

Chinese Bulletin of Botany ›› 2020, Vol. 55 ›› Issue (5): 613-622.DOI: 10.11983/CBB19236 cstr: 32102.14.CBB19236
• SPECIAL TOPICS • Previous Articles Next Articles
Fei Qi1, Piyi Xing2, Yinguang Bao1,2, Honggang Wang1,2, Xingfeng Li1,2,*( )
)
Received:2019-12-11
Accepted:2020-03-23
Online:2020-09-01
Published:2020-09-03
Contact:
Xingfeng Li
Fei Qi, Piyi Xing, Yinguang Bao, Honggang Wang, Xingfeng Li. Advances in Genetic Studies of the Awn in Cereal Crops[J]. Chinese Bulletin of Botany, 2020, 55(5): 613-622.
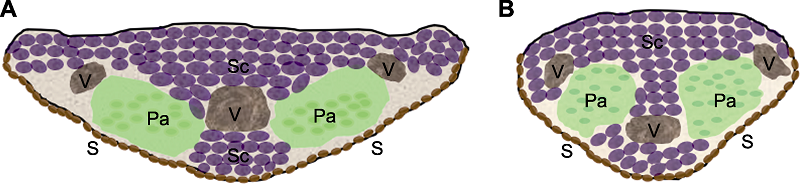
Figure 1 Cross-section of the awn (A) Cross-section of the awn of Hordeum vulgare; (B) Cross-section of the awn of Triticum aestivum. S: Stoma; V: Vascular bundle; Pa: Parenchyma; Sc: Sclerenchyma
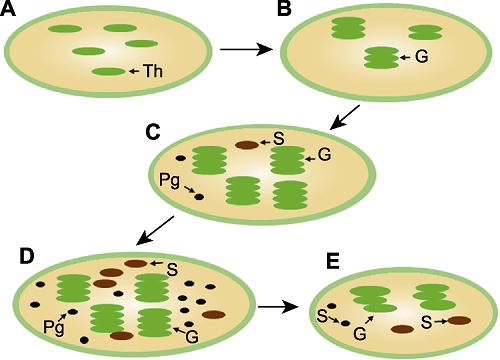
Figure 2 Chloroplast structure of the awn at different developmental stages (A) Heading stage; (B) Anthesis stage; (C) Filling stage; (D) Dry matter formation stage; (E) Ripening stage. Th: Thylakoid; G: Granum; Pg: Plastoglobuli; S: Starch
| 基因 | 染色体 | 功能 | 参考文献 |
|---|---|---|---|
| B1 | 5AL | 分生组织的 维持 | |
| B2 | 6BL | 抑制芒伸长 | |
| Hd (Wknox1a) | 4AL | 抑制芒伸长 | |
| Wknox1b Wknox1d | 4BS 4DS | 茎尖分生组织的形成和维持 |
Table 1 The genes involved in the function of the awn in Triticum aestivum identified by genetic mapping
| 基因 | 染色体 | 功能 | 参考文献 |
|---|---|---|---|
| B1 | 5AL | 分生组织的 维持 | |
| B2 | 6BL | 抑制芒伸长 | |
| Hd (Wknox1a) | 4AL | 抑制芒伸长 | |
| Wknox1b Wknox1d | 4BS 4DS | 茎尖分生组织的形成和维持 |
| 基因 | 染色体 | 功能 | 参考文献 |
|---|---|---|---|
| AN1 | 4 | 促进细胞分裂和芒原基形成、谷粒伸长及穗粒数减少 | |
| AN2 | 4 | 促进细胞分裂素的合成 | |
| LABA1 | 4 | 增强芒原基细胞分裂活性及促进芒伸长和芒刺形成 | |
| GAD1 | 8 | 促进芒原基细胞分裂和芒形成 | |
| GLA | 8 | 促进谷粒伸长 | |
| TOB1 | 4 | 促进外稃和内稃形成和生长、分生组织的维持及花器官数量减少 | |
| DL | 3 | 分生组织细胞的激活、促进芒形成和伸长及调节花器官的发育 | |
| SHO2 | - | 参与TAS3途径中ta-siRNA的合成 | |
| SHL2 | 1 | 参与TAS3途径中ta-siRNA的合成 | |
| SHO1 | 4 | 参与TAS3途径中ta-siRNA的合成 | |
| WAF1 | 7 | 参与TAS3途径中ta-siRNA的合成 | |
| OsETT2 | 1 | 外稃生长活化 |
Table 2 The genes involved in the function of the awn in Oryza sativa identified by genetic mapping
| 基因 | 染色体 | 功能 | 参考文献 |
|---|---|---|---|
| AN1 | 4 | 促进细胞分裂和芒原基形成、谷粒伸长及穗粒数减少 | |
| AN2 | 4 | 促进细胞分裂素的合成 | |
| LABA1 | 4 | 增强芒原基细胞分裂活性及促进芒伸长和芒刺形成 | |
| GAD1 | 8 | 促进芒原基细胞分裂和芒形成 | |
| GLA | 8 | 促进谷粒伸长 | |
| TOB1 | 4 | 促进外稃和内稃形成和生长、分生组织的维持及花器官数量减少 | |
| DL | 3 | 分生组织细胞的激活、促进芒形成和伸长及调节花器官的发育 | |
| SHO2 | - | 参与TAS3途径中ta-siRNA的合成 | |
| SHL2 | 1 | 参与TAS3途径中ta-siRNA的合成 | |
| SHO1 | 4 | 参与TAS3途径中ta-siRNA的合成 | |
| WAF1 | 7 | 参与TAS3途径中ta-siRNA的合成 | |
| OsETT2 | 1 | 外稃生长活化 |
| 基因 | 染色体 | 功能 | 参考文献 |
|---|---|---|---|
| HvKNOX3 | 4H | 分生组织的维持 | |
| Lks2 | 7H | 抑制芒伸长和控制雌蕊形态 | |
| SuKD SuKB SuKC SuKE SuKF | 5H 7H 7H 7H 7H | 抑制HvKNOX3的表达 | |
| ROUGH AWN1 | 5H | 控制芒倒钩 |
Table 3 The genes involved in the function of the awn in Hordeum vulgare identified by genetic mapping
| 基因 | 染色体 | 功能 | 参考文献 |
|---|---|---|---|
| HvKNOX3 | 4H | 分生组织的维持 | |
| Lks2 | 7H | 抑制芒伸长和控制雌蕊形态 | |
| SuKD SuKB SuKC SuKE SuKF | 5H 7H 7H 7H 7H | 抑制HvKNOX3的表达 | |
| ROUGH AWN1 | 5H | 控制芒倒钩 |
| [1] | 巴青松, 傅兆麟, 白凡杰 ( 2010). 小麦芒的研究. 淮北师范大学学报 31, 29-33. |
| [2] | 陈培元, 李英 ( 1981). 小麦芒的功能及去芒对籽粒重的影响. 作物学报 7, 279-282. |
| [3] | 杜斌, 崔法, 王洪刚, 李兴锋 ( 2010). 小麦芒长抑制基因B1近等基因系的鉴定及遗传分析. 分子植物育种 8, 259-264. |
| [4] | 金迪, 王冬至, 王焕雪, 李润枝, 陈树林, 阳文龙, 张爱民, 刘冬成, 詹克慧 ( 2019). 小麦芒长抑制基因B2的精细定位与候选基因分析. 作物学报 45, 807-817. |
| [5] | 李寒冰, 胡玉熹, 白克智, 匡廷云, 周馥, 林金星 ( 2002). 小麦芒和旗叶叶绿体结构及低温荧光发射光谱的比较研究. 电子显微学报 21, 97-101. |
| [6] | 李晓娟 ( 2006). 高产小麦旗叶和芒细胞结构及其光合性能的研究. 硕士论文. 北京: 中国科学院植物研究所. pp. 37-42. |
| [7] | 王忠, 顾蕴洁, 高煜珠 ( 1993). 麦芒的结构及其光合特性. 植物学报 35, 921-928. |
| [8] | 张永平, 王志敏, 吴永成, 张霞 ( 2006). 不同供水条件下小麦不同绿色器官的气孔特性研究. 作物学报 32, 70-75. |
| [9] | Abe M, Yoshikawa T, Nosaka M, Sakakibara H, Sato Y, Nagato Y, Itoh J ( 2010). WAVY LEAF1, an ortholog of Arabidopsis HEN1, regulates shoot development by maintaining microRNA and trans-acting small interfering RNA accumulation in rice. Plant Physiol 154, 1335-1346. |
| [10] |
Abebe T, Melmaiee K, Berg V, Wise RP ( 2010). Drought response in the spikes of barley: gene expression in the lemma, palea, awn, and seed. Funct Integr Genomic 10, 191-205.
DOI URL |
| [11] |
Abebe T, Wise RP, Skadsen RW ( 2009). Comparative transcriptional profiling established the awn as the major photosynthetic organ of the barley spike while the lemma and the palea primarily protect the seed. Plant Genome 2, 247-259.
DOI URL |
| [12] | Antonyuk MZ, Prokopyk DO, Martynenko VS, Ternovska TK ( 2012). Identification of the genes promoting awnedness in the Triticum aestivum/Aegilops umbellulata introgressive line. Cytol Genet 46, 136-143. |
| [13] |
Biscoe PV, Littleton EJ, Scott RK ( 1973). Stomatal control of gas exchange in barley awns. Ann Appl Biol 75, 285-297.
DOI URL |
| [14] | Brown HT, Escombe F ( 1901). Static diffusion of gases and liquids in relation to the assimilation of carbon and translocation in plants. Proc Royal Soc Lond 67, 124-128. |
| [15] | Cuthbert JL, Somers DJ, Brûlé-Babel A, Brown PD, Crow GH ( 2008). Molecular mapping of quantitative trait loci for yield and yield components in spring wheat (Triticum aestivum L.). Theor Appl Genet 117, 595-608. |
| [16] | DeWitt N, Guedira M, Lauer E, Sarinelli M, Tyagi P, Fu DL, Hao QQ, Murphy JP, Marshall D, Akhunova A, Jordan K, Akhunov E, Brown-Guedira G ( 2020). Sequence-based mapping identifies a candidate transcription repressor underlying awn suppression at the B1 locus in wheat. New Phytol 225, 326-339. |
| [17] |
Elbaum R, Zaltzman L, Burgert I, Fratzl P ( 2007). The role of wheat awns in the seed dispersal unit. Science 316, 884-886.
DOI URL PMID |
| [18] |
Evans LT, Bingham J, Jackson P, Sutherland J ( 1972). Effect of awns and drought on the supply of photosynthate and its distribution within wheat ears. Ann Appl Biol 70, 67-76.
DOI URL |
| [19] | Evans LT, Rawson HM ( 1970). Photosynthesis and respiration by the flag leaf and components of the ear during grain development in wheat. Austr J Biol Sci 23, 245-254. |
| [20] |
Grundbacher FJ ( 1963). The physiological function of the cereal awn. Bot Rev 29, 366-381.
DOI URL |
| [21] |
Gu BG, Zhou TY, Luo JH, Liu H, Wang YC, Shangguan YY, Zhu JJ, Li Y, Sang T, Wang ZX, Han B ( 2015). An-2 encodes a cytokinin synthesis enzyme that regulates awn length and grain production in rice. Mol Plant 8, 1635-1650.
URL PMID |
| [22] |
Guo ZF, Schnurbusch T ( 2016). Costs and benefits of awns. J Exp Bot 67, 2533-2535.
DOI URL PMID |
| [23] |
Hua L, Wang DR, Tan LB, Fu YC, Liu FX, Xiao LT, Zhu ZF, Fu Q, Sun XY, Gu P, Cai HW, McCouch SR, Sun CQ ( 2015). LABA1, a domestication gene associated with long, barbed awns in wild rice. Plant Cell 27, 1875-1888.
DOI URL PMID |
| [24] | Huang DQ, Zheng Q, Melchkart T, Bekkaoui Y, Konkin DJF, Kagale S, Martucci M, You FM, Clarke M, Adamski NM, Chinoy C, Steed A, McCartney CA, Cutler AJ, Nicholson P, Feurtado JA ( 2020). Dominant inhibition of awn development by a putative zinc-finger transcriptional repressor expressed at the B1 locus in wheat. New Phytol 225, 340-355. |
| [25] |
Hudson ME, Quail PH ( 2003). Identification of promoter motifs involved in the network of phytochrome a-regulated gene expression by combined analysis of genomic sequence and microarray data. Plant Physiol 133, 1605-1616.
DOI URL PMID |
| [26] |
Itoh JI, Kitano H, Matsuoka M, Nagato Y ( 2000). SHOOT ORGANIZATION genes regulate shoot apical meristem organization and the pattern of leaf primordium initiation in rice. Plant Cell 12, 2161-2174.
DOI URL PMID |
| [27] | Itoh JI, Sato Y, Nagato Y ( 2008). The SHOOT ORGANIZATION 2 gene coordinates leaf domain development along the central-marginal axis in rice. Plant Cell Physiol 49, 1226-1236. |
| [28] |
Jin J, Hua L, Zhu ZF, Tan LB, Zhao XH, Zhang WF, Liu FX, Fu YC, Cai HW, Sun XY, Gu P, Xie DX, Sun CQ ( 2016). GAD1 encodes a secreted peptide that regulates grain number, grain length, and awn development in rice domestication. Plant Cell 28, 2453-2463.
DOI URL PMID |
| [29] | Lipavská H, Mašková P, Vojvodová P ( 2011). Regulatory dephosphorylation of CDK at G2/M in plants: yeast mitotic phosphatase cdc25 induces cytokinin-like effects in transgenic tobacco morphogenesis. Ann Bot 107, 1071-1086. |
| [30] |
Luo JH, Liu H, Zhou TY, Gu BG, Huang XH, Shangguan YY, Zhu JJ, Li Y, Zhao Y, Wang YC, Zhao Q, Wang AH, Wang ZQ, Sang T, Wang ZX, Han B ( 2013). An-1 encodes a basic helix-loop-helix protein that regulates awn development, grain size, and grain number in rice. Plant Cell 25, 3360-3376.
DOI URL PMID |
| [31] |
Mackay IJ, Bansept-Basler P, Barber T, Bentley AR, Cockram J, Gosman N, Greenland AJ, Horsnell R, Howells R, O’Sullivan DM, Rose GA, Howell PJ ( 2014). An eight-parent multiparent advanced generation inter- cross population for winter-sown wheat: creation, properties, and validation. G3 4, 1603-1610.
DOI URL PMID |
| [32] | Maydup ML, Antonietta M, Graciano C, Guiamet JJ, Tambussi EA ( 2014). The contribution of the awns of bread wheat (Triticum aestivum L.) to grain filling: responses to water deficit and the effects of awns on ear temperature and hydraulic conductance. Field Crops Res 167, 102-111. |
| [33] |
Milner SG, Jost M, Taketa S, Mazón ER, Himmelbach A, Oppermann M, Weise S, Knüpffer H, Basterrechea M, König P, Schüler D, Sharma R, Pasam RK, Rutten T, Guo GG, Xu DD, Zhang J, Herren G, Müller T, Krattinger SG, Keller B, Jiang Y, González MY, Zhao YS, Habekuß A, Färber S, Ordon F, Lange M, Börner A, Graner A, Reif JC, Scholz U, Mascher M, Stein N ( 2019). Genebank genomics highlights the diversity of a global barley collection. Nat Genet 51, 319-326.
DOI URL PMID |
| [34] | Morimoto R, Kosugi T, Nakamura C, Takumi S ( 2005). Intragenic diversity and functional conservation of the three homoeologous loci of the KN1-type homeobox gene Wknox1 in common wheat. Plant Mol Biol 57, 907-924. |
| [35] |
Motzo R, Giunta F ( 2002). Awnedness affects grain yield and kernel weight in near-isogenic lines of durum wheat. Aust J Agric Res 53, 1285-1293.
DOI URL |
| [36] |
Müller KJ, Pozzi C, Müller J, Salamini F, Rohde W ( 2000). Molecular analysis of homeotic genes involved in barley development. Pflügers Arch Eur J Physiol 439, R14-R15.
DOI URL |
| [37] | Müller KJ, Romano N, Gerstner O, Garcia-Maroto F, Pozzi C, Salamini F, Rohde W ( 1995). The barley Hooded mutation caused by a duplication in a homeobox gene intron. Nature 374, 727-730. |
| [38] |
Olugbemi LB ( 1978). Distribution of carbon-14 assimilated by wheat awns. Ann Appl Biol 90, 111-114.
DOI URL |
| [39] | Olugbemi LB, Bingham J, Austin RB ( 1976). Ear and flag leaf photosynthesis of awned and awnless Triticum species. Ann Appl Biol 84, 231-240. |
| [40] | Qureshi N, Bariana HS, Zhang P, McIntosh R, Bansal UK, Wong D, Hayden MJ, Dubcovsky J, Shankar M ( 2018). Genetic relationship of stripe rust resistance genes Yr34 and Yr48 in wheat and identification of linked KASP markers. Plant Dis 102, 413-420. |
| [41] |
Rebetzke GJ, Bonnett DG, Reynolds MP ( 2016). Awns reduce grain number to increase grain size and harvestable yield in irrigated and rainfed spring wheat. J Exp Bot 67, 2573-2586.
DOI URL PMID |
| [42] | Roig C, Pozzi C, Santi L, Müller J, Wang YM, Stile MR, Rossini L, Stanca M, Salamini F ( 2004). Genetics of barley hooded suppression. Genetics 167, 439-448. |
| [43] | Santi L, Wang YL, Stile MR, Berendzen KW, Wanke D, Roig C, Pozzi C, Müller K, Müller J, Rohde W, Salamini F ( 2003). The GA octodinucleotide repeat binding factor BBR participates in the transcriptional regulation of the homeobox gene BKn3. Plant J 34, 813-826. |
| [44] | Satoh N, Itoh J, Nagato Y ( 2003). The SHOOTLESS2 and SHOOTLESS1 genes are involved in both initiation and maintenance of the shoot apical meristem through regulating the number of indeterminate cells. Genetics 164, 335-346. |
| [45] | Sorensen AE ( 1986). Seed dispersal by adhesion. Ann Rev Ecol Evol Syst 17, 443-463. |
| [46] | Sourdille P, Singh S, Cadalen T, Brown-Guedira GL, Qi LL, Gill BS, Dufour P, Murigneux A, Bernard M ( 2004). Microsatellite-based deletion bin system for the establishment of genetic-physical map relationships in wheat (Triticum aestivum L.). Funct Integr Genomic 4, 12-25. |
| [47] |
Takumi S, Kosugi T, Murai K, Mori N, Nakamura C ( 2000). Molecular cloning of three homoeologous cDNAs encoding orthologs of the maize KNOTTED1 homeobox protein from young spikes of hexaploid wheat. Gene 249, 171-181.
DOI URL PMID |
| [48] | Tanaka W, Toriba T, Ohmori Y, Yoshida A, Kawai A, Mayama-Tsuchida T, Ichikawa H, Mitsuda N, Ohme- Takagi M, Hirano HY ( 2012). The YABBY gene TONGARI-BOUSHI1 is involved in lateral organ development and maintenance of meristem organization in the rice spikelet. Plant Cell 24, 80-95. |
| [49] |
Teare ID, Sij JW, Waldren RP, Goltz SM ( 1972). Comparative data on the rate of photosynthesis, respiration, and transpiration of different organs in awned and awnless isogenic lines of wheat. Can J Plant Sci 52, 965-971.
DOI URL |
| [50] | Toriba T, Hirano HY ( 2014). The DROOPING LEAF and OsETTIN2 genes promote awn development in rice. Plant J 77, 616-626. |
| [51] | Wang DZ, Yu K, Jin D, Sun LH, Chu JF, Wu WY, Xin PY, Gregová E, Li X, Sun JZ, Yang WL, Zhan KH, Zhang AM, Liu DC ( 2020). Natural variations in the promoter of Awn Length Inhibitor 1(ALI-1) are associated with awn elongation and grain length in common wheat. Plant J 101, 1075-1090. |
| [52] | Watkins AE, Ellerton S ( 1940). Variation and genetics of the awn in Triticum. J Genet 40, 243-270. |
| [53] |
Welchen E, Gonzalez DH ( 2006). Overrepresentation of elements recognized by TCP-domain transcription factors in the upstream regions of nuclear genes encoding components of the mitochondrial oxidative phosphorylation machinery. Plant Physiol 141, 540-545.
DOI URL PMID |
| [54] | Yamaguchi T, Nagasawa N, Kawasaki S, Matsuoka M, Nagato Y, Hirano HY ( 2004). The YABBY gene DROOPING LEAF regulates carpel specification and midrib development in Oryza sativa. Plant Cell 16, 500-509. |
| [55] |
Yoshioka M, Iehisa JCM, Ohno R, Kimura T, Enoki H, Nishimura S, Nasuda S, Takumi S ( 2017). Three dominant awnless genes in common wheat: fine mapping, interaction and contribution to diversity in awn shape and length. PLoS One 12, e0176148.
DOI URL PMID |
| [56] | Yuo T, Yamashita Y, Kanamori H, Matsumoto T, Lundqvist U, Sato K, Ichii M, Jobling SA, Taketa S ( 2012). A SHORT INTERNODES (SHI) family transcription factor gene regulates awn elongation and pistil morphology in barley. J Exp Bot 63, 5223-5232. |
| [57] | Zhang YP, Zhang ZY, Sun XM, Zhu XY, Li B, Li JJ, Guo HF, Chen C, Pan YH, Liang YT, Xu ZJ, Zhang HL, Li ZC ( 2019). Natural alleles of GLA for grain length and awn development were differently domesticated in rice subspecies japonica and indica. Plant Biotechnol J 17, 1547-1559. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||