

Chinese Bulletin of Botany ›› 2023, Vol. 58 ›› Issue (4): 656-667.DOI: 10.11983/CBB22160 cstr: 32102.14.CBB22160
• SPECIAL TOPICS • Previous Articles
Yanhong Yin1, Wansheng Chen1,2( ), Ying Xiao1(
), Ying Xiao1( )
)
Received:2022-07-20
Accepted:2022-12-13
Online:2023-07-01
Published:2023-01-10
Contact:
*E-mail: chenwansheng@shutcm.edu.cn;xiaoyingtcm@shutcm.edu.cn
Yanhong Yin, Wansheng Chen, Ying Xiao. Research Progress on Catalytic Characteristics of Pinoresinol-lariciresinol Reductase in Plants[J]. Chinese Bulletin of Botany, 2023, 58(4): 656-667.
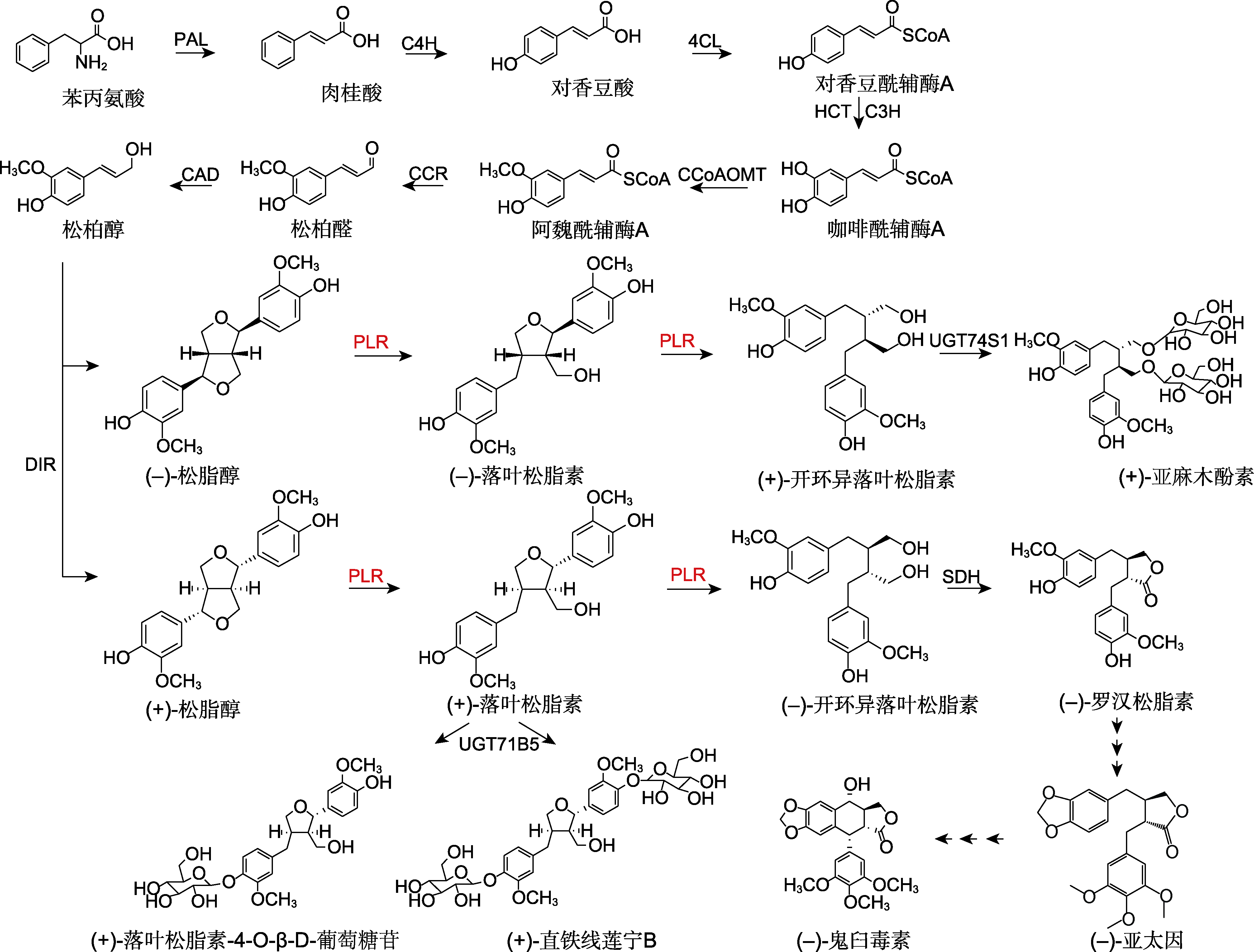
Figure 1 Lignan biosynthetic pathways PAL: Phenylalanine ammonia-lyase; C4H: Cinnamate-4-hydroxylase; 4CL: 4-coumarate:coenzyme A ligase; HCT: Hydroxycinnamoyltransferase; C3H: Coumarate-3-hydroxylase; CCoAOMT: Caffeoyl coenzyme A-O-methyltransferase; CCR: Cinnamoyl-CoA reductase; CAD: Cinnamyl alcohol dehydrogenase; DIR: Dirigent protein; PLR: Pinoresinol-lariciresinol reductase; SDH: Secoisolariciresinol dehydrogenase; UGT: Uridine diphosphatedependent glycosyltransferase
| 植物名称 | 基因名称 | GenBank 登录号 | cDNA长度(bp) | 开放阅读框 长度(bp) | 参考文献 |
|---|---|---|---|---|---|
| 金钟连翘(Forsythia intermedia) | FiPLR1 | U81158 | 1047 | 939 | Dinkova-Kostova et al., |
| 拟南芥(Arabidopsis thaliana) | AtPrR1 | NM102944 | 1129 | 954 | Nakatsubo et al., |
| AtPrR2 | NM117440 | 1206 | 954 | ||
| 菘蓝(Isatis indigotica) | IiPLR1 | JF264893 | 1062 | 954 | Xiao et al., |
| 亚麻(Linum usitatissimum) | LuPLR1 | AJ849359 | - | 939 | von Heimendahl et al., |
| LuPLR2 | EU029951 | 1203 | 993 | Hemmati et al., | |
| 白亚麻(L. album) | LaPLR1 | AJ849358 | 1482 | 981 | von Heimendahl et al., |
| 黄亚麻(L. flavum) | LfPLR | MK599138 | - | 975 | Akira et al., 2016 |
| 长萼亚麻(L. corymbulosum) | LcPLR1 | EU107358 | 1244 | 948 | Bayindir et al., 2010 |
| 宿根亚麻(L. perenne) | LpPLR1 | EF050530 | 1145 | 945 | Hemmati et al., |
| 桃儿七(Sinopodophyllum hexandrum) | ShPLR | EU855792 | 983 | 936 | Wankhede et al., |
| 六角莲(Dysosma pleiantha) | DpPLR | KJ000045 | - | 933 | Kuo et al., |
| 北美乔柏(Thuja plicata) | TpPLR1 | AF242503 | 1190 | 942 | Fujita et al., |
| TpPLR2 | AF242504 | 1151 | 939 | ||
| TpPLR3 | AF242505 | 1308 | 945 | ||
| TpPLR4 | AF242506 | 1287 | 939 | ||
| 台湾杉(Taiwania cryptomerioides) | TcPLR1 | MG264424 | - | 975 | Chiang et al., |
| TcPLR2.2 | MG264425 | 1138 | 942 | ||
| TcPLR3 | MG264426 | 1102 | 939 | ||
| 山茶(Camellia sinensis) | CsPLR1 | MH037247 | 1325 | 939 | Wu et al., |
| CsPLR2 | MH037248 | 1126 | 939 |
Table 1 PLR genes from different plant species
| 植物名称 | 基因名称 | GenBank 登录号 | cDNA长度(bp) | 开放阅读框 长度(bp) | 参考文献 |
|---|---|---|---|---|---|
| 金钟连翘(Forsythia intermedia) | FiPLR1 | U81158 | 1047 | 939 | Dinkova-Kostova et al., |
| 拟南芥(Arabidopsis thaliana) | AtPrR1 | NM102944 | 1129 | 954 | Nakatsubo et al., |
| AtPrR2 | NM117440 | 1206 | 954 | ||
| 菘蓝(Isatis indigotica) | IiPLR1 | JF264893 | 1062 | 954 | Xiao et al., |
| 亚麻(Linum usitatissimum) | LuPLR1 | AJ849359 | - | 939 | von Heimendahl et al., |
| LuPLR2 | EU029951 | 1203 | 993 | Hemmati et al., | |
| 白亚麻(L. album) | LaPLR1 | AJ849358 | 1482 | 981 | von Heimendahl et al., |
| 黄亚麻(L. flavum) | LfPLR | MK599138 | - | 975 | Akira et al., 2016 |
| 长萼亚麻(L. corymbulosum) | LcPLR1 | EU107358 | 1244 | 948 | Bayindir et al., 2010 |
| 宿根亚麻(L. perenne) | LpPLR1 | EF050530 | 1145 | 945 | Hemmati et al., |
| 桃儿七(Sinopodophyllum hexandrum) | ShPLR | EU855792 | 983 | 936 | Wankhede et al., |
| 六角莲(Dysosma pleiantha) | DpPLR | KJ000045 | - | 933 | Kuo et al., |
| 北美乔柏(Thuja plicata) | TpPLR1 | AF242503 | 1190 | 942 | Fujita et al., |
| TpPLR2 | AF242504 | 1151 | 939 | ||
| TpPLR3 | AF242505 | 1308 | 945 | ||
| TpPLR4 | AF242506 | 1287 | 939 | ||
| 台湾杉(Taiwania cryptomerioides) | TcPLR1 | MG264424 | - | 975 | Chiang et al., |
| TcPLR2.2 | MG264425 | 1138 | 942 | ||
| TcPLR3 | MG264426 | 1102 | 939 | ||
| 山茶(Camellia sinensis) | CsPLR1 | MH037247 | 1325 | 939 | Wu et al., |
| CsPLR2 | MH037248 | 1126 | 939 |
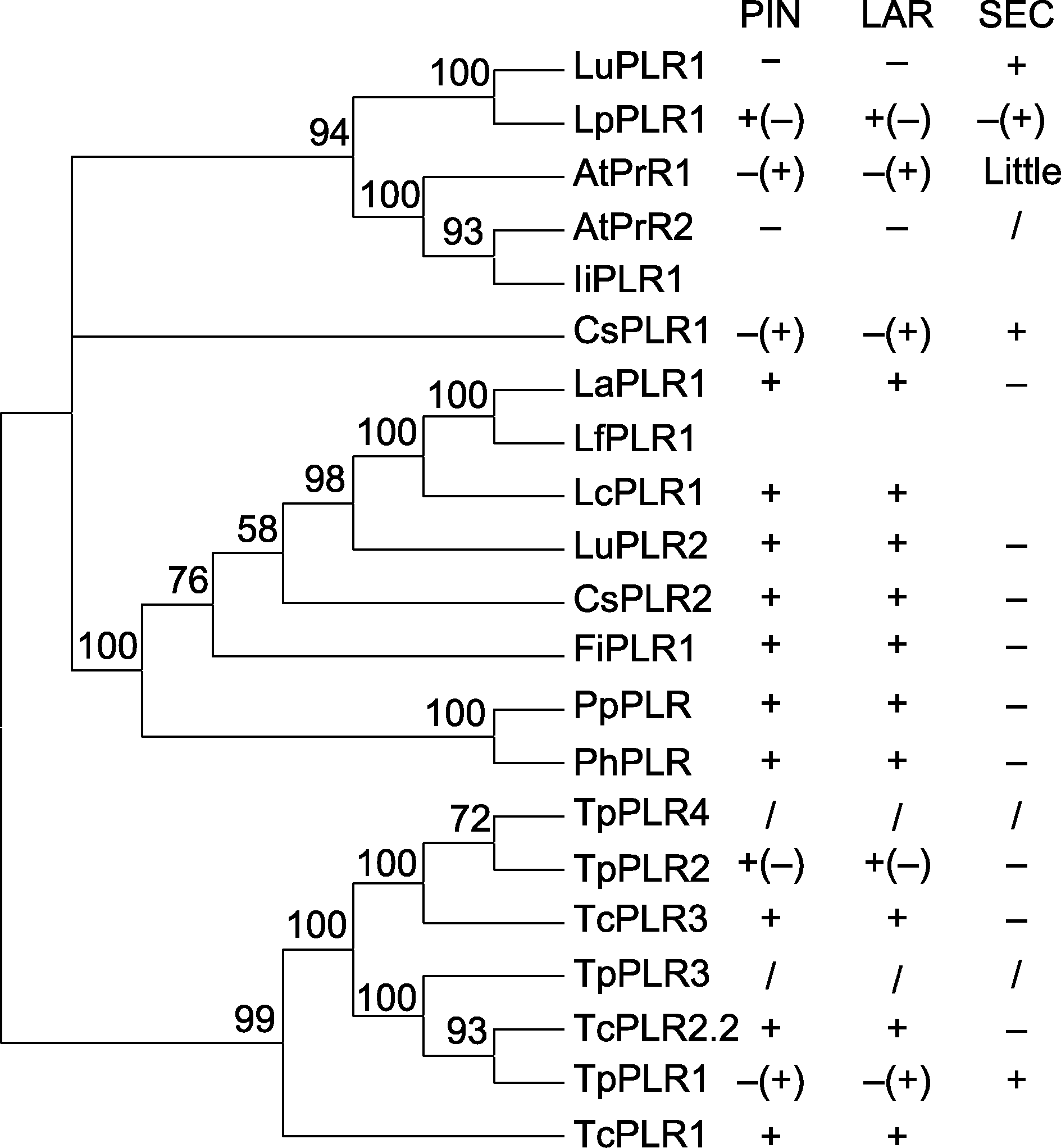
Figure 3 Phylogenetic tree and enantioselectivity of PLRs in plants PIN: Pinoresinol; LAR: Lariciresinol; SEC: Secoisolariciresinol. On the right side, specific forms of enantioselectivity of PLRs in plants to pinoresinol and lariciresinol ( / indicate no catalytic activity)
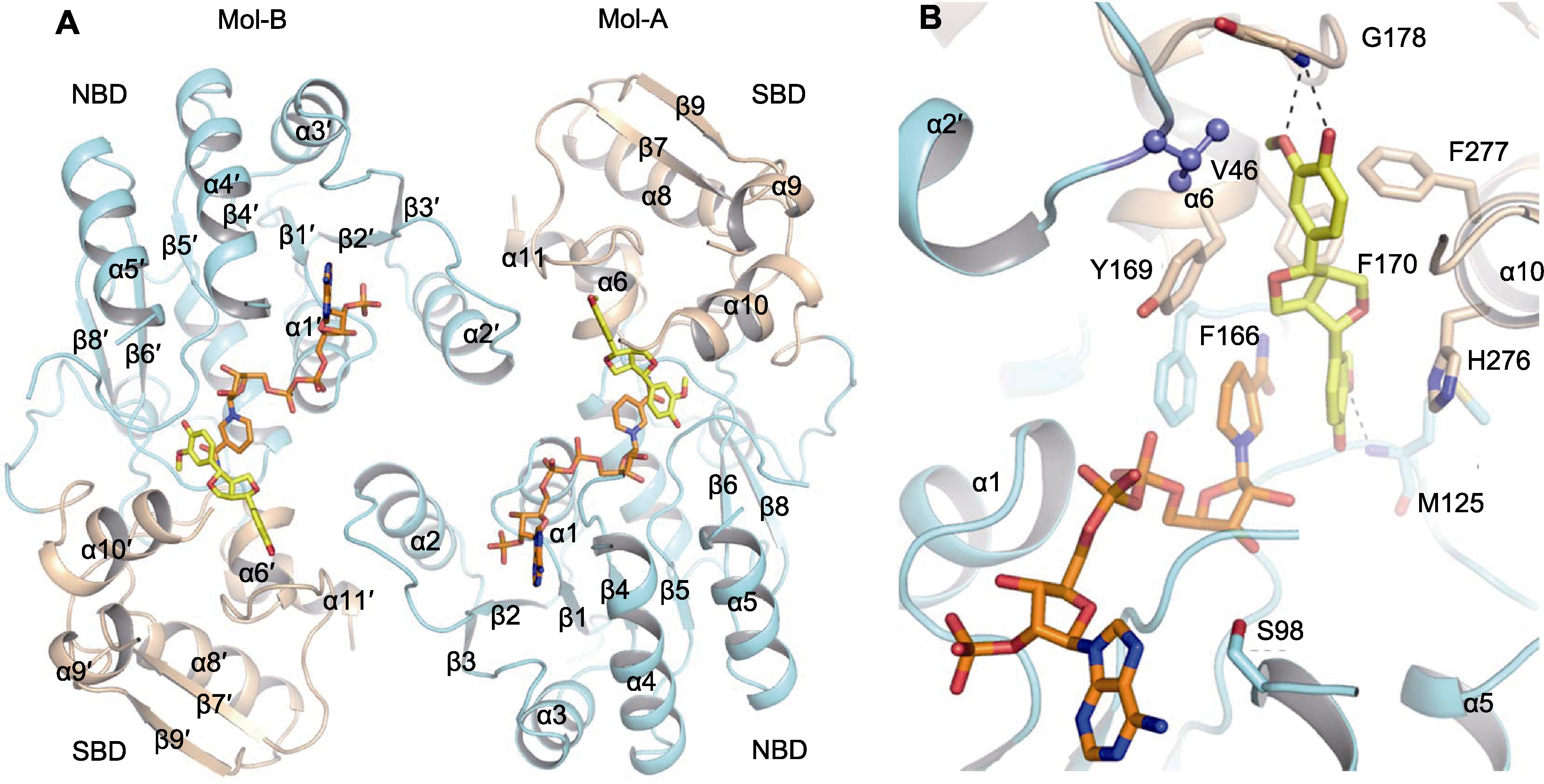
Figure 4 Protein crystal structure of IiPLR1_NAP_+PIN (A) and detail of substrate bonding groove (B) α: α-helix; β: β-sheet. NADPH and +PIN were highlighted in orange and yellow, respectively; the N-terminal NADP+ binding domain (NBD) and the C-terminal substrate binding domain (SBD) were colored in light green and ginger, respectively; dotted lines denote possible hydrogen bonds between +PIN and active residues around substrate; Mol-A and Mol-B are two monomer of homodimer.
| [1] | 陈瑞兵 (2018). Dirigent蛋白催化菘蓝有效成分木脂素生物合成的机制研究. 博士论文. 上海: 中国人民解放军海军军医大学. pp. 20-152. |
| [2] | 程丽姣, 丁羽佳, 翟永功, 赵长琦 (2006). 植物中新的木脂素类化合物及其生物活性. 国外医药·植物药分册 21(3), 93-100. |
| [3] | 刘长军, 侯嵩生 (1997). 抗癌活性物质鬼臼类木脂素的研究进展. 天然产物研究与开发 9(3), 81-89. |
| [4] |
Allen KL, Tschantz DR, Awad KS, Lynch WP, Delucia AL (2007). A plant lignan, 3′-O-methyl-nordihydroguaiaretic acid, suppresses papillomavirus E6 protein function, stabilizes p53 protein, and induces apoptosis in cervical tumor cells. Mol Carcinog 46, 564-575.
DOI URL |
| [5] |
Ayella AK, Trick HN, Wang WQ (2007). Enhancing lignan biosynthesis by over-expressing pinoresinol lariciresinol reductase in transgenic wheat. Mol Nutr Food Res 51, 1518-1526.
PMID |
| [6] |
Bayindir Ü, Alfermann AW, Fuss E (2008). Hinokinin biosynthesis in Linum corymbulosum Reichenb. Plant J 55, 810-820.
DOI URL |
| [7] |
Bohlin L, Rosen B (1996). Podophyllotoxin derivatives: drug discovery and development. Drug Discovery Today 1, 343-351.
DOI URL |
| [8] |
Calado A, Neves PM, Santos T, Ravasco P (2018). The effect of flaxseed in breast cancer: a literature review. Front Nutr 5, 4.
DOI PMID |
| [9] |
Céspedes CL, Avila JG, García AM, Becerra J, Flores C, Aqueveque P, Bittner M, Hoeneisen M, Martinez M, Silva M (2006). Antifungal and antibacterial activities of Araucaria araucana (Mol.) K. Koch heartwood lignans. Z Naturforsch C J Biosci 61, 35-43.
DOI PMID |
| [10] |
Chen X, Chen JF, Feng JX, Wang Y, Li SN, Xiao Y, Diao Y, Zhang L, Chen WS (2021). Tandem UGT71B5s catalyze lignan glycosylation in Isatis indigotica with substrates promiscuity. Front Plant Sci 12, 637695.
DOI URL |
| [11] |
Chiang NT, Ma LT, Lee YR, Tsao NW, Yang CK, Wang SY, Chu FH (2018). The gene expression and enzymatic activity of pinoresinol-lariciresinol reductase during wood formation in Taiwania cryptomerioides Hayata. Holzforschung 73, 197-208.
DOI URL |
| [12] |
Corbin C, Drouet S, Markulin L, Auguin D, Lainé É, Davin LB, Cort JR, Lewis NG, Hano C (2018). A genome-wide analysis of the flax (Linum usitatissimum L.) dirigent protein family: from gene identification and evolution to differential regulation. Plant Mol Biol 97, 73-101.
DOI |
| [13] |
Corbin C, Drouet S, Mateljak I, Markulin L, Decourtil C, Renouard S, Lopez T, Doussot J, Lamblin F, Auguin D, Lainé E, Fuss E, Hano C (2017). Functional characterization of the pinoresinol-lariciresinol reductase-2 gene reveals its roles in yatein biosynthesis and flax defense response. Planta 246, 405-420.
DOI PMID |
| [14] |
Cullmann F, Becker H (1999). Lignans from the liverwort Lepicolea ochroleuca. Phytochemistry 52, 1651-1656.
DOI URL |
| [15] | Davin LB, Lewis NG (1992). Phenylpropanoid metabolism:biosynthesis of monolignols, lignans and neolignans, lignins and suberins. In: Stafford HA, Ibrahim RK, eds. Phenolic Metabolism in Plants. Boston: Springer. pp. 325-375. |
| [16] |
Dinkova-Kostova AT, Gang DR, Davin LB, Bedgar DL, Chu A, Lewis NG (1996). (+)-pinoresinol/(+)-lariciresinol reductase from Forsythia intermedia. Protein purification, cDNA cloning, heterologous expression and comparison to isoflavone reductase. J Biol Chem 271, 29473-29482.
DOI PMID |
| [17] |
Favela-Hernández JMJ, García A, Garza-González E, Rivas-Galindo VM, Camacho-Corona MR (2012). Antibacterial and antimycobacterial lignans and flavonoids from Larrea tridentata. Phytother Res 26, 1957-1960.
DOI PMID |
| [18] |
Fujita M, Gang DR, Davin LB, Lewis NG (1999). Recombinant pinoresinol-lariciresinol reductases from western red cedar (Thuja plicata) catalyze opposite enantiospecific conversions. J Biol Chem 274, 618-627.
DOI PMID |
| [19] |
Gordaliza M, García PA, del Corral JMM, Castro MA, Gómez-Zurita MA (2004). Podophyllotoxin: distribution, sources, applications and new cytotoxic derivatives. Toxicon 44, 441-459.
DOI PMID |
| [20] |
Guo R, Lv TM, Shang XY, Yao GD, Lin B, Wang XB, Huang XX, Song SJ (2019). Racemic neolignans from Crataegus pinnatifida: chiral resolution, configurational assignment, and cytotoxic activities against human hepatoma cells. Fitoterapia 137, 104287.
DOI URL |
| [21] |
Hano C, Corbin C, Drouet S, Quéro A, Rombaut N, Savoire R, Molinié R, Thomasset B, Mesnard F, Lainé E (2017). The lignan (+)-secoisolariciresinol extracted from flax hulls is an effective protectant of linseed oil and its emulsion against oxidative damage. Eur J Lipid Sci Technol 119, 1600219.
DOI URL |
| [22] |
Hano C, Martin I, Fliniaux O, Legrand B, Gutierrez L, Arroo RRJ, Mesnard F, Lamblin F, Lainé E (2006). Pinoresinol-lariciresinol reductase gene expression and secoisolariciresinol diglucoside accumulation in developing flax (Linum usitatissimum) seeds. Planta 224, 1291-1301.
DOI PMID |
| [23] |
Hemmati S, Schmidt TJ, Fuss E (2007). (+)-pinoresinol/ (-)-lariciresinol reductase from Linum perenne Himmelszelt involved in the biosynthesis of justicidin B. FEBS Lett 581, 603-610.
PMID |
| [24] |
Hemmati S, von Heimendahl CB, Klaes M, Alfermann AW, Schmidt TJ, Fuss E (2010). Pinoresinol-lariciresinol reductases with opposite enantiospecificity determine the enantiomeric composition of lignans in the different organs of Linum usitatissimum L. Planta Med 76, 928-934.
DOI PMID |
| [25] |
Kassuya CAL, Leite DFP, de Melo LV, Rehder VLG, Calixto JB (2005). Anti-inflammatory properties of extracts, fractions and lignans isolated from Phyllanthus amarus. Planta Med 71, 721-726.
DOI URL |
| [26] |
Kraus C, Spiteller G (1997). Comparison of phenolic compounds from galls and shoots of Picea glauca. Phytochemistry 44, 59-67.
DOI URL |
| [27] |
Kumar S, Stecher G, Tamura K (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33, 1870-1874.
DOI PMID |
| [28] |
Kuo HJ, Wei ZY, Lu PC, Huang PL, Lee KT (2014). Bioconversion of pinoresinol into matairesinol by use of recombinant Escherichia coli. Appl Environ Microbiol 80, 2687-2692.
DOI URL |
| [29] |
Kuo YC, Kuo YH, Lin YL, Tsai WJ (2006). Yatein from Chamaecyparis obtusa suppresses herpes simplex virus type 1 replication in HeLa cells by interruption the immediate-early gene expression. Antiviral Res 70, 112-120.
DOI URL |
| [30] |
Lau W, Sattely ES (2015). Six enzymes from mayapple that complete the biosynthetic pathway to the etoposide aglycone. Science 349, 1224-1228.
DOI PMID |
| [31] |
Li KM, Dong X, Ma YN, Wu ZH, Yan YM, Cheng YX (2019). Antifungal coumarins and lignans from Artemisia annua. Fitoterapia 134, 323-328.
DOI URL |
| [32] |
Min T, Kasahara H, Bedgar DL, Youn B, Lawrence PK, Gang DR, Halls SC, Park H, Hilsenbeck JL, Davin LB, Lewis NG, Kang C (2003). Crystal structures of pinoresinol-lariciresinol and phenylcoumaran benzylic ether reductases and their relationship to isoflavone reductases. J Biol Chem 278, 50714-50723.
DOI PMID |
| [33] |
Nakatsubo T, Mizutani M, Suzuki S, Hattori T, Umezawa T (2008). Characterization of Arabidopsis thaliana pinoresinol reductase, a new type of enzyme involved in lignan biosynthesis. J Biol Chem 283, 15550-15557.
DOI PMID |
| [34] |
Rahman MMA, Dewick PM, Jackson DE, Lucas JA (1990). Lignans of Forsythia intermedia. Phytochemistry 29, 1971-1980.
DOI URL |
| [35] |
Renouard S, Tribalatc MA, Lamblin F, Mongelard G, Fliniaux O, Corbin C, Marosevic D, Pilard S, Demailly H, Gutierrez L, Hano C, Mesnard F, Lainé E (2014). RNAi-mediated pinoresinol lariciresinol reductase gene silencing in flax (Linum usitatissimum L.) seed coat: consequences on lignans and neolignans accumulation. J Plant Physiol 171, 1372-1377.
DOI URL |
| [36] |
Robert X, Gouet P (2014). Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res 42, W320-W324.
DOI URL |
| [37] |
Scher JM, Zapp J, Becker H (2003). Lignan derivatives from the liverwort Bazzania trilobata. Phytochemistry 62, 769-777.
DOI URL |
| [38] | Shiaishi A, Murata J, Matsumoto E, Matsubara S, Ono E, Satake H (2016). De novo transcriptomes of Forsythia koreana using a novel assembly method: insight into tissue- and species-specific expression of lignan biosynthesis-related gene. PLoS One 11, e0164805. |
| [39] |
Stadler D, Bach T (2008). Concise stereoselective synthesis of (-)-podophyllotoxin by an intermolecular iron(III)- catalyzed Friedel-Crafts alkylation. Angew Chem Int Ed Engl 47, 7557-7559.
DOI URL |
| [40] |
Suzuki S, Umezawa T, Shimada M (2002). Stereochemical diversity in lignan biosynthesis of Arctium lappa L. Biosci Biotechnol Biochem 66, 1262-1269.
DOI URL |
| [41] |
Takeda R, Hasegawa J, Shinozaki M (1990). The first isolation of lignans, megacerotonic acid and anthocerotonic acid, from non-vascular plants, anthocerotae (hornworts). Tetrahedron Lett 31, 4159-4162.
DOI URL |
| [42] |
Teponno RB, Kusari S, Spiteller M (2016). Recent advances in research on lignans and neolignans. Nat Prod Rep 33, 1044-1092.
DOI PMID |
| [43] |
Umezawa T (2003). Diversity in lignan biosynthesis. Phytochem Rev 2, 371-390.
DOI URL |
| [44] |
Umezawa T, Davin LB, Lewis NG (1991). Formation of lignans (-)-secoisolariciresinol and (-)-matairesinol with Forsythia intermedia cell-free extracts. J Biol Chem 266, 10210-10217.
PMID |
| [45] |
van Fürden B, Humburg A, Fuss E (2005). Influence of methyl jasmonate on podophyllotoxinand 6-methoxypodophyllotoxin accumulation in Linum album cell suspension cultures. Plant Cell Rep 24, 312-317.
DOI URL |
| [46] |
von Heimendahl CBI, Schäfer KM, Eklund P, Sjöholm R, Schmidt TJ, Fuss E (2005). Pinoresinol-lariciresinol reductases with different stereospecificity from Linum album and Linum usitatissimum. Phytochemistry 66, 1254-1263.
DOI PMID |
| [47] |
Wada H, Kido T, Tanaka N, Murakami T, Saiki Y, Chen CM (1992). Chemical and chemotaxonomical studies of ferns. LXXXI. Characteristic lignans of Blechnaceous ferns. Chem Pharm Bull 40, 2099-2101.
DOI URL |
| [48] |
Wankhede DP, Biswas DK, Rajkumar S, Sinha AK (2013). Expressed sequence tags and molecular cloning and characterization of gene encoding pinoresinol/lariciresinol reductase from Podophyllum hexandrum. Protoplasma 250, 1239-1249.
DOI URL |
| [49] |
Wu YL, Xing DW, Ma GL, Dai XL, Gao LP, Xia T (2019). A variable loop involved in the substrate selectivity of pinoresinol/lariciresinol reductase from Camellia sinensis. Phytochemistry 162, 1-9.
DOI URL |
| [50] |
Xiao Y, Ji Q, Gao SH, Tan HX, Chen RB, Li Q, Chen JF, Yang YB, Zhang L, Wang ZT, Chen WS, Hu ZB (2015). Combined transcriptome and metabolite profiling reveals that IiPLR1 plays an important role in lariciresinol accumulation in Isatis indigotica. J Exp Bot 66, 6259-6271.
DOI URL |
| [51] |
Xiao Y, Shao K, Zhou JW, Wang L, Ma XQ, Wu D, Yang YB, Chen JF, Feng JX, Qiu S, Lv ZY, Zhang L, Zhang P, Chen WS (2021). Structure-based engineering of substrate specificity for pinoresinol-lariciresinol reductases. Nat Commun 12, 2828.
DOI PMID |
| [52] |
Zheng CJ, Zhang XW, Han T, Jiang YP, Tang JY, Brömme D, Qin LP (2014). Anti-inflammatory and anti-osteoporotic lignans from Vitex negundo seeds. Fitoterapia 93, 31-38.
DOI URL |
| [53] |
Zhou L, Yao GD, Lu LW, Song XY, Lin B, Wang XB, Huang XX, Song SJ (2018). Neolignans from red raspberry (Rubus idaeus L.) exhibit enantioselective neuroprotective effects against H2O2-induced oxidative injury in SH-SY5Y cells. J Agric Food Chem 66, 11390-11397.
DOI URL |
| [1] | WANG Guo-Dong CHEN Xiao-Ya. The Properties, Functions, Catalytic Mechanism and Applicability of Laccase [J]. Chinese Bulletin of Botany, 2003, 20(04): 469-475. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||