

Chinese Bulletin of Botany ›› 2023, Vol. 58 ›› Issue (2): 248-260.DOI: 10.11983/CBB22026 cstr: 32102.14.CBB22026
• EXPERIMENTAL COMMUNICATION • Previous Articles Next Articles
Jinbo Bao, Zhijie Ding, Haoyu Miao, Xueli Li, Shuxian Ren, Ruoyan Jiao, Hao Li;Qianqian Deng, Yingzi Li, Xinmin Tian
Received:2022-02-10
Accepted:2022-05-10
Online:2023-03-01
Published:2023-03-15
Contact:
*E-mail: Jinbo Bao, Zhijie Ding, Haoyu Miao, Xueli Li, Shuxian Ren, Ruoyan Jiao, Hao Li, Qianqian Deng, Yingzi Li, Xinmin Tian. Analysis of Chloroplast Genomes of Aleurites moluccana[J]. Chinese Bulletin of Botany, 2023, 58(2): 248-260.
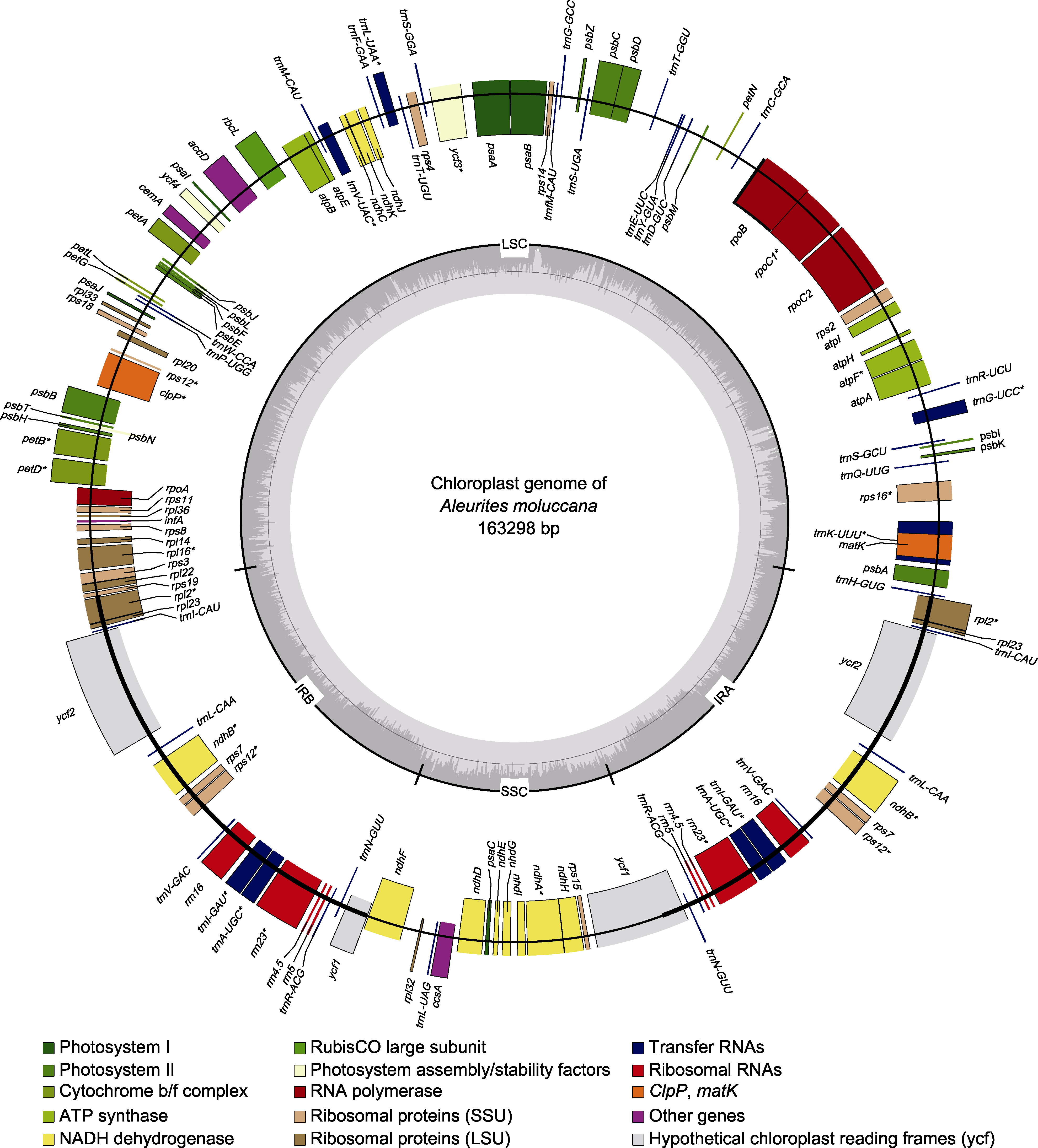
Figure 2 The chloroplast genome map of Aleurites moluccana Genes on the outside of the large circle are transcribed clockwise and those on the inside are transcribed counterclockwise. The genes are color-coded based on their function. The dashed area on the inside represents the GC composition of the A. moluccana chloroplast genome. LSC: Large single-copy region; SSC: Small single-copy region; IRA: Inverted repeat region A; IRB: Inverted repeat region B. * for genes containing introns.
| Categories of genes | Group of genes | Name of genes |
|---|---|---|
| Genes for photosynthesis | Subunits of photosystem I | psaA, psaB, psaC, psaI, psaJ |
| Subunits of photosystem II | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbN, psbT, psbZ | |
| Subunits of ATP synthase | atpA, atpB, atpE, atpF, atpH, atpI | |
| Subunits of cytochrome | petA, petB, petD, petG, petL, petN | |
| ATP-dependent protease subunits P gene | clpP | |
| Large subunits of Rubisco | rbcL | |
| Subunits of NADH dehydrogenase | ndhA, ndhB, ndhC, ndhD, ndhE, ndhF, ndhG, ndhH, ndhI, ndhJ, ndhK | |
| Self replication | Small subunit of ribosome | rps2, rps3, rps4, rps7, rps8, rps11, rps12, rps14, rps15, rps16, rps18, rps19 |
| Large subunit of ribosome | rpl2, rpl14, rpl16, rpl20, rpl2, rpl23, rpl32, rpl33, rpl36, rpl22 | |
| DNA dependent RNA polymerase | rpoA, rpoB, rpoC1, rpoC2 | |
| Ribosomal RNA genes | rrn5, rrn4.5, rrn16, rrn23 | |
| Transfer RNA genes | trnN-GUU, trnR-ACG, trnH-GUG, trnL-CAU, trnA-UGC, trnL-GAU, trnV-GAC, trnL- CAA, trnL-CAU, trnP-UGG, trnW-CCA, trnM-CAU, trnV-UAC, trnF-GAA, trnL-UAA, trnT-UGU, trnS-GGA, trnfM-CAU, trnG-GCC, trnS-UGA, trnT-GGU, trnE-UUC, trnY-GUA, trnD-GUC, trnC-GCA, trnR-UCU, trnS-GCU, trnQ-UUG, trnK-UUU, trnL-CAA, trnV-GAC, trnL-GAU, trnR-UGC, trnL-UAG, trnR-ACG, trnN-GUU | |
| Other genes | Maturase | matK |
| Envelop membrane protein | cemA | |
| Translation initiation factor IF-1 | infA | |
| C-type cytochrome synthesis gene | ccsA | |
| Unknown function | Conserved open reading frames | ycf1, ycf2, ycf3, ycf4, ycf15 |
Table 1 Annotation of functional genes in the chloroplast genome of Aleurites moluccana
| Categories of genes | Group of genes | Name of genes |
|---|---|---|
| Genes for photosynthesis | Subunits of photosystem I | psaA, psaB, psaC, psaI, psaJ |
| Subunits of photosystem II | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbN, psbT, psbZ | |
| Subunits of ATP synthase | atpA, atpB, atpE, atpF, atpH, atpI | |
| Subunits of cytochrome | petA, petB, petD, petG, petL, petN | |
| ATP-dependent protease subunits P gene | clpP | |
| Large subunits of Rubisco | rbcL | |
| Subunits of NADH dehydrogenase | ndhA, ndhB, ndhC, ndhD, ndhE, ndhF, ndhG, ndhH, ndhI, ndhJ, ndhK | |
| Self replication | Small subunit of ribosome | rps2, rps3, rps4, rps7, rps8, rps11, rps12, rps14, rps15, rps16, rps18, rps19 |
| Large subunit of ribosome | rpl2, rpl14, rpl16, rpl20, rpl2, rpl23, rpl32, rpl33, rpl36, rpl22 | |
| DNA dependent RNA polymerase | rpoA, rpoB, rpoC1, rpoC2 | |
| Ribosomal RNA genes | rrn5, rrn4.5, rrn16, rrn23 | |
| Transfer RNA genes | trnN-GUU, trnR-ACG, trnH-GUG, trnL-CAU, trnA-UGC, trnL-GAU, trnV-GAC, trnL- CAA, trnL-CAU, trnP-UGG, trnW-CCA, trnM-CAU, trnV-UAC, trnF-GAA, trnL-UAA, trnT-UGU, trnS-GGA, trnfM-CAU, trnG-GCC, trnS-UGA, trnT-GGU, trnE-UUC, trnY-GUA, trnD-GUC, trnC-GCA, trnR-UCU, trnS-GCU, trnQ-UUG, trnK-UUU, trnL-CAA, trnV-GAC, trnL-GAU, trnR-UGC, trnL-UAG, trnR-ACG, trnN-GUU | |
| Other genes | Maturase | matK |
| Envelop membrane protein | cemA | |
| Translation initiation factor IF-1 | infA | |
| C-type cytochrome synthesis gene | ccsA | |
| Unknown function | Conserved open reading frames | ycf1, ycf2, ycf3, ycf4, ycf15 |
| SSR repeat type (number of copies) | SSR repeat sequence | Number of copies | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16-30 | Total | ||
| Mononucleotide (80) | A/T | 24 | 17 | 7 | 14 | 3 | 3 | 68 | ||||||
| C/G | 7 | 3 | 2 | 12 | ||||||||||
| Dinucleotide (53) | AT/TA/AC/CA/AG/GA | 18 | 7 | 6 | 4 | 6 | 2 | 2 | 45 | |||||
| CG/GC | 4 | 2 | 1 | 1 | 8 | |||||||||
| Trinucleotide (10) | CGG/AAG | 2 | 2 | 1 | 5 | |||||||||
| TGT/TTA | 1 | 2 | 3 | |||||||||||
| ATT/AAT | 1 | 1 | 2 | |||||||||||
| Tetranucleotide (2) | TACA/TGGT | 1 | 1 | 2 | ||||||||||
Table 2 Information of simple sequence repeat (SSR) identified in the chloroplast genome of Aleurites moluccana
| SSR repeat type (number of copies) | SSR repeat sequence | Number of copies | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16-30 | Total | ||
| Mononucleotide (80) | A/T | 24 | 17 | 7 | 14 | 3 | 3 | 68 | ||||||
| C/G | 7 | 3 | 2 | 12 | ||||||||||
| Dinucleotide (53) | AT/TA/AC/CA/AG/GA | 18 | 7 | 6 | 4 | 6 | 2 | 2 | 45 | |||||
| CG/GC | 4 | 2 | 1 | 1 | 8 | |||||||||
| Trinucleotide (10) | CGG/AAG | 2 | 2 | 1 | 5 | |||||||||
| TGT/TTA | 1 | 2 | 3 | |||||||||||
| ATT/AAT | 1 | 1 | 2 | |||||||||||
| Tetranucleotide (2) | TACA/TGGT | 1 | 1 | 2 | ||||||||||
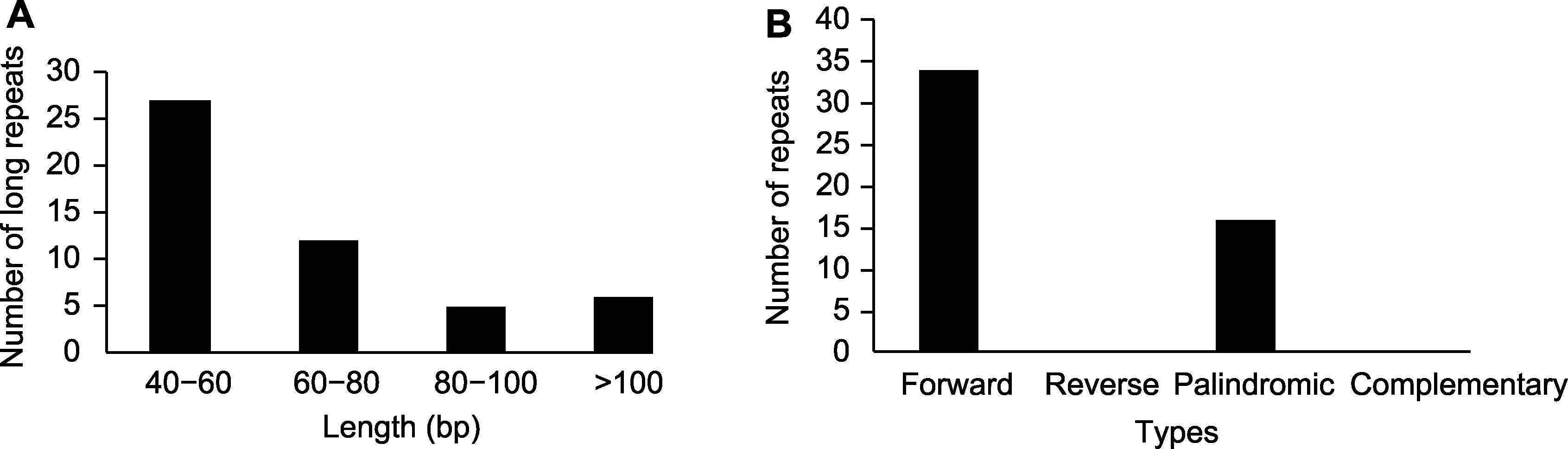
Figure 3 The length and type of repeat sequences in the chloroplast genome of Aleurites moluccana (A) The length of repeat sequences; (B) The type of repeat sequences
| Amino acid | Codon | No. of codon | RSCU | Amino acid | Codon | No. of codon | RSCU |
|---|---|---|---|---|---|---|---|
| Ala | GCU | 431 | 1.20 | Cys | UGU | 698 | 1.20 |
| GCC | 306 | 0.86 | UGC | 468 | 0.80 | ||
| GCA | 427 | 1.19 | Glu | GAA | 1416 | 1.40 | |
| GCG | 267 | 0.75 | GAG | 611 | 0.60 | ||
| Asp | GAU | 1073 | 1.41 | Gly | GGU | 534 | 0.93 |
| GAC | 448 | 0.59 | GGC | 382 | 0.67 | ||
| Phe | UUU | 2457 | 1.26 | GGA | 803 | 1.40 | |
| UUC | 1452 | 0.74 | GGG | 572 | 1.00 | ||
| His | CAU | 874 | 1.36 | Ile | AUU | 1927 | 1.19 |
| CAC | 412 | 0.64 | AUC | 1184 | 0.73 | ||
| Lys | AAA | 2578 | 1.38 | AUA | 1728 | 1.07 | |
| AAG | 1162 | 0.62 | Leu | UUA | 1299 | 1.46 | |
| Trp | UGG | 748 | 1.00 | UUG | 1125 | 1.26 | |
| Asn | AAU | 2122 | 1.43 | CUU | 1021 | 1.15 | |
| AAC | 843 | 0.57 | CUC | 577 | 0.65 | ||
| Arg | CGU | 341 | 0.58 | CUA | 844 | 0.95 | |
| CGC | 246 | 0.42 | CUG | 442 | 0.50 | ||
| CGA | 590 | 1.00 | Pro | CCU | 568 | 1.02 | |
| CGG | 412 | 0.70 | CCC | 556 | 1.00 | ||
| AGA | 1253 | 2.12 | CCA | 699 | 1.25 | ||
| AGG | 702 | 1.19 | CCG | 408 | 0.73 | ||
| Thr | ACU | 683 | 1.18 | Met | AUG | 887 | 1.00 |
| ACC | 550 | 0.95 | Gln | CAA | 1041 | 1.38 | |
| ACA | 696 | 1.20 | CAG | 463 | 0.62 | ||
| ACG | 383 | 0.66 | Ser | UCU | 1070 | 1.36 | |
| Val | GUU | 737 | 1.34 | UCC | 840 | 1.07 | |
| GUC | 402 | 0.73 | UCA | 1002 | 1.27 | ||
| GUA | 667 | 1.21 | UCG | 565 | 0.72 | ||
| GUG | 393 | 0.71 | AGU | 720 | 0.92 | ||
| Tyr | UAU | 1842 | 1.43 | AGC | 521 | 0.66 | |
| UAC | 737 | 0.57 |
Table 3 Codon usage bias in the chloroplast genome of Aleurites moluccana
| Amino acid | Codon | No. of codon | RSCU | Amino acid | Codon | No. of codon | RSCU |
|---|---|---|---|---|---|---|---|
| Ala | GCU | 431 | 1.20 | Cys | UGU | 698 | 1.20 |
| GCC | 306 | 0.86 | UGC | 468 | 0.80 | ||
| GCA | 427 | 1.19 | Glu | GAA | 1416 | 1.40 | |
| GCG | 267 | 0.75 | GAG | 611 | 0.60 | ||
| Asp | GAU | 1073 | 1.41 | Gly | GGU | 534 | 0.93 |
| GAC | 448 | 0.59 | GGC | 382 | 0.67 | ||
| Phe | UUU | 2457 | 1.26 | GGA | 803 | 1.40 | |
| UUC | 1452 | 0.74 | GGG | 572 | 1.00 | ||
| His | CAU | 874 | 1.36 | Ile | AUU | 1927 | 1.19 |
| CAC | 412 | 0.64 | AUC | 1184 | 0.73 | ||
| Lys | AAA | 2578 | 1.38 | AUA | 1728 | 1.07 | |
| AAG | 1162 | 0.62 | Leu | UUA | 1299 | 1.46 | |
| Trp | UGG | 748 | 1.00 | UUG | 1125 | 1.26 | |
| Asn | AAU | 2122 | 1.43 | CUU | 1021 | 1.15 | |
| AAC | 843 | 0.57 | CUC | 577 | 0.65 | ||
| Arg | CGU | 341 | 0.58 | CUA | 844 | 0.95 | |
| CGC | 246 | 0.42 | CUG | 442 | 0.50 | ||
| CGA | 590 | 1.00 | Pro | CCU | 568 | 1.02 | |
| CGG | 412 | 0.70 | CCC | 556 | 1.00 | ||
| AGA | 1253 | 2.12 | CCA | 699 | 1.25 | ||
| AGG | 702 | 1.19 | CCG | 408 | 0.73 | ||
| Thr | ACU | 683 | 1.18 | Met | AUG | 887 | 1.00 |
| ACC | 550 | 0.95 | Gln | CAA | 1041 | 1.38 | |
| ACA | 696 | 1.20 | CAG | 463 | 0.62 | ||
| ACG | 383 | 0.66 | Ser | UCU | 1070 | 1.36 | |
| Val | GUU | 737 | 1.34 | UCC | 840 | 1.07 | |
| GUC | 402 | 0.73 | UCA | 1002 | 1.27 | ||
| GUA | 667 | 1.21 | UCG | 565 | 0.72 | ||
| GUG | 393 | 0.71 | AGU | 720 | 0.92 | ||
| Tyr | UAU | 1842 | 1.43 | AGC | 521 | 0.66 | |
| UAC | 737 | 0.57 |
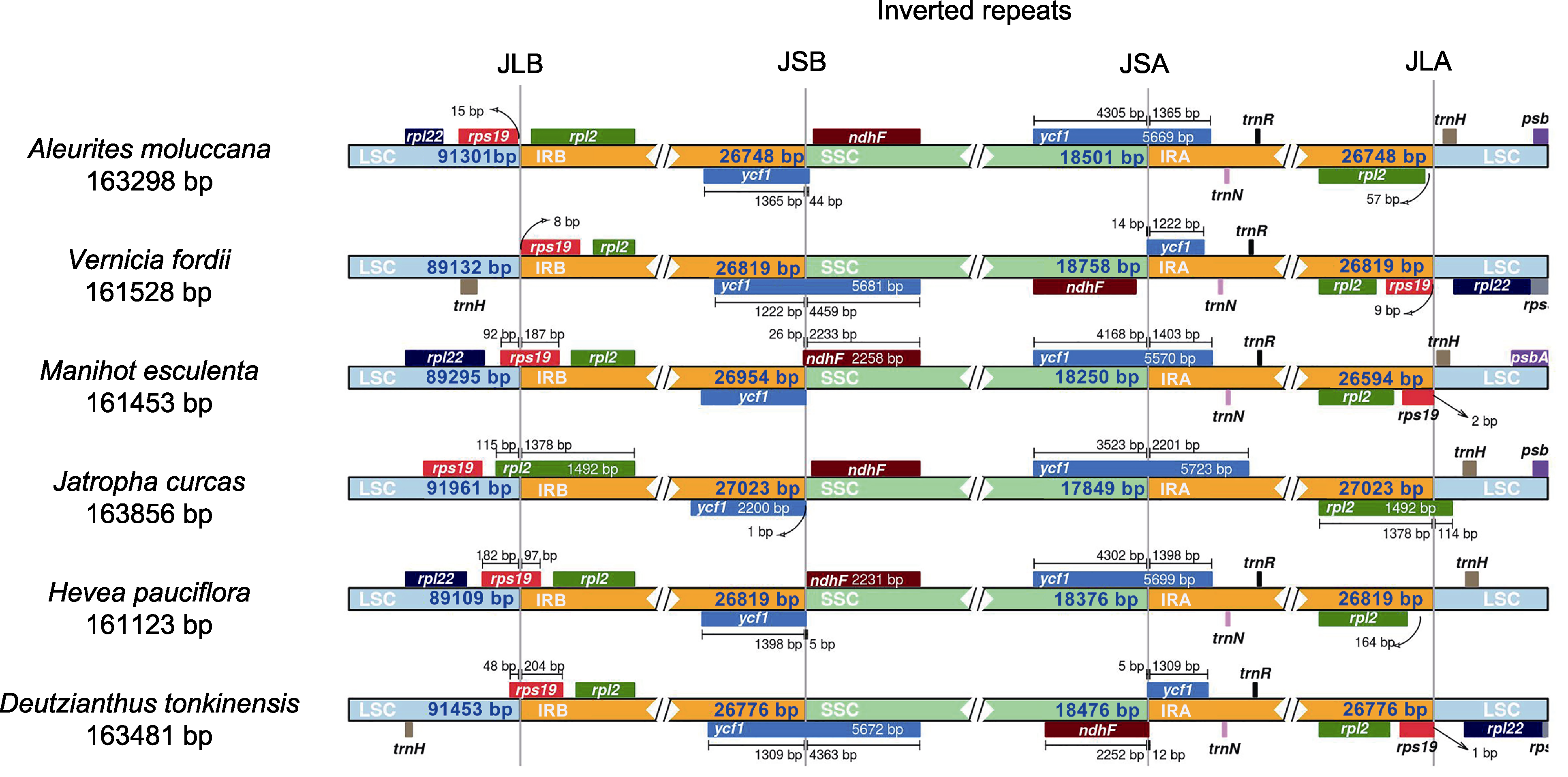
Figure 6 Contraction and expansion of inverted repeat region in the chloroplast genome of Aleurites moluccana LSC, SSC, IRA, and IRB are the same as in Figure 2. JLB: Boundary between LSC and IRB; JSB: Boundary between SSC and IRB; JSA: Boundary between SSC and IRA; JLA: Boundary between LSC and IRA
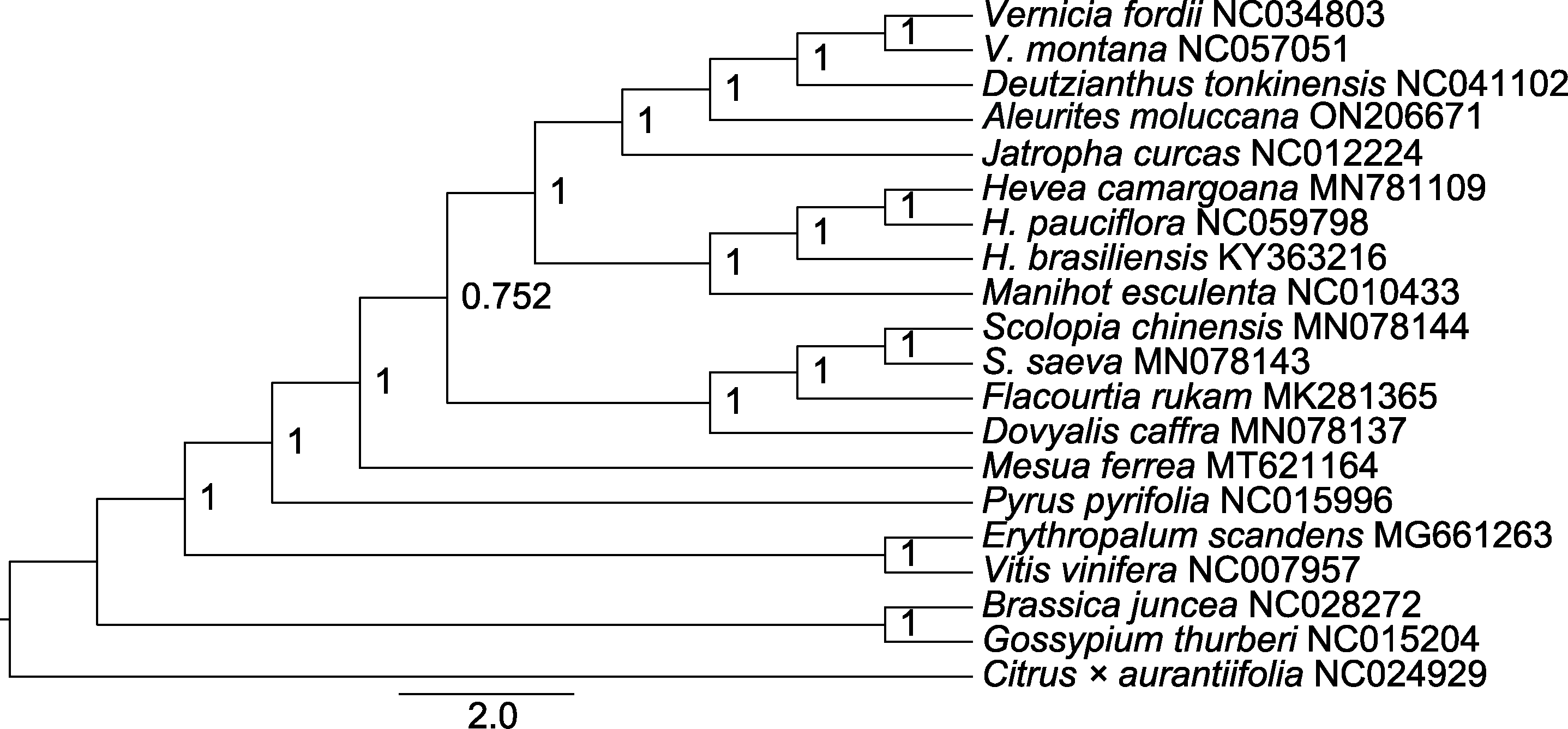
Figure 7 Bayesian phylogenetic tree of Aleurites moluccana and other 19 species based on protein coding sequence The values on the branch are posteriori probability.
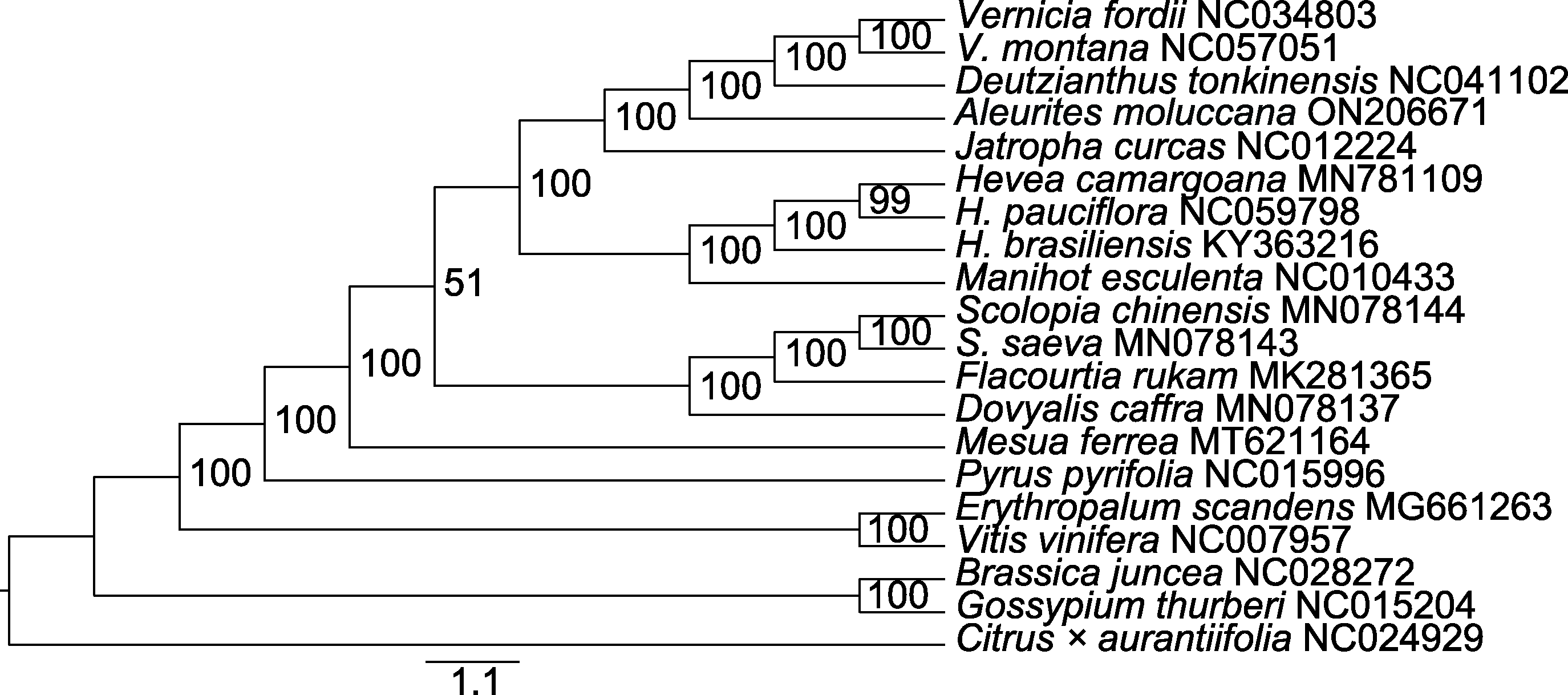
Figure 8 Maximum likelihood (ML) phylogenetic tree of Aleurites moluccana and other 19 species based on protein coding sequence The values on the branch are posteriori probability.
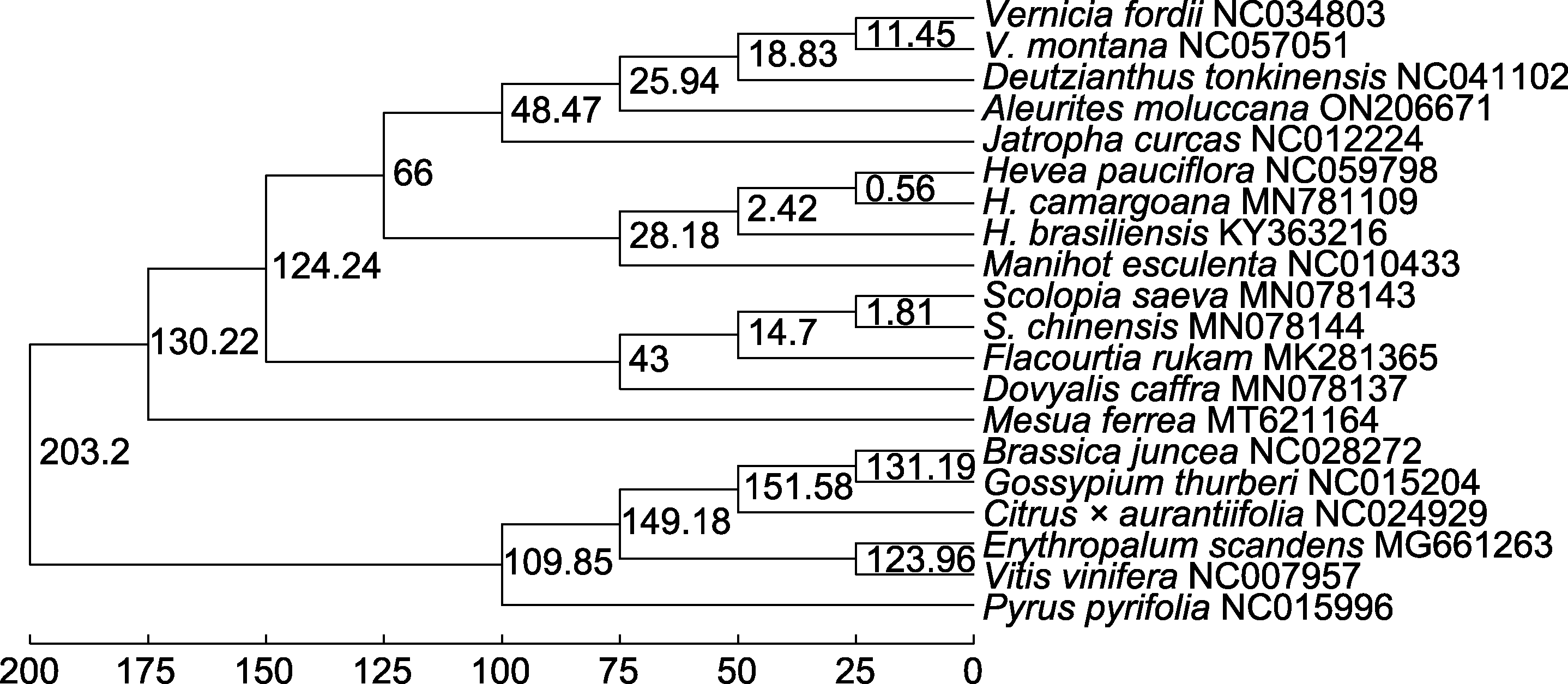
Figure 9 Phylogenetic dating tree of Aleurites moluccana and other 19 species based on relax molecular clock model The values on the branch are the differentiation time (unit: million years ago).
| [1] | 蔡金标, 丁建祖, 陈必勇 (1997). 中国油桐品种、类型的分类. 经济林研究 15(4), 47-50. |
| [2] | 曹晖, 肖艳华, 王绍云 (2007). 石栗属和油桐属的化学成分和生物活性. 凯里学院学报 25(006), 43-45. |
| [3] | 陈琴怡 (2017). 两种五加科植物的叶绿体全基因组研究及其系统发育分析. 硕士论文. 杭州: 浙江大学. pp. 22-34. |
| [4] |
李巧丽, 延娜, 宋琼, 郭军战 (2018). 鲁桑叶绿体基因组序列及特征分析. 植物学报 53, 94-103.
DOI |
| [5] | 梁文汇, 李开祥, 邓力, 曾祥艳, 邓福春 (2011). 广西生物柴油原料树种石栗的综合评价. 广西林业科学 40, 333-335. |
| [6] | 凌建群, 张新英, 陈耀堂 (1995). 油桐、千年桐和石栗的木材比较解剖. 北京大学学报(自然科学版) 31, 745-751. |
| [7] | 刘昌盛, 黄凤洪, 李重屹, 王明霞, 南占东, 韩伟, 廖李 (2008). 木本油料石栗的初步研究. 中国油料作物学报 30, 106-107, 111. |
| [8] | 罗群凤, 冯源恒, 贾婕, 陈虎, 杨章旗 (2018). 马尾松叶绿体基因组测序及特征分析. 广西林业科学 47, 7. |
| [9] | 苏梦云, 周国璋 (1988). 油桐属与石栗属叶绿体的核酸、蛋白质及超微结构的初步研究. 林业科学研究 1, 424-427. |
| [10] | 孙雨晴 (2018). 四种葱蒜类蔬菜叶绿体DNA提取优化及比较基因组学研究. 硕士论文. 长春: 吉林农业大学. pp. 36-38. |
| [11] | 王劲风, 方嘉兴, 刘兴温, 周国璋, 苏梦云, 成小飞 (1986). 油桐属种分类及其品种类型鉴别方法的探讨. 全国林木遗传育种第五次学术报告会论文汇编. pp. 119-121. |
| [12] | 王磊 (2013). 石栗种子内含物变化及accD基因的克隆研究. 硕士论文. 南宁: 广西大学. pp. 45-68. |
| [13] |
谢海坤, 焦健, 樊秀彩, 张颖, 姜建福, 孙海生, 刘崇怀 (2017). 基于高通量测序组装赤霞珠叶绿体基因组及其特征分析. 中国农业科学 50, 1655-1665.
DOI |
| [14] |
杨亚蒙, 焦健, 樊秀彩, 张颖, 姜建福, 李民, 刘崇怀 (2019). 桑叶葡萄叶绿体基因组及其特征分析. 园艺学报 46, 635-648.
DOI |
| [15] | 杨艳婷 (2018). 羊草叶绿体全基因组分析及分子标记开发. 硕士论文. 扬州: 扬州大学. pp. 55-65. |
| [16] |
赵月梅, 杨振艳, 赵永平, 李筱玲, 赵志新, 赵桂仿 (2019). 木犀科植物叶绿体基因组结构特征和系统发育关系. 植物学报 54, 441-454.
DOI |
| [17] | 周会, 荆胜利, 李刚, 张磊, 覃瑞, 刘虹 (2014). 叶绿体基因组分析在植物系统发育中的应用. 植物学研究 3, 1-9. |
| [18] |
Cesca TG, Faqueti LG, Rocha LW, Meira NA, Meyre-Silva C, De Souza MM, Quintão NLM, Silva RML, Filho VC, Bresolin TMB (2012). Antinociceptive, anti-inflammatory and wound healing features in animal models treated with a semisolid herbal medicine based on Aleurites moluccana L. Willd. Euforbiaceae standardized leaf extract: semisolid herbal. J Ethnopharmacol 143, 355-362.
DOI PMID |
| [19] |
Daniell H, Lin CS, Yu M, Chang WJ (2016). Chloroplast genomes: diversity, evolution, and applications in genetic engineering. Genome Biol 17, 134.
DOI PMID |
| [20] |
Fukuda Y, Tomita M, Washio T (1999). Comparative study of overlapping genes in the genomes of Mycoplasma gen- italium and Mycoplasma pneumoniae. Nucleic Acids Res 27, 1847-1853.
PMID |
| [21] |
Jin JJ, Yu WB, Yang JB, Song Y, dePamphilis CW, Yi TS, Li DZ (2020). GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol 21, 241.
DOI |
| [22] |
Krause K (2008). From chloroplasts to “cryptic” plastids: evolution of plastid genomes in parasitic plants. Curr Genet 54, 111-121.
DOI URL |
| [23] | Li PR, Zhang SJ, Li F, Zhang SF, Zhang H, Wang XW, Sun RF, Bonnema G, Borm TJA (2017a). A phylogenetic analysis of chloroplast genomes elucidates the relationships of the six economically important Brassica species comprising the triangle of U. Front Plant Sci 8, 111. |
| [24] |
Li Z, Long HX, Zhang L, Liu ZM, Cao HP, Shi MW, Tan XF (2017b). The complete chloroplast genome sequence of tung tree (Vernicia fordii): organization and phylogenetic relationships with other angiosperms. Sci Rep 7, 1869.
DOI |
| [25] | Liu L, Hao ZZ, Liu YY, Wei XX, Cun YZ, Wang XQ (2014). Phylogeography of Pinus armandii and its relatives: heterogeneous contributions of geography and climate changes to the genetic differentiation and diversification of Chinese white pines. PLoS One 9, e85920. |
| [26] | Martin G, Baurens FC, Cardi C, Aury JM, D’Hont A (2013). The complete chloroplast genome of banana (Musa acuminata, Zingiberales): insight into plastid monocotyledon evolution. PLoS One 8, e67350. |
| [27] | Nie XJ, Lv SZ, Zhang YX, Du XH, Wang L, Biradar SS, Tan XF, Wan FH, Song WN (2012). Complete chloroplast genome sequence of a major invasive species, crofton weed (Ageratina adenophora). PLoS One 7, e36869. |
| [28] |
Niu YF, Hu YS, Zheng C, Liu ZY, Liu J (2020). The complete chloroplast genome of Hevea camargoana. Mitochondrial DNA Part B 5, 607-608.
DOI URL |
| [29] |
Quintão NLM, Pastor MVD, de-Souza Antonialli C, da Silva GF, Rocha LW, Berté TE, de Souza MM, Meyre- Silva C, Lucinda-Silva RM, Bresolin TMB, Filho VC (2019). Aleurites moluccanus and its main active constituent, the flavonoid 2″-O-rhamnosylswertisin, in experimental model of rheumatoid arthritis. J Ethnopharmacol 235, 248-254.
DOI PMID |
| [30] |
Radunz A, He P, Schmid GH (1998). Analysis of the seed lipids of Aleurites montana. Z Naturforsch C 53, 305-310.
DOI URL |
| [31] |
Reback RG, Kapgate DK, Wurdack K, Manchester SR (2022). Fruits of euphorbiaceae from the late cretaceous Deccan intertrappean beds of India. Int J Plant Sci 183, 128-138.
DOI URL |
| [32] | Shaw J, Lickey EB, Schilling EE, Small RL (2007). Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: the tortoise and the hare III. Am J Bot 94, 275-288. |
| [33] |
Song Y, Dong WP, Liu B, Xu C, Yao X, Gao J, Corlett RT (2015). Comparative analysis of complete chloroplast genome sequences of two tropical trees Machilus yunnanensis and Machilus balansae in the family Lauraceae. Front Plant Sci 6, 662.
DOI PMID |
| [34] |
Villarante NR, Davila RAE, Sumalapao DEP (2018). Removal of lead (ΙΙ) by Lumbang, Aleurites moluccana activated carbon carboxymethylcellulose composite crosslinked with epichlorohydrin. Orient J Chem 34, 693-703.
DOI URL |
| [35] |
Villarante NR, Ibarrientos CH (2021). Physicochemical characterization of candlenut (Aleurites moluccana)-derived biodiesel purified with deed eutectic solvents. J Oleo Sci 70, 113-123.
DOI PMID |
| [36] |
Wang YL, Jian X, Wang S (2022). Characterization of the complete chloroplast genome of Rupr. (Euphorbiaceae). Mitochondrial DNA Part B 7, 1550-1552.
DOI URL |
| [37] | Zhang QY, Chen X, Guo MB, Guo R, Xu YP, Yang M, Guo HY (2017). Screening and development of chloroplast polymorphic molecular markers on wild hemp (Cannabis sativa L.). Mol Plant Breed 15, 979-985. |
| [1] | Chuanyong Wang, Dian Zhuang, Zhengda Song, Henghua Zhai, Naiwei Li, Fan Zhang. Structural and Comparative Analysis of the Complete Chloroplast Genome and Phylogenetic Inference of the Aronia melanocarpa [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [2] | Hong Deyuan. A brief discussion on methodology in taxonomy [J]. Biodiv Sci, 2025, 33(2): 24541-. |
| [3] | Yajun Sun. What do higher or lower organisms mean—Clarify the meaning and validity of the biological ladder implied by On the Origin of Species [J]. Biodiv Sci, 2025, 33(1): 24394-. |
| [4] | Hua He, Dunyan Tan, Xiaochen Yang. Cryptic dioecy in angiosperms: Diversity, phylogeny and evolutionary significance [J]. Biodiv Sci, 2024, 32(6): 24149-. |
| [5] | Yanyu Ai, Haixia Hu, Ting Shen, Yuxuan Mo, Jinhua Qi, Liang Song. Vascular epiphyte diversity and the correlation analysis with host tree characteristics: A case in a mid-mountain moist evergreen broad-leaved forest, Ailao Mountains [J]. Biodiv Sci, 2024, 32(5): 24072-. |
| [6] | Yanwen Lv, Ziyun Wang, Yu Xiao, Zihan He, Chao Wu, Xinsheng Hu. Advances in lineage sorting theories and their detection methods [J]. Biodiv Sci, 2024, 32(4): 23400-. |
| [7] | Zhi Yang, Yong Yang. Research Advances on Nuclear Genomes of Economically Important Trees of Lauraceae [J]. Chinese Bulletin of Botany, 2024, 59(2): 302-318. |
| [8] | Chen-Kun Jiang, Wen-Bin Yu, Guang-Yuan Rao, Huaicheng Li, Julien B. Bachelier, Hartmut H. Hilger, Theodor C. H. Cole. Plant Phylogeny Posters—An educational project on plant diversity from an evolutionary perspective [J]. Biodiv Sci, 2024, 32(11): 24210-. |
| [9] | Zhenzhou Chu, Gulbar Yisilam, Zezhong Qu, Xinmin Tian. Comparative Analyses on the Chloroplast Genome of Three Sympatric Atraphaxis Species [J]. Chinese Bulletin of Botany, 2023, 58(3): 417-432. |
| [10] | Huiyin Song, Zhengyu Hu, Guoxiang Liu. Assessing advances in taxonomic research on Chlorellaceae (Chlorophyta) [J]. Biodiv Sci, 2023, 31(2): 22083-. |
| [11] | Zhizhong Li, Shuai Peng, Qingfeng Wang, Wei Li, Shichu Liang, Jinming Chen. Cryptic diversity of the genus Ottelia in China [J]. Biodiv Sci, 2023, 31(2): 22394-. |
| [12] | Ting Wang, Jiangping Shu, Yufeng Gu, Yanqing Li, Tuo Yang, Zhoufeng Xu, Jianying Xiang, Xianchun Zhang, Yuehong Yan. Insight into the studies on diversity of lycophytes and ferns in China [J]. Biodiv Sci, 2022, 30(7): 22381-. |
| [13] | Jianming Wang, Mengjun Qu, Yin Wang, Yiming Feng, Bo Wu, Qi Lu, Nianpeng He, Jingwen Li. The drivers of plant taxonomic, functional, and phylogenetic β-diversity in the gobi desert of northern Qinghai-Tibet Plateau [J]. Biodiv Sci, 2022, 30(6): 21503-. |
| [14] | XIANG Wei, HUANG Dong-Liu, ZHU Shi-Dan. Absorptive root anatomical traits of 26 tropical and subtropical fern species [J]. Chin J Plant Ecol, 2022, 46(5): 593-601. |
| [15] | WANG Chun-Cheng, ZHANG Yun-Ling, MA Song-Mei, HUANG Gang, ZHANG Dan, YAN Han. Phylogeny and species differentiation of four wild almond species of subgen. Amygdalus in China [J]. Chin J Plant Ecol, 2021, 45(9): 987-995. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||