

基于H2DCFDA荧光探针的植物活性氧检测方法
收稿日期: 2022-03-10
录用日期: 2022-05-11
网络出版日期: 2022-05-11
基金资助
浙江省自然科学基金(LY19C130003);浙江师范大学2022年度实验技术开发项目(SJ202218)
Detection of Reactive Oxygen Species Using H2DCFDA Probe in Plant
Received date: 2022-03-10
Accepted date: 2022-05-11
Online published: 2022-05-11
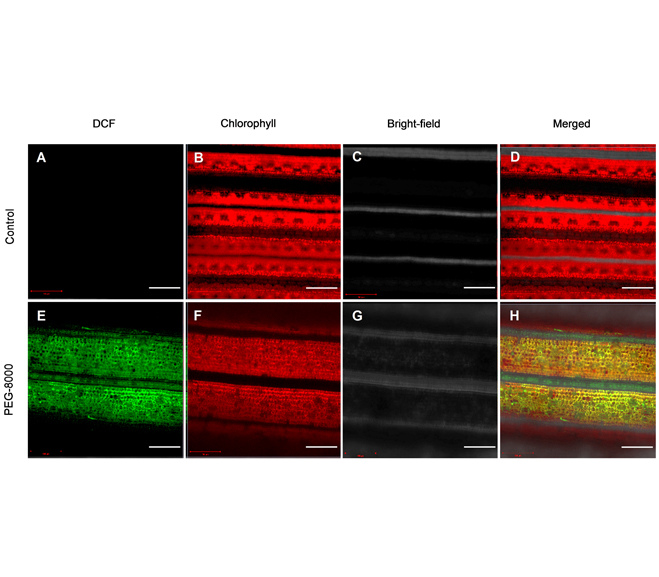
胡海涛 , 钱婷婷 , 杨玲 . 基于H2DCFDA荧光探针的植物活性氧检测方法[J]. 植物学报, 2022 , 57(3) : 320 -326 . DOI: 10.11983/CBB22043

| [1] | Akter S, Khan MS, Smith EN, Flashman E (2021). Measuring ROS and redox markers in plant cells. RSC Chem Biol 2, 1384-1401. |
| [2] | Anjum NA, Amreen N, Tantray AY, Khan NA, Ahmad A (2020). Reactive oxygen species detection-approaches in plants: insights into genetically encoded FRET-based sen- sors. J Biotechnol 308, 108-117. |
| [3] | Apel K, Hirt H (2004). REACTIVE OXYGEN SPECIES: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55, 373-399. |
| [4] | Castro B, Citterico M, Kimura S, Stevens DM, Wrzaczek M, Coaker G (2021). Stress-induced reactive oxygen species compartmentalization, perception and signaling. Nat Plants 7, 403-412. |
| [5] | Chan ZL, Yokawa K, Kim WY, Song CP (2016). Editorial: ROS regulation during plant abiotic stress responses. Front Plant Sci 7, 1536. |
| [6] | Choudhury FK, Rivero RM, Blumwald E, Mittler R (2017). Reactive oxygen species, abiotic stress and stress combination. Plant J 90, 856-867. |
| [7] | Considine MJ, Foyer CH (2021a). Oxygen and reactive oxygen species-dependent regulation of plant growth and development. Plant Physiol 186, 79-92. |
| [8] | Considine MJ, Foyer CH (2021b). Stress effects on the reactive oxygen species-dependent regulation of plant growth and development. J Exp Bot 72, 5795-5806. |
| [9] | Duanghathaipornsuk S, Farrell EJ, Alba-Rubio AC, Zelenay P, Kim DS (2021). Detection technologies for reactive oxygen species: fluorescence and electrochemical methods and their applications. Biosensors 11, 30. |
| [10] | Eruslanov E, Kusmartsev S (2010). Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol Biol 594, 57-72. |
| [11] | Fichman Y, Miller G, Mittler R (2019). Whole-plant live imaging of reactive oxygen species. Mol Plant 12, 1203- 1210. |
| [12] | Gomes A, Fernandes E, Lima JLFC (2005). Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Methods 65, 45-80. |
| [13] | Hasanuzzaman M, Bhuyan MHMB, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fujita M, Fotopoulos V (2020). Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9, 681. |
| [14] | Hu HT, Ren DY, Hu J, Jiang HZ, Chen P, Zeng DL, Qian Q, Guo LB (2021). WHITE AND LESION-MIMIC LEAF1, encoding a lumazine synthase, affects reactive oxygen species balance and chloroplast development in rice. Plant J 108, 1690-1703. |
| [15] | Kristiansen KA, Jensen PE, Møller IM, Schulz A (2009). Monitoring reactive oxygen species formation and localisation in living cells by use of the fluorescent probe CM- H2DCFDA and confocal laser microscopy. Physiol Plant 136, 369-383. |
| [16] | Li HX, Liu Y, Qin HH, Lin XL, Tang D, Wu ZJ, Luo W, Shen Y, Dong FQ, Wang YL, Feng TT, Wang LL, Li LY, Chen DD, Zhang Y, Murray JD, Chao DY, Chong K, Cheng ZK, Meng Z (2020). A rice chloroplast-localized ABC transporter ARG1 modulates cobalt and nickel homeostasis and contributes to photosynthetic capacity. New Phytol 228, 163-178. |
| [17] | Liu XY, Zhang ZG (2022). A double-edged sword: reactive oxygen species (ROS) during the rice blast fungus and host interaction. FEBS J doi:10.1111/febs.16171 |
| [18] | Maulucci G, Bačić G, Bridal L, Schmidt HH, Tavitian B, Viel T, Utsumi H, Yalçın AS, De Spirito M (2016). Imaging reactive oxygen species-induced modifications in living systems. Antioxid Redox Signal 24, 939-958. |
| [19] | Mhamdi A, van Breusegem F (2018). Reactive oxygen species in plant development. Development 145, dev164376. |
| [20] | Oparka M, Walczak J, Malinska D, van Oppen LMPE, Szczepanowska J, Koopman WJH, Wieckowski MR (2016). Quantifying ROS levels using CM-H2DCFDA and HyPer. Methods 109, 3-11. |
| [21] | Ortega-Villasante C, Burén S, Barón-Sola Á, Martínez F, Hernández LE (2016). In vivo ROS and redox potential fluorescent detection in plants: present approaches and future perspectives. Methods 109, 92-104. |
| [22] | Ortega-Villasante C, Burén S, Blázquez-Castro A, Barón- Sola Á, Hernández LE (2018). Fluorescent in vivo imaging of reactive oxygen species and redox potential in plants. Free Radic Biol Med 122, 202-220. |
| [23] | Qi JS, Song CP, Wang BS, Zhou JM, Kangasjärvi J, Zhu JK, Gong ZZ (2018). Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J Integr Plant Biol 60, 805- 826. |
| [24] | Rajneesh, Pathak J, Chatterjee A, Singh SP, Sinha RP (2017). Detection of reactive oxygen species (ROS) in cyanobacteria using the oxidant-sensing probe 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA). Bio Protoc 7, e2545. |
| [25] | Robles V, Riesco MF, Martínez-Vázquez JM, Valcarce DG (2021). Flow cytometry and confocal microscopy for ROS evaluation in fish and human spermatozoa. Methods Mol Biol 2202, 93-102. |
| [26] | Tripathy BC, Oelmüller R (2012). Reactive oxygen species generation and signaling in plants. Plant Signal Behav 7, 1621-1633. |
| [27] | Waszczak C, Carmody M, Kangasjärvi J (2018). Reactive oxygen species in plant signaling. Annu Rev Plant Biol 69, 209-236. |
| [28] | Xia SS, Liu H, Cui YJ, Yu HP, Rao YC, Yan YP, Zeng DL, Hu J, Zhang GH, Gao ZY, Zhu L, Shen L, Zhang Q, Li Q, Dong GJ, Guo LB, Qian Q, Ren DY (2022). UDP-N-acetylglucosamine pyrophosphorylase enhances rice survival at high temperature. New Phytol 233, 344- 359. |
| [29] | Xiong HY, Yu JP, Miao JL, Li JJ, Zhang HL, Wang X, Liu PL, Zhao Y, Jiang CH, Yin ZG, Li Y, Guo Y, Fu BY, Wang WS, Li ZK, Ali J, Li ZC (2018). Natural variation in OsLG3 increases drought tolerance in rice by inducing ROS scavenging. Plant Physiol 178, 451-467. |
/
| 〈 |
|
〉 |