[1]郭泽, 李子绅, 代晓燕, 王英锋(2019).低钾胁迫下外源生长素对烟草根系生长及钾吸收的影响.植物营养与肥料学报, 25:1173-1184.
[2]罗海斌, 黄诚梅, 曹辉庆, 蒋胜理, 吴兴剑, 叶丽萍, 魏源文(2022).不同浓度钾元素对西番莲组培苗根系生长和内源激素含量的影响., 中国果树:53-58.
[3]王立梅, 刘奕清, 阮玉娟 (2015).植物钾素研究进展..中国园艺文摘, 31:71+148.-.
[4]闫慧峰, 石屹, 李乃会, 张永春(2013).烟草钾素营养研究进展.中国农业科技导报, 15:123-129.
[5]张标, 吴健, 张杨, 董小卫, 韩硕, 高昕, 杜从伍, 李慧英, 种学法, 朱莹莹, 刘海伟(2023).木栓层在水和溶质运输中的生理功能研究进展.植物学报, 58:1008-1018.
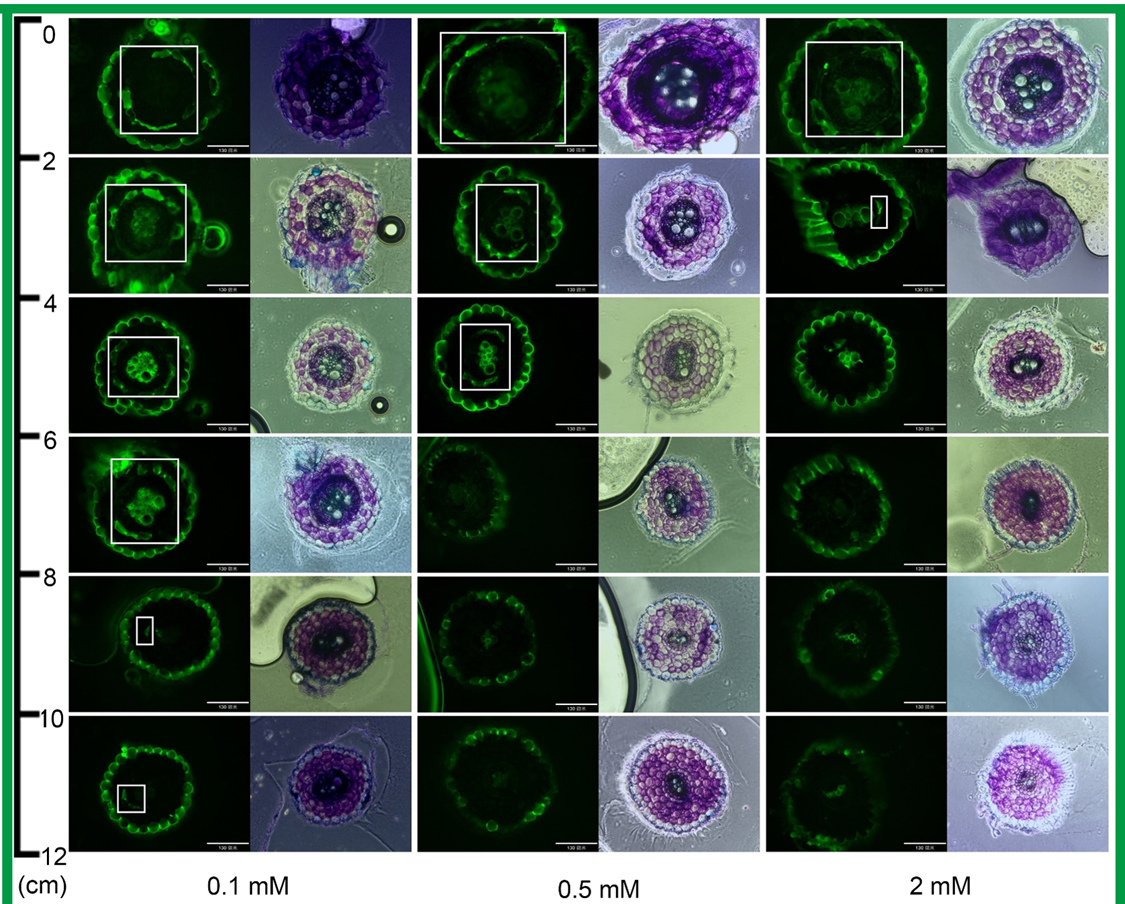
[6]张标, 许耘祥, 张莉汶, 朱莹莹, 刘海伟(2024).低-胁迫抑制烟草根系质外体运输的机制研究.中国烟草科学, 45:25-34.
[7]Andersen T G, Barberon M, Geldner N(2015).Suberization - the second life of an endodermal cell.Curr Opin Plant Biol, 28:9-15.
[8]Barberon M(2017).The endodermis as a checkpoint for nutrients.New Phytol, 213:1604-1610.
[9]Barberon M, Vermeer JE, De Bellis D, Wang P, Naseer S, Andersen TG, Humbel BM, Nawrath C, Takano J, Salt DE, Geldner N(2016).Adaptation of Root Function by Nutrient-Induced Plasticity of Endodermal Differentiation.Cell, 164:447-459.
[10]Baxter I, Hosmani PS, Rus A, Lahner B, Borevitz JO, Muthukumar B, Mickelbart MV, Schreiber L, Franke RB, Salt DE (2009).Root suberin forms an extracellular barrier that affects water relations and mineral nutrition in Arabidopsis. PLoS Genet, 5, e1000492.., :-.
[11]Chen A, Liu T, Deng Y, Xiao R, Zhang T, Wang Y, Yang Y, Lakshmanan P, Shi X, Zhang F, Chen X (2023).Nitrate_dependent suberization regulates cadmium uptake and accumulation in maize. Science of The Total Environment, 878, 162848.., :-.
[12]Leide J, Hildebrandt U, Hartung W, Riederer M, Vogg G(2012).Abscisic acid mediates the formation of a suberized stem scar tissue in tomato fruits.New Phytologist, 194:402-415.
[13]Liu Y, Lu M, Persson DP, Luo J, Liang Y, Li T (2022).The involvement of nitric oxide and ethylene on the formation of endodermal barriers in response to Cd in hyperaccumulator Sedum alfredii. Environ Pollut, 307, 119530.., :-.
[14]Liu Y, Tao Q, Li J, Guo X, Luo J, Jupa R, Liang Y, Li T (2021).Ethylene-mediated apoplastic barriers development involved in cadmium accumulation in root of hyperaccumulator Sedum alfredii. J Hazard Mater, 403, 123729.., :-.
[15]Lulai EC, Suttle JC, Pederson SM(2008).Regulatory involvement of abscisic acid in potato tuber wound-healing.Journal of Experimental Botany, 59:1175-1186.
[16]Lux A, Morita S, Abe J, Ito K(2005).An improved method for clearing and staining free-hand sections and whole-mount samples.Ann Bot, 96:989-996.
[17]Melino VJ, Plett DC, Bendre P, Thomsen HC, Zeisler-Diehl VV, Schreiber L, Kronzucker HJ (2021).Nitrogen depletion enhances endodermal suberization without restricting transporter-mediated root NO3(-) influx. J Plant Physiol, 257, 153334.., :-.
[18]Naseer S, Lee Y, Lapierre C, Franke R, Nawrath C, Geldner N 2012.Casparian strip diffusion barrier in Arabidopsis is made of a lignin polymer without suberin. Proc Natl Acad Sci USA, 109, 10101-10106.., :-.
[19]Pfister A, Barberon M, Alassimone J, Kalmbach L, Lee Y, Vermeer J E, Yamazaki M, Li G, Maurel C, Takano J, Kamiya T, Salt D E, Roppolo D, Geldner N (2014).A receptor-like kinase mutant with absent endodermal diffusion barrier displays selective nutrient homeostasis defects. eLife, 3, e03115.., :-.
[20]Tao Q, Jupa R, Liu Y, Luo J, Li J, Kovac J, Li B, Li Q, Wu K, Liang Y, Lux A, Wang C, Li T(2019).Abscisic acid-mediated modifications of radial apoplastic transport pathway play a key role in cadmium uptake in hyperaccumulator Sedum alfredii.Plant Cell Environ, 42:1425-1440.
[21]Tao Q, Li M, Xu Q, Kovac J, Yuan S, Li B, Li Q, Huang R, Gao X, Wang C (2022).Radial transport difference mediated by root endodermal barriers contributes to differential cadmium accumulation between japonica and indica subspecies of rice (Oryza sativa L.). J Hazard Mater, 425, 128008.., :-.
[22]Vestenaa MW, Husted S, Minutello F, Persson DP (2024).Endodermal suberin restricts root leakage of cesium: a suitable tracer for potassium. Physiologia Plantarum, 176.., :-.
[23]Wang P, Wang C-M, Gao L, Cui YN, Yang HL, de Silva N D G, Ma Q, Bao AK, Flowers TJ, Rowland O, Wang SM(2020).Aliphatic suberin confers salt tolerance to Arabidopsis by limiting Na+ influx,K+ efflux and water backflow.Plant and Soil, 448:603-620.
[24]Wei X, Liu L, Jin X, Xue J, Geng P, Xu Z, Zhang L, Wang X, Zong W, Zhang L, Mao L (2024a).Exogenous methyl jasmonate promotes wound healing of Chinese yam tubers (Dioscorea opposita) through the deposition of suberin polyaliphatics at the wound sites. Postharvest Biology and Technology, 207.., :-.
[25]Wei X, Liu L, Liu G, Geng P, Wei X, Yao X, Chen J, Gong W, Ge Z, Liu M, Mao L (2024b.) Methyl jasmonate promotes suberin biosynthesis by stimulating transcriptional activation of AchMYC2 on AchFHT in wound healing of kiwifruit. Postharvest Biology and Technology, 210.., :-.
[26]Wei X, Liu L, Xu Z, Xue J, Geng P, Ge Z, Wang X, Zhang L, Zong W, Mao L (2023).Methyl jasmonate facilitates wound healing of Chinese yam tubers via positively regulating the biosynthesis and polymerization of suberin polyphenolics. Scientia Horticulturae, 312.., :-.
[27]Zhang B, Xu Y, Zhang L, Yu S, Zhu Y, Liu C, Wang P, Shi Y, Li L, Liu H (2024).Root endodermal suberization induced by nitrate stress regulate apoplastic pathway rather than nitrate uptake in tobacco (Nicotiana tabacum L.). Plant Physiology and Biochemistry, 216, 109166.?., :-.