

Chinese Bulletin of Botany ›› 2023, Vol. 58 ›› Issue (6): 917-925.DOI: 10.11983/CBB22228 cstr: 32102.14.CBB22228
• TECHNIQUES AND METHODS • Previous Articles Next Articles
Xiaohui Feng1,2,3, Xuetong Yan2,3, Keyuan Zheng2,3, Qiang Zhou1, Weizhong Zhang4, Quanyong Wang4, Mulan Zhu2,3,*( )
)
Received:2022-09-29
Accepted:2023-02-28
Online:2023-11-01
Published:2023-11-27
Contact:
* E-mail: mlzhu@cemps.ac.cn
Xiaohui Feng, Xuetong Yan, Keyuan Zheng, Qiang Zhou, Weizhong Zhang, Quanyong Wang, Mulan Zhu. In vitro Culture of Taxus Rich in Taxanes[J]. Chinese Bulletin of Botany, 2023, 58(6): 917-925.
| Treatments | 75% ethanol (s) | Disinfectant (min) | Pollution rate (%) | Survival rate (%) |
|---|---|---|---|---|
| 1 | 10 | 3 | 68.9±5.09a | 31.1±5.09f |
| 2 | 10 | 5 | 35.6±3.85c | 57.8±3.85bcd |
| 3 | 10 | 7 | 20.0±3.33de | 62.2±3.85bc |
| 4 | 30 | 3 | 54.4±5.09b | 45.6±5.09e |
| 5 | 30 | 5 | 25.6±5.09d | 74.4±5.09a |
| 6 | 30 | 7 | 12.2±1.92ef | 64.4±8.38b |
| 7 | 50 | 3 | 41.1±5.09c | 51.1±1.92de |
| 8 | 50 | 5 | 16.7±3.33e | 64.4±5.09b |
| 9 | 50 | 7 | 5.6±1.92f | 53.3±5.77de |
Table 1 Effects of disinfection treatments on the contamination rate and survival rate of explants
| Treatments | 75% ethanol (s) | Disinfectant (min) | Pollution rate (%) | Survival rate (%) |
|---|---|---|---|---|
| 1 | 10 | 3 | 68.9±5.09a | 31.1±5.09f |
| 2 | 10 | 5 | 35.6±3.85c | 57.8±3.85bcd |
| 3 | 10 | 7 | 20.0±3.33de | 62.2±3.85bc |
| 4 | 30 | 3 | 54.4±5.09b | 45.6±5.09e |
| 5 | 30 | 5 | 25.6±5.09d | 74.4±5.09a |
| 6 | 30 | 7 | 12.2±1.92ef | 64.4±8.38b |
| 7 | 50 | 3 | 41.1±5.09c | 51.1±1.92de |
| 8 | 50 | 5 | 16.7±3.33e | 64.4±5.09b |
| 9 | 50 | 7 | 5.6±1.92f | 53.3±5.77de |
| Treatments | NAA (mg·L-1) | 6-BA (mg·L-1) | Proline (g·L-1) | Expansion rate (%) |
|---|---|---|---|---|
| 1 | 0.1 | 1 | 0 | 28.9±5.09g |
| 2 | 0.1 | 2 | 0.1 | 53.3±8.82ef |
| 3 | 0.1 | 3 | 0.3 | 62.3±2.02de |
| 4 | 0.2 | 1 | 0.5 | 58.9±5.09ef |
| 5 | 0.2 | 2 | 0.7 | 76.7±6.67abc |
| 6 | 0.2 | 3 | 1 | 86.7±5.77ab |
| 7 | 0.3 | 1 | 0 | 47.8±6.93f |
| 8 | 0.3 | 2 | 0.1 | 51.1±15.40ef |
| 9 | 0.3 | 3 | 0.3 | 86.7±5.77ab |
| 10 | 0.4 | 1 | 0.5 | 55.6±5.09ef |
| 11 | 0.4 | 2 | 0.7 | 90.0±6.67a |
| 12 | 0.4 | 3 | 1 | 63.3±3.33cde |
| 13 | 0.5 | 1 | 0 | 52.2±1.92ef |
| 14 | 0.5 | 2 | 0.1 | 75.6±8.39bcd |
| 15 | 0.5 | 3 | 0.3 | 81.1±10.18ab |
| 16 | 0.6 | 1 | 0.5 | 64.4±11.71cde |
| 17 | 0.6 | 2 | 0.7 | 57.8±6.94ef |
| 18 | 0.6 | 3 | 1 | 46.7±5.77f |
Table 2 Effect of different additive combinations on the bottom absorbent surface
| Treatments | NAA (mg·L-1) | 6-BA (mg·L-1) | Proline (g·L-1) | Expansion rate (%) |
|---|---|---|---|---|
| 1 | 0.1 | 1 | 0 | 28.9±5.09g |
| 2 | 0.1 | 2 | 0.1 | 53.3±8.82ef |
| 3 | 0.1 | 3 | 0.3 | 62.3±2.02de |
| 4 | 0.2 | 1 | 0.5 | 58.9±5.09ef |
| 5 | 0.2 | 2 | 0.7 | 76.7±6.67abc |
| 6 | 0.2 | 3 | 1 | 86.7±5.77ab |
| 7 | 0.3 | 1 | 0 | 47.8±6.93f |
| 8 | 0.3 | 2 | 0.1 | 51.1±15.40ef |
| 9 | 0.3 | 3 | 0.3 | 86.7±5.77ab |
| 10 | 0.4 | 1 | 0.5 | 55.6±5.09ef |
| 11 | 0.4 | 2 | 0.7 | 90.0±6.67a |
| 12 | 0.4 | 3 | 1 | 63.3±3.33cde |
| 13 | 0.5 | 1 | 0 | 52.2±1.92ef |
| 14 | 0.5 | 2 | 0.1 | 75.6±8.39bcd |
| 15 | 0.5 | 3 | 0.3 | 81.1±10.18ab |
| 16 | 0.6 | 1 | 0.5 | 64.4±11.71cde |
| 17 | 0.6 | 2 | 0.7 | 57.8±6.94ef |
| 18 | 0.6 | 3 | 1 | 46.7±5.77f |

Figure 1 Tissue culture of Taxus (A) Image of bottom absorbent surface expands from the front (bar=10 mm); (B) Image of bottom absorbent surface expands from the underside (bar=5 mm); (C) Axillary bud induction (bar=10 mm); (D) The original basis of adventitious bud induction (bar=10 mm); (E) High-frequency synchronous growth (bar=10 mm); (F) Adventitious bud elongation (bar=10 mm); (G) Biomass amplification (bar=20 mm)
| Treatments | 6-BA (mg·L-1) | NAA (mg·L-1) | Axillary buds induction rate (%) |
|---|---|---|---|
| 1 | 0.5 | 0.05 | 63.3±3.33ef |
| 2 | 0.5 | 0.1 | 48.9±5.09g |
| 3 | 1 | 0.05 | 81.1±1.92ab |
| 4 | 1 | 0.1 | 88.9±5.09a |
| 5 | 1 | 0.2 | 68.9±5.09cde |
| 6 | 1 | 0.3 | 64.4±5.09ef |
| 7 | 2 | 0.05 | 73.3±3.34bcd |
| 8 | 2 | 0.1 | 75.6±3.85bc |
| 9 | 2 | 0.2 | 65.6±5.09def |
| 10 | 2 | 0.3 | 58.9±5.09f |
| 11 | 3 | 0.1 | 60.0±3.33f |
| 12 | 3 | 0.2 | 43.3±3.34g |
Table 3 Effect of different concentrations of 6-BA and NAA on axillary bud induction
| Treatments | 6-BA (mg·L-1) | NAA (mg·L-1) | Axillary buds induction rate (%) |
|---|---|---|---|
| 1 | 0.5 | 0.05 | 63.3±3.33ef |
| 2 | 0.5 | 0.1 | 48.9±5.09g |
| 3 | 1 | 0.05 | 81.1±1.92ab |
| 4 | 1 | 0.1 | 88.9±5.09a |
| 5 | 1 | 0.2 | 68.9±5.09cde |
| 6 | 1 | 0.3 | 64.4±5.09ef |
| 7 | 2 | 0.05 | 73.3±3.34bcd |
| 8 | 2 | 0.1 | 75.6±3.85bc |
| 9 | 2 | 0.2 | 65.6±5.09def |
| 10 | 2 | 0.3 | 58.9±5.09f |
| 11 | 3 | 0.1 | 60.0±3.33f |
| 12 | 3 | 0.2 | 43.3±3.34g |
| Treatments | 6-BA (mg·L-1) | NAA (mg·L-1) | Adventitious buds induction rate (%) |
|---|---|---|---|
| 1 | 0.2 | 0.02 | 32.2±5.09e |
| 2 | 0.4 | 0.04 | 47.8±3.85d |
| 3 | 0.6 | 0.06 | 62.2±1.92bc |
| 4 | 0.8 | 0.08 | 68.9±3.84b |
| 5 | 1.0 | 0.10 | 77.8±1.92a |
| 6 | 1.2 | 0.12 | 67.8±5.09bc |
| 7 | 1.4 | 0.14 | 61.1±3.84c |
Table 4 Effect of different concentration of 6-BA and NAA on the original basis of adventitious bud induction
| Treatments | 6-BA (mg·L-1) | NAA (mg·L-1) | Adventitious buds induction rate (%) |
|---|---|---|---|
| 1 | 0.2 | 0.02 | 32.2±5.09e |
| 2 | 0.4 | 0.04 | 47.8±3.85d |
| 3 | 0.6 | 0.06 | 62.2±1.92bc |
| 4 | 0.8 | 0.08 | 68.9±3.84b |
| 5 | 1.0 | 0.10 | 77.8±1.92a |
| 6 | 1.2 | 0.12 | 67.8±5.09bc |
| 7 | 1.4 | 0.14 | 61.1±3.84c |
| Treatments | NAA (mg·L-1) | 6-BA (mg·L-1) | High-frequency synchronous growth rate (%) | Average number of adventitious buds |
|---|---|---|---|---|
| 1 | 0.05 | 0.25 | 38.9±5.09e | 12.2±2.65c |
| 2 | 0.05 | 0.5 | 55.6±5.09cd | 14.2±2.78bc |
| 3 | 0.1 | 0.5 | 50.0±6.67d | 20.1±3.66b |
| 4 | 0.1 | 1 | 82.2±1.92a | 31.5±2.93a |
| 5 | 0.2 | 1 | 72.2±3.85b | 33.4±3.67a |
| 6 | 0.2 | 2 | 63.3±3.34bc | 29.7±2.94a |
Table 5 Effect of different medium formulations on the original basis of adventitious bud high-frequency synchronous growth
| Treatments | NAA (mg·L-1) | 6-BA (mg·L-1) | High-frequency synchronous growth rate (%) | Average number of adventitious buds |
|---|---|---|---|---|
| 1 | 0.05 | 0.25 | 38.9±5.09e | 12.2±2.65c |
| 2 | 0.05 | 0.5 | 55.6±5.09cd | 14.2±2.78bc |
| 3 | 0.1 | 0.5 | 50.0±6.67d | 20.1±3.66b |
| 4 | 0.1 | 1 | 82.2±1.92a | 31.5±2.93a |
| 5 | 0.2 | 1 | 72.2±3.85b | 33.4±3.67a |
| 6 | 0.2 | 2 | 63.3±3.34bc | 29.7±2.94a |
| Treatments | 6-BA (mg·L-1) | NAA (mg·L-1) | Adventitious bud elongation rate (%) | Average seedling height (mm) |
|---|---|---|---|---|
| 1 | 0.1 | 0.01 | 35.5±6.94c | 17.3±0.85d |
| 2 | 0.3 | 0.03 | 40.0±3.33c | 22.7±1.34c |
| 3 | 0.5 | 0.05 | 74.4±5.09b | 28.4±0.94b |
| 4 | 0.7 | 0.07 | 92.2±1.92a | 33.4±1.56a |
| 5 | 1 | 0.1 | 80.0±5.77b | 29.9±0.69ab |
Table 6 Effect of medium formulations on the elongation of adventitious buds
| Treatments | 6-BA (mg·L-1) | NAA (mg·L-1) | Adventitious bud elongation rate (%) | Average seedling height (mm) |
|---|---|---|---|---|
| 1 | 0.1 | 0.01 | 35.5±6.94c | 17.3±0.85d |
| 2 | 0.3 | 0.03 | 40.0±3.33c | 22.7±1.34c |
| 3 | 0.5 | 0.05 | 74.4±5.09b | 28.4±0.94b |
| 4 | 0.7 | 0.07 | 92.2±1.92a | 33.4±1.56a |
| 5 | 1 | 0.1 | 80.0±5.77b | 29.9±0.69ab |
| Treatments | PG (mg·L-1) | Average stem thickness (mm) | Average seedling biomass (mg) |
|---|---|---|---|
| 1 | 0 | 0.6±0.05c | 283±15d |
| 2 | 20 | 0.8±0.10b | 483±12b |
| 3 | 50 | 1.1±0.15a | 542±13a |
| 4 | 80 | 0.9±0.06ab | 490±15b |
| 5 | 100 | 0.8±0.15bc | 353±20c |
Table 7 Effects of different concentrations of phloroglucinol (PG) on Taxus biomass amplification phase I
| Treatments | PG (mg·L-1) | Average stem thickness (mm) | Average seedling biomass (mg) |
|---|---|---|---|
| 1 | 0 | 0.6±0.05c | 283±15d |
| 2 | 20 | 0.8±0.10b | 483±12b |
| 3 | 50 | 1.1±0.15a | 542±13a |
| 4 | 80 | 0.9±0.06ab | 490±15b |
| 5 | 100 | 0.8±0.15bc | 353±20c |
| Treatments | AC (g·L-1) | Average seedling height (mm) | Average seedling biomass (mg) |
|---|---|---|---|
| 1 | 0 | 7.6±0.15d | 1318±12d |
| 2 | 1 | 7.9±0.21d | 1434±18c |
| 3 | 1.5 | 9.8±0.06b | 1496±24b |
| 4 | 2 | 10.4±0.21a | 1612±23a |
| 5 | 2.5 | 9.9±0.15b | 1531±15b |
| 6 | 3 | 8.7±0.12c | 1423±18c |
Table 8 Effects of different concentrations of active charcoal (AC) on biomass amplification phase II
| Treatments | AC (g·L-1) | Average seedling height (mm) | Average seedling biomass (mg) |
|---|---|---|---|
| 1 | 0 | 7.6±0.15d | 1318±12d |
| 2 | 1 | 7.9±0.21d | 1434±18c |
| 3 | 1.5 | 9.8±0.06b | 1496±24b |
| 4 | 2 | 10.4±0.21a | 1612±23a |
| 5 | 2.5 | 9.9±0.15b | 1531±15b |
| 6 | 3 | 8.7±0.12c | 1423±18c |
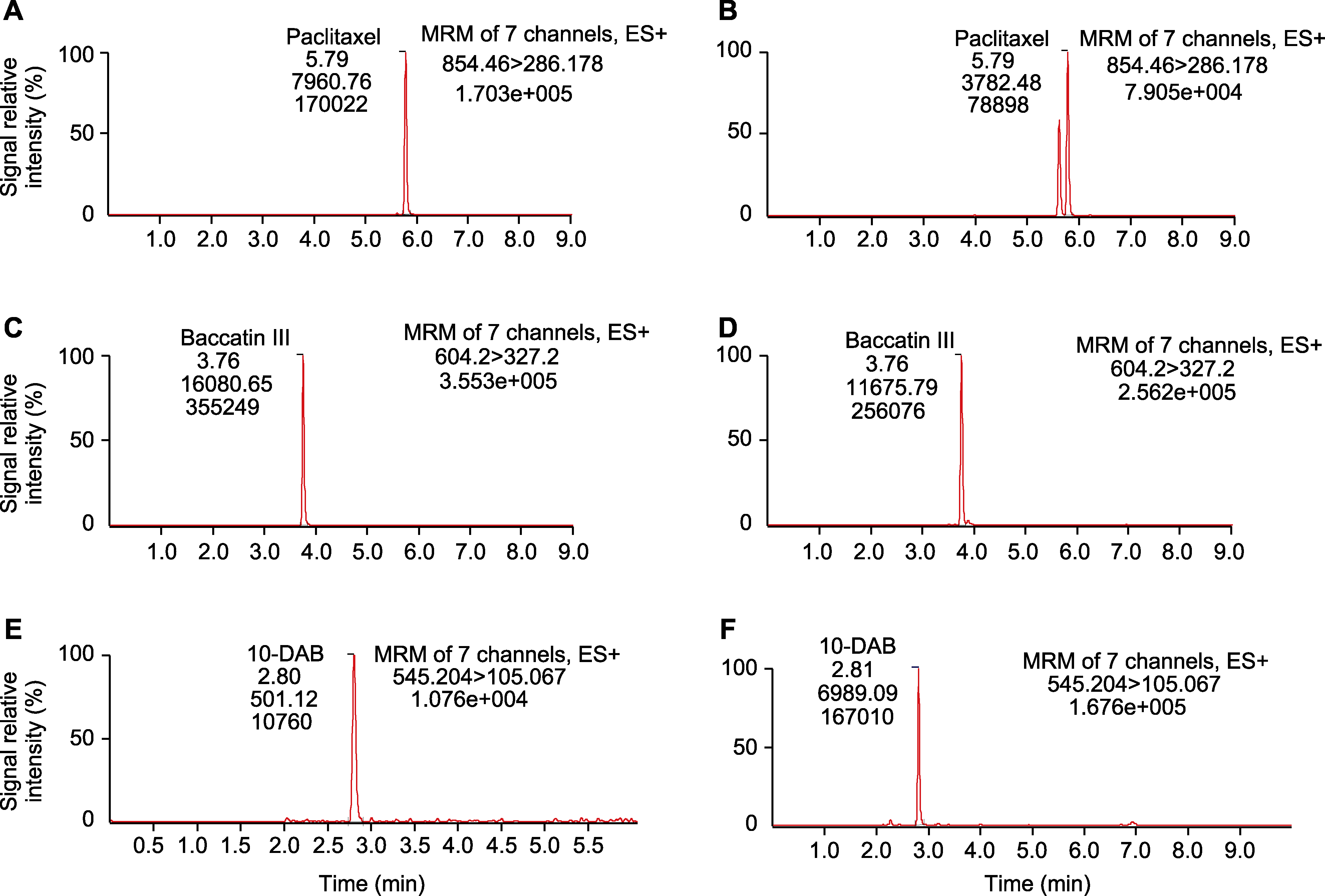
Figure 2 HPLC image of taxane standards and Taxus tissue culture samples (A) HPLC of standard paclitaxel; (B) HPLC of sample paclitaxel; (C) HPLC of standard Baccatin III; (D) HPLC of sample Baccatin III; (E) HPLC of standard 10-DAB; (F) HPLC of sample 10-DAB
| Paclitaxel (μg·g-1) | Baccatin III (μg·g-1) | 10-DAB (μg·g-1) | |
|---|---|---|---|
| Natural materials | 15.7 | 16.6 | 29.9 |
| Tissue culture materials | 95.4 | 135.9 | 2036.0 |
Table 9 Contents of paclitaxel and taxane compounds
| Paclitaxel (μg·g-1) | Baccatin III (μg·g-1) | 10-DAB (μg·g-1) | |
|---|---|---|---|
| Natural materials | 15.7 | 16.6 | 29.9 |
| Tissue culture materials | 95.4 | 135.9 | 2036.0 |
| [1] |
董明珠, 王立涛, 吕慕洁, 孟冬, 杨春雨, 赵春建, 付玉杰, 杨清 (2020). 濒危植物野生东北红豆杉群落特征及保护策略. 植物研究 40, 416-423.
DOI |
| [2] | 丰美静, 张恺恺, 黄中文, 邱德有, 陈段芬, 杨艳芳 (2020). 东北红豆杉温室扦插繁殖试验. 北方园艺 (13), 65-70. |
| [3] | 何晓明 (2011). 天然抗癌药紫杉醇及其半合成类似物的研究进展. 中国实用医药 6(24), 236-237. |
| [4] | 贺新强, 林金星, 胡玉熹, 王献溥, 李法曾 (1996). 中国松杉类植物濒危等级划分的比较. 生物多样性 4, 47-53. |
| [5] |
焦骄, 刘婧, 付玉杰, 王馨, 盖庆岩, 鹿瑶, 徐晓杰, 王紫莹 (2021). 3种红豆杉茎段腋芽启动组培繁育技术研究. 植物研究 41, 721-728.
DOI |
| [6] | 李干雄, 李志良, 曾腾锋, 何艳宇, 黄巧明 (2008). 几种促进剂的添加时间对中国红豆杉细胞悬浮培养紫杉醇合成的影响. 天然产物研究与开发 20, 876-879. |
| [7] | 梁敬钰 (1993). 国外紫杉醇研究进展. 生物工程进展 13(4), 19-20. |
| [8] | 师春娟, 李平英, 于永明, 董菊兰, 韩云花, 高淑琴 (2007). 红豆杉愈伤组织诱导试验初探. 甘肃林业科技 32(4), 19-22. |
| [9] | 王威, 白江平, 王清, 黄惠英 (2019). 中国红豆杉植株再生体系优化. 草原与草坪 39(5), 102-106. |
| [10] | 王莹, 王姝, 刘盈盈, 刘燕, 向准, 任春光, 孙超 (2016). 生物技术在药用植物次生代谢产物方面的应用进展. 现代园艺 39(11), 14-16. |
| [11] | 吴秀兰, 贾艳, 朱波, 周杰 (2014). 天然抗癌药物紫杉醇的研究进展. 中国野生植物资源 33(5), 42-45, 60. |
| [12] | 杨文婷, 匡倩 (2016). 红豆杉组织培养的防褐变措施研究. 北方园艺 (17), 111-114. |
| [13] | 袁云香 (2018). 硅对南方红豆杉愈伤组织诱导及增殖培养的影响. 科学技术与工程 18(35), 121-123. |
| [14] | 翟合欢 (2010). 曼地亚红豆杉组培快繁技术的研究. 硕士论文. 合肥: 安徽农业大学. pp. 1-57. |
| [15] | 张翔宇, 杜亚填, 龚雪元 (2012). 南方红豆杉愈伤组织生长动力学及紫杉醇代谢动力学研究. 中草药 43, 990-994. |
| [16] | 赵春芳, 余龙江, 刘智, 孙友平, 李文兵 (2005). 中国红豆杉中主要紫杉烷类物质的分布研究. 林产化学与工业 25, 89-93. |
| [17] | 周华, 朱祺, 杨艳芳, 余发新, 邱德有 (2014). 红豆杉分子生物学研究进展. 植物生理学报 50, 373-381. |
| [18] | Furmanowa M, Glowniak K, Syklowska-Baranek K, Zgórka G, Józefczyk A (1997). Effect of picloram and methyl jasmonate on growth and taxane accumulation in callus culture of Taxus × media var. hatfieldii. Plant Cell Tissue Organ Cult 49, 75-79. |
| [19] | Li YL, Huang SW, Zhang JY, Bu FJ, Lin T, Zhang ZH, Xiong XY (2016). A protocol of homozygous haploid callus induction from endosperm of Taxus chinensis Rehd. var. mairei. Springerplus 5, 659. |
| [20] | Ross S, Castillo A (2009). Mass propagation of Vaccinium corymbosum in bioreactors. Agrociencia 13, 1-8. |
| [21] |
Ross S, Castillo A (2010). Micropropagation of Achyrocline flaccida (Weinm.) DC. in liquid culture media. Agrociencia 14, 1-7.
DOI URL |
| [22] | Ross S, Grasso R (2010). In vitro propagation of ‘Guayabo del país’ (Acca sellowiana (Berg.) Burret). Fruit Veg Cereal Sci Biotechnol 4, 83-87. |
| [23] |
Sarmadi M, Karimi N, Palazón J, Ghassempour A, Mirjalili MH (2019). Improved effects of polyethylene glycol on the growth, antioxidative enzymes activity and taxanes production in a Taxus baccata L. callus culture. Plant Cell Tissue Organ Cult 137, 319-328.
DOI |
| [24] |
Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT (1971). Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc 93, 2325-2327.
DOI PMID |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||