

一种提升流式细胞术分析效果的前处理方法
收稿日期: 2022-02-23
录用日期: 2022-09-07
网络出版日期: 2022-09-30
基金资助
国家重点实验室专项经费(Y7106F1001)
A Plant Sample Optimal Pretreatment for Flow Cytometric Analysis
Received date: 2022-02-23
Accepted date: 2022-09-07
Online published: 2022-09-30
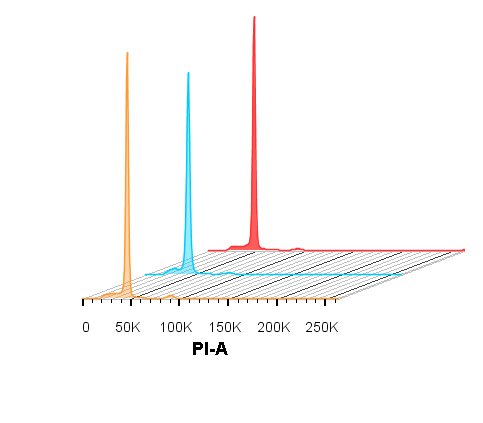
提取完整植物细胞核是流式细胞术检测前处理的关键步骤。分别以不同种属植物的新鲜叶片和大量硅胶快速干燥的植物叶片为研究材料, 比较新优化的细胞核提取液PVPK12-mGB2与其它2种常见提取液(LB01和CyStain® PI Absolute P)的提取效果。结果表明, 新优化的PVPK12-mGB2细胞核提取液在测试多种富含干扰性次生代谢物的植物类群新鲜材料时效果最佳, 提取的细胞核质量基本能满足流式细胞术分析的要求。此外, 实验结果证实该细胞核提取液能够提升大量硅胶快速干燥保存植物材料的提取效果, 测试结果明显优于其它2种细胞核提取液。研究建立了用于DNA流式细胞术的植物样品前处理方法, 为野外采样分析提供了参考方法。
张晋丹, 冯旻 . 一种提升流式细胞术分析效果的前处理方法[J]. 植物学报, 2023 , 58(2) : 285 -297 . DOI: 10.11983/CBB22034
Flow cytometry requires preparation of suspensions of intact nuclei, which is crucial step for analysis. A newly formulated buffer, PVPK12-mGB2 compared with two common buffers (LB01 and CyStain® PI Absolute P), were used to isolate nuclei from fresh or silica gel desiccated leaf tissues of different species. PVPK12-mGB2 exhibited the best performance on all fresh leaves tested of plant species, of which the interfering secondary metabolites highly accumulated, the quality of extracted nuclei were able to satisfy demands for flow cytometric analysis. Moreover, results of the present study substantiate the enhanced effectiveness of PVPK12-mGB2, for silica gel desiccated plant tissue, compared to other buffers tested. The study established an optimal pretreatment of plants for DNA flow cytometry, which provided reference method for sampling and analysis in remote regions.

| [1] | 陈西娟, 王成章, 叶建中 (2008). 银杏叶化学成分及其应用研究进展. 生物质化学工程 42(4), 57-62. |
| [2] | 金永日, 桂明玉, 李绪文, 陆娟, 马场正树, 奥山徹, 徐吉庆 (2007). 狗枣猕猴桃叶化学成分研究. 高等学校化学学报 28, 2060-2064. |
| [3] | 赖娟华, 徐丽瑛, 饶华, 李玉云 (2004). 杜仲叶化学成分和药理作用研究概况. 实用中西医结合临床 4(2), 67-68, 78. |
| [4] | 李军集, 孟忠磊, 黎贵卿 (2012). 广西白玉兰花和叶片挥发油化学成分的GC/MS分析. 西南林业大学学报 32(6), 102-106. |
| [5] | 马广莹, 史小华, 邹清成, 田丹青, 朱开元, 詹菁, 周江华 (2018). 8个玉簪品种幼叶的营养成分测定及品质分析. 浙江农业科学 59, 814-820. |
| [6] | 潘秋文 (2004). 金银花叶的研究进展. 浙江中医学院学报 28(4), 90. |
| [7] | 宋二颖, 雷荣爱 (1997). 水杉叶挥发油成分分析. 中药材 20, 514-515. |
| [8] | 孙兴姣, 李红娇, 刘婷, 李骁 (2018). 麻黄属植物化学成分及临床应用的研究进展. 中国药事 32, 201-209. |
| [9] | 王庆菊, 胡艳丽, 李晓磊, 王磊, 沈向 (2007). 紫叶稠李叶片不同叶序花青苷与化学成分的相关性. 山东农业大学学报(自然科学版) 38, 557-560. |
| [10] | 吴忆微, 蒋立勤 (2013). 红薯叶功效成分及抗肿瘤作用研究进展. 中国食物与营养 19(12), 63-65. |
| [11] | 邢全, 石雷, 刘保东, 崔洪霞, 张金政 (2004). 枇杷叶荚蓬叶片解剖结构及其生态学意义. 园艺学报 31, 526-528. |
| 12 | 严旭, 左艳春, 王红林, 李杨, 李影正, 寇晶, 周晓康, 唐祈林, 杜周和 (2021). 禾本科三倍体: 形成、鉴定与利用. 植物学报 56, 372-387. |
| [13] | Bainard JD, Husband BC, Baldwin SJ, Fazekas AJ, Gregory TR, Newmaster SG, Kron P (2011). The effects of rapid desiccation on estimates of plant genome size. Chromosome Res 19, 825-842. |
| [14] | Bennett MD, Leitch IJ (2005). Plant genome size research: a field in focus. Ann Bot 95, 1-6. |
| [15] | Bühler V (2005). Polyvinylpyrrolidone Excipients for Pharmaceuticals:Povidone, Crospovidone and Copovidone. Ber- lin, Heidelberg: Springer. pp. 5-124. |
| [16] | ?ertner M, Lu?anová M, Sliwinska E, Kolá? F, Loureiro J (2022). Plant material selection, collection, preservation, and storage for nuclear DNA content estimation. Cytometry A 101, 737-748. |
| [17] | Chase MW, Hills HH (1991). Silica gel: an ideal material for field preservation of leaf samples for DNA studies. Taxon 40, 215-220. |
| [18] | Dole?el J, Barto? J (2005). Plant DNA flow cytometry and estimation of nuclear genome size. Ann Bot 95, 99-110. |
| [19] | Dole?el J, Binarová P, Lucretti S (1989). Analysis of nuclear DNA content in plant cells by flow cytometry. Biol Plant 31, 113-120. |
| [20] | Galbraith DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabady E (1983). Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220, 1049-1051. |
| [21] | Galbraith DW, Lambert GM, Macas J, Dolezel J (2001). Analysis of nuclear DNA content and ploidy in higher plants. Curr Protoc Cytom doi: 10.1002/0471142956.cy-0706s02. |
| [22] | Kabera JN, Semana E, Mussa AR, He X (2014). Plant secondary metabolites: biosynthesis, classification, function and pharmacological properties. J Pharm Pharmacol 2, 377-392. |
| [23] | Loureiro J, Kron P, Temsch EM, Koutecky P, Lopes S, Castro M, Castro S (2021). Isolation of plant nuclei for estimation of nuclear DNA content: overview and best practices. Cytometry A 99, 318-327. |
| [24] | Loureiro J, Rodriguez E, Dole?el J, Santos C (2006a). Flow cytometric and microscopic analysis of the effect of tannic acid on plant nuclei and estimation of DNA content. Ann Bot 98, 515-527. |
| [25] | Loureiro J, Rodriguez E, Dole?el J, Santos C (2006b). Comparison of four nuclear isolation buffers for plant DNA flow cytometry. Ann Bot 98, 679-689. |
| [26] | Loureiro J, Rodriguez E, Dole?el J, Santos C (2007). Two new nuclear isolation buffers for plant DNA flow cytometry: a test with 37 species. Ann Bot 100, 875-888. |
| [27] | Noirot M, Barre P, Duperray C, Louarn J, Hamon S (2003). Effects of caffeine and chlorogenic acid on propidium iodide accessibility to DNA: consequences on genome size evaluation in coffee tree. Ann Bot 92, 259-264. |
| [28] | Sadhu A, Bhadra S, Bandyopadhyay M (2016). Novel nuclei isolation buffer for flow cytometric genome size estimation of Zingiberaceae: a comparison with common isolation buffers. Ann Bot 118, 1057-1070. |
/
| 〈 |
|
〉 |