分配对最大净光合速率的影响[J]. 植物生态学报, 2025, 49(3): 460-474. |
| [4] | 刘柯言, 韩璐, 宋午椰, 张初蕊, 胡旭, 许行, 陈立欣. 基于日光诱导叶绿素荧光探测干旱对黄土高原植被光合稳定性的影响[J]. 植物生态学报, 2025, 49(3): 415-431. |
| [5] | 邵畅畅, 段洪浪, 赵熙州, 丁贵杰. 树木干旱死亡点预测及致死生理机制研究进展[J]. 植物生态学报, 2025, 49(2): 221-231. |
| [6] | 樊蓓, 任敏, 王延峰, 党峰峰, 陈国梁, 程国亭, 杨金雨, 孙会茹. 番茄SlWRKY45转录因子在响应低温和干旱胁迫中的功能(长英文摘要)[J]. 植物学报, 2025, 60(2): 186-203. |
| [7] | 王堃莹, 邱贵福, 刘子赫, 孟君, 刘宇轩, 贾国栋. 气候变化对不同退化程度小叶杨林分生长和内在水分利用效率的调节[J]. 植物生态学报, 2025, 49(2): 343-355. |
| [8] | 李若月, 杨小超, 郝占庆, 贾仕宏. 高温热浪和虫食对校园植物的作用强度及其与叶功能性状的关系[J]. 生物多样性, 2025, 33(1): 24283-. |
| [9] | 田建红, 刘燕, 尹梦琪, 王静, 陈婷, 汪燕, 姜孝成. 水稻OsWAK16通过调节抗氧化酶活性调控种子抗老化能力(长英文摘要)[J]. 植物学报, 2025, 60(1): 17-32. |
| [10] | 吴风燕, 吴永胜, 陈晓涵, 冯骥, 卢丽媛, 查斯娜, 王超宇, 孟元发, 尹强. 鄂尔多斯高原3种固沙灌木水分利用效率的时空变化特征[J]. 植物生态学报, 2024, 48(9): 1180-1191. |
| [11] | 王音, 同小娟, 张劲松, 李俊, 孟平, 刘沛荣, 张静茹. 干旱对栓皮栎人工林碳水通量及其耦合的影响[J]. 植物生态学报, 2024, 48(9): 1157-1171. |
| [12] | 马煦晗, 黄菊莹, 余海龙, 韩翠, 李冰. 降水量变化及氮添加下荒漠草原土壤有机碳及其易分解组分研究[J]. 植物生态学报, 2024, 48(8): 1065-1077. |
| [13] | 张鹏, 焦亮, 薛儒鸿, 魏梦圆, 杜达石, 吴璇, 王旭鸽, 李倩. 干旱强度影响祁连山西段不同海拔青海云杉的生长恢复[J]. 植物生态学报, 2024, 48(8): 977-987. |
| [14] | 龙吉兰, 蒋铮, 刘定琴, 缪宇轩, 周灵燕, 冯颖, 裴佳宁, 刘瑞强, 周旭辉, 伏玉玲. 干旱下植物根系分泌物及其介导的根际激发效应研究进展[J]. 植物生态学报, 2024, 48(7): 817-827. |
| [15] | 王涛, 冯敬磊, 张翠. 高温胁迫影响玉米生长发育的分子机制研究进展[J]. 植物学报, 2024, 59(6): 963-977. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||