

植物学报 ›› 2017, Vol. 52 ›› Issue (6): 774-782.DOI: 10.11983/CBB16171 cstr: 32102.14.CBB16171
胡添源1, 王睿1, 陈上1, 马宝伟1, 高伟1,2,*( ), 黄璐琦3
), 黄璐琦3
收稿日期:2016-08-21
接受日期:2017-01-10
出版日期:2017-11-01
发布日期:2018-02-22
通讯作者:
高伟
基金资助:
Hu Tianyuan1, Wang Rui1, Chen Shang1, Ma Baowei1, Gao Wei1,2,*( ), Huang Luqi3
), Huang Luqi3
Received:2016-08-21
Accepted:2017-01-10
Online:2017-11-01
Published:2018-02-22
Contact:
Gao Wei
摘要: 为探索药用植物雷公藤(Tripterygium wilfordii)悬浮细胞原生质体提取的最优条件, 并建立雷公藤原生质体瞬时转化体系, 以雷公藤悬浮细胞为材料, 对酶解液配比、酶解时间、甘露醇浓度及处理转速进行考察。用PEG介导的瞬时转化法将外源基因转化到雷公藤原生质体中。结果表明, 以雷公藤悬浮细胞为材料提取原生质体的最佳条件是酶液配比为2.0%纤维素酶+0.5%果胶酶+0.5%离析酶, 甘露醇浓度为0.6 mol?L-1, 酶解10小时, 处理转速为67×g; 用PEG介导法将含有编码GFP的植物表达载体转化雷公藤悬浮细胞原生质体, 激光共聚焦扫描显微镜下细胞显示绿色荧光。通过实验筛选得到雷公藤悬浮细胞原生质体的最佳提取条件, 建立了雷公藤悬浮细胞原生质体的瞬时转化体系, 为进一步开展雷公藤功能基因及合成生物学研究奠定了基础。
胡添源, 王睿, 陈上, 马宝伟, 高伟, 黄璐琦. 雷公藤悬浮细胞原生质体的制备及瞬时转化体系的建立. 植物学报, 2017, 52(6): 774-782.
Hu Tianyuan, Wang Rui, Chen Shang, Ma Baowei, Gao Wei, Huang Luqi. Protoplast Isolation and Establishment of Transient Expression System of Tripterygium wilfordii Suspension Culture Cells. Chinese Bulletin of Botany, 2017, 52(6): 774-782.
| Group | Cellulase (%) | Pectinase (%) | Macerozyme (%) |
|---|---|---|---|
| 1 | 1.5 | 0.3 | 0.5 |
| 2 | 2.0 | 0.3 | 0.5 |
| 3 | 2.0 | 0.5 | 0.5 |
| 4 | 2.0 | 0.7 | 0.5 |
| 5 | 2.5 | 0.3 | 0.5 |
表1 雷公藤悬浮细胞细胞壁酶解液配比
Table 1 Proportion of enzymic digestion to remove Triptery- gium wilfordii suspension cells cytoderm
| Group | Cellulase (%) | Pectinase (%) | Macerozyme (%) |
|---|---|---|---|
| 1 | 1.5 | 0.3 | 0.5 |
| 2 | 2.0 | 0.3 | 0.5 |
| 3 | 2.0 | 0.5 | 0.5 |
| 4 | 2.0 | 0.7 | 0.5 |
| 5 | 2.5 | 0.3 | 0.5 |
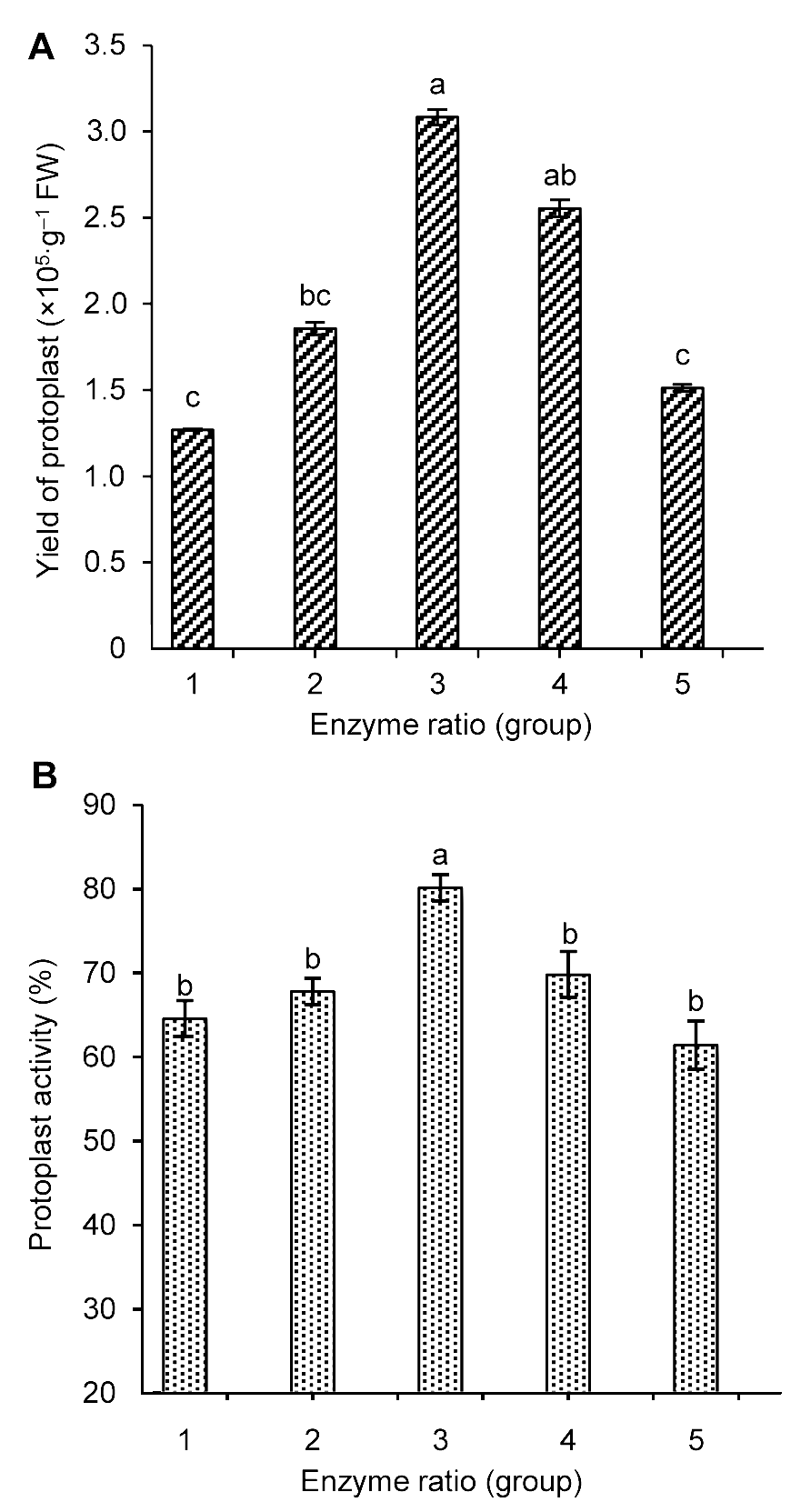
图1 酶解液浓度对雷公藤悬浮细胞原生质体产量(A)和活力(B)的影响不同小写字母表示在P<0.05水平上差异显著。
Figure 1 The influence of enzyme concentration on Tripterygium wilfordii suspension cells protoplast yield (A) and activity (B) Different lowercase letters indicate significant differences at P<0.05.
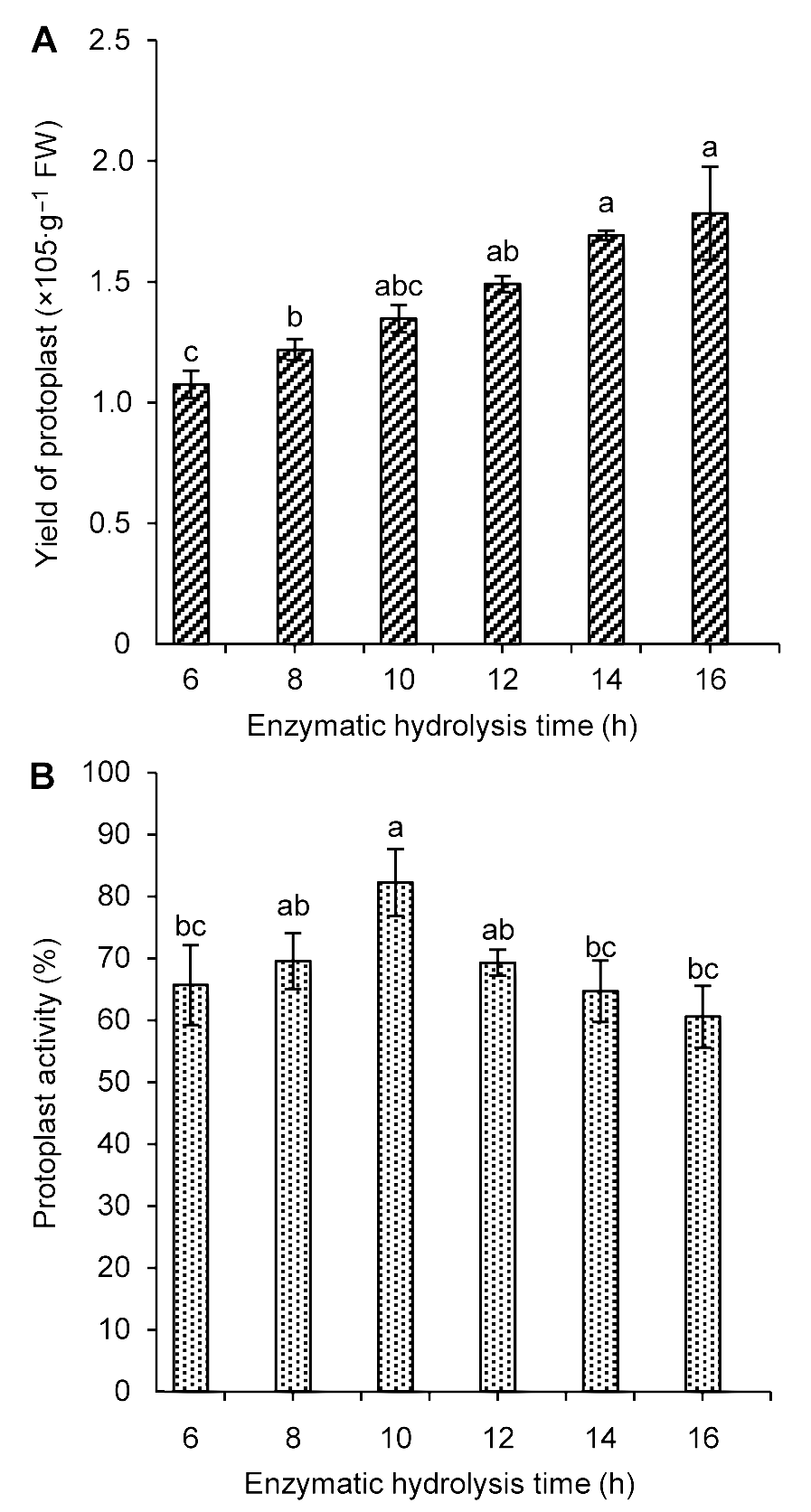
图2 不同酶解时间对雷公藤悬浮细胞原生质体产量(A)及活力(B)的影响不同小写字母表示在P<0.05水平上差异显著。
Figure 2 The influence of enzymatic hydrolysis time on Trip- terygium wilfordii suspension cells protoplast yield (A) and activity (B)Different lowercase letters indicate significant differences at P<0.05.
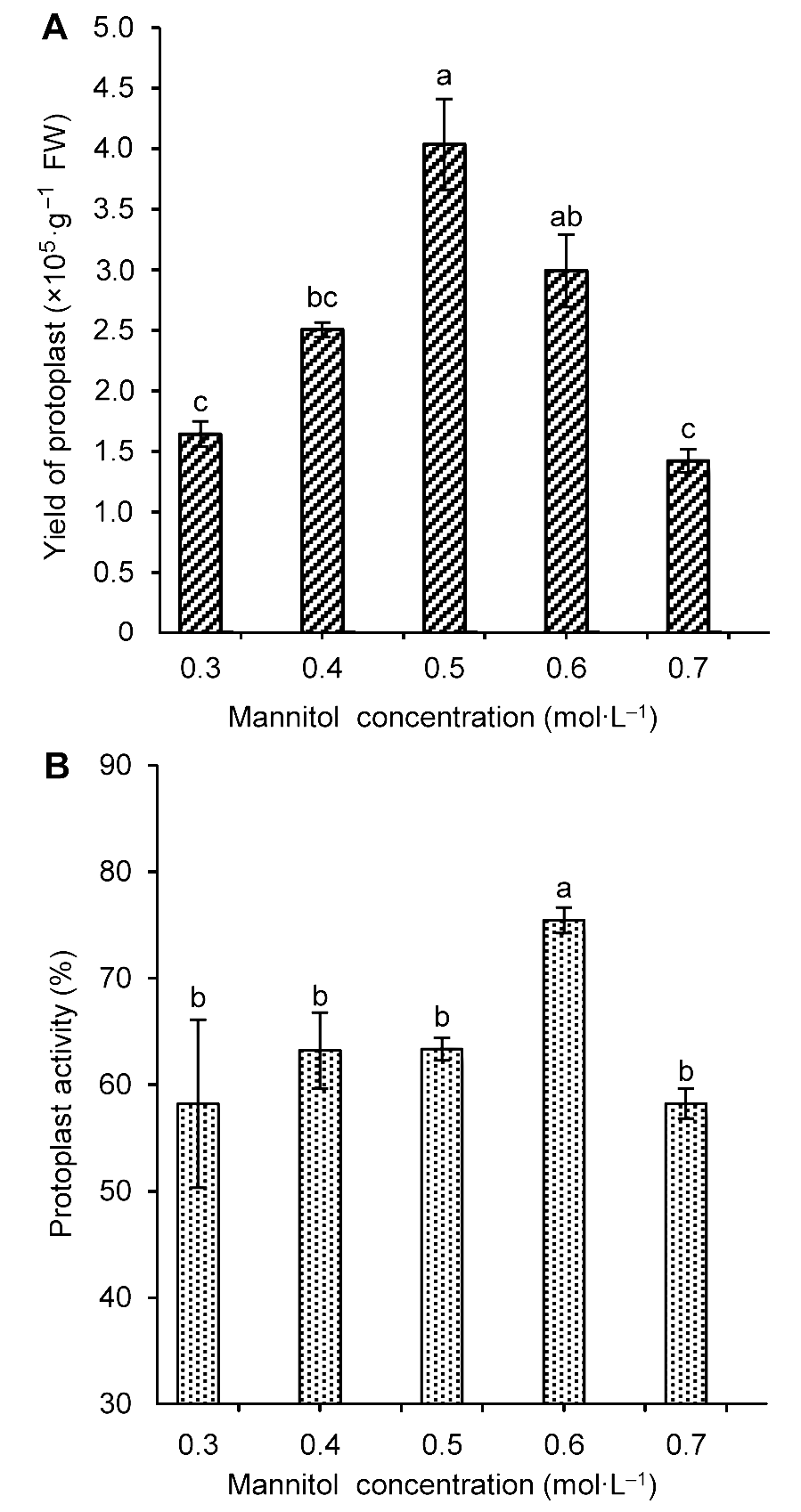
图3 不同甘露醇浓度对雷公藤悬浮细胞原生质体产量(A)和活力(B)的影响不同小写字母表示在P<0.05水平上差异显著。
Figure 3 The influence of mannitol concentration on Tripterygium wilfordii suspension cells protoplast yield (A) and activity (B)Different lowercase letters indicate significant differences at P<0.05.
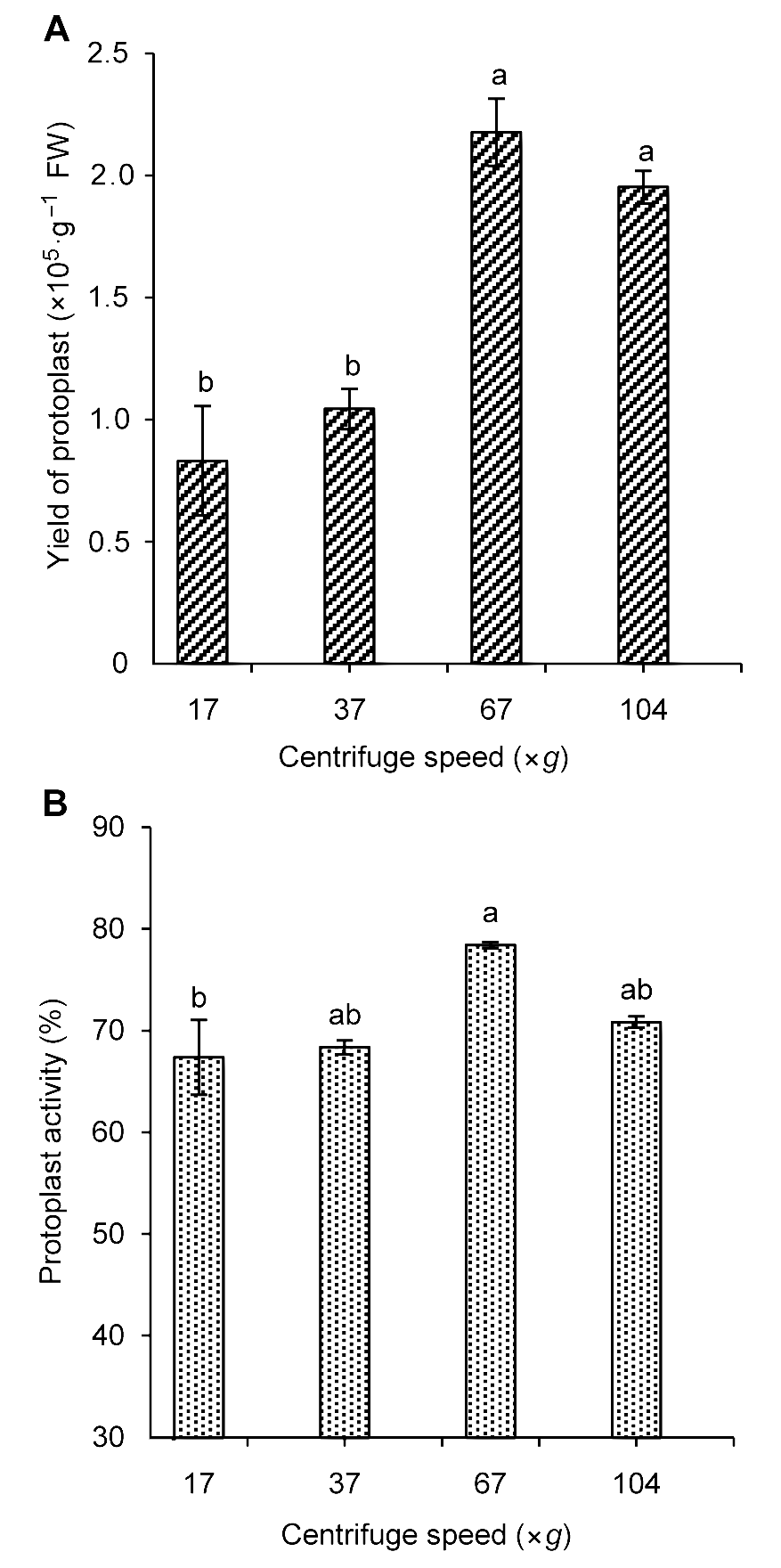
图4 不同转速对雷公藤悬浮细胞原生质体的产量(A)及活力(B)的影响不同小写字母表示在P<0.05水平上差异显著。
Figure 4 The influence of centrifugal speed on Tripterygium wilfordii suspension cells protoplast yield (A) and activity (B)Different lowercase letters indicate significant differences at P<0.05.
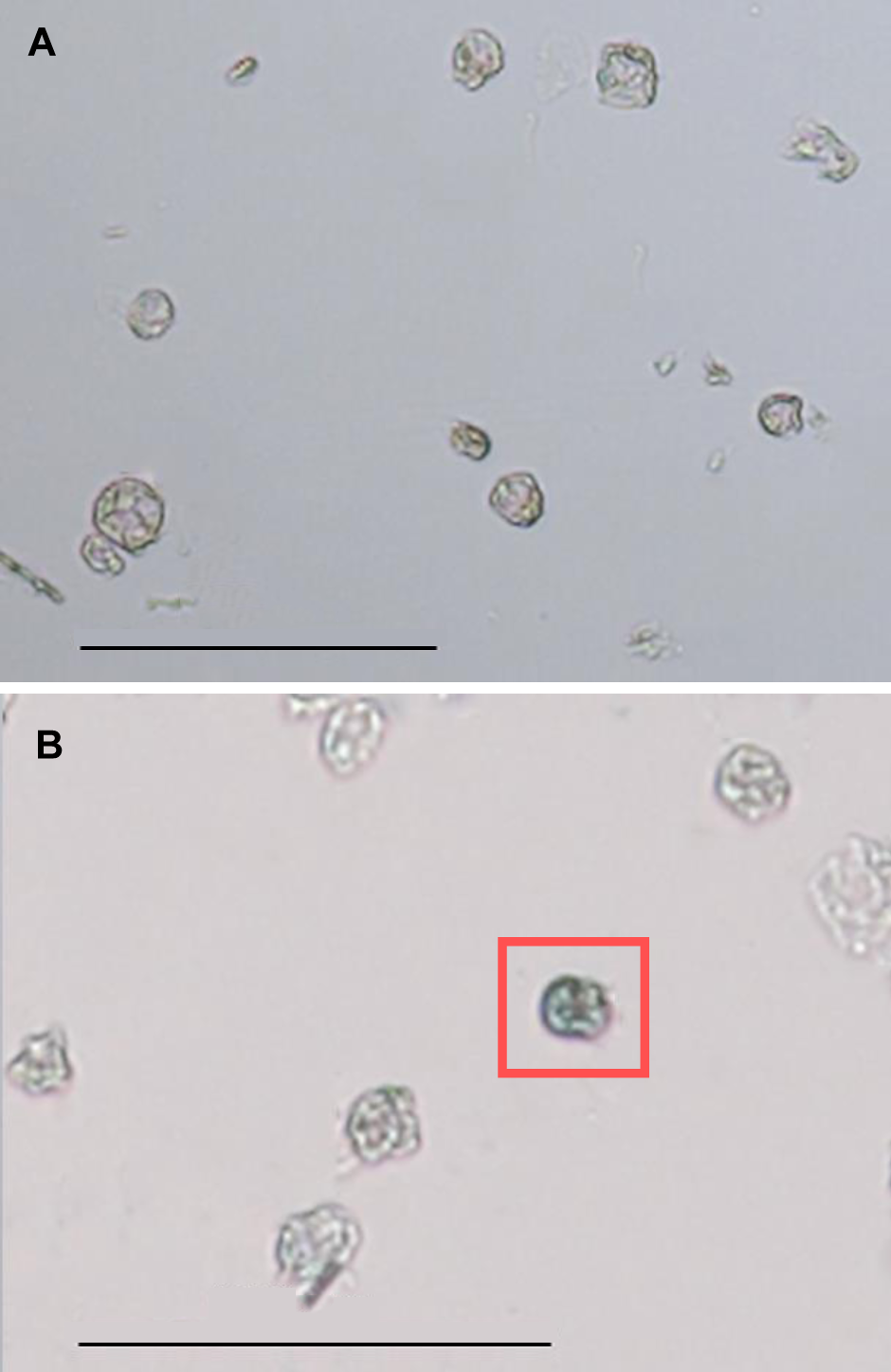
图5 光学显微镜下雷公藤悬浮细胞原生质体形态(A) 台盼蓝染色前的原生质体; (B) 台盼蓝染色后的原生质体(红框标出的为死细胞)。Bars=100 μm
Figure 5 Morphology of Tripterygium wilfordii suspension cells protoplast under optical microscope(A) Morphology of protoplast before trypan blue staining; (B) Morphology of protoplast after trypan blue staining, and the blue cell in red box was dead. Bars=100 μm
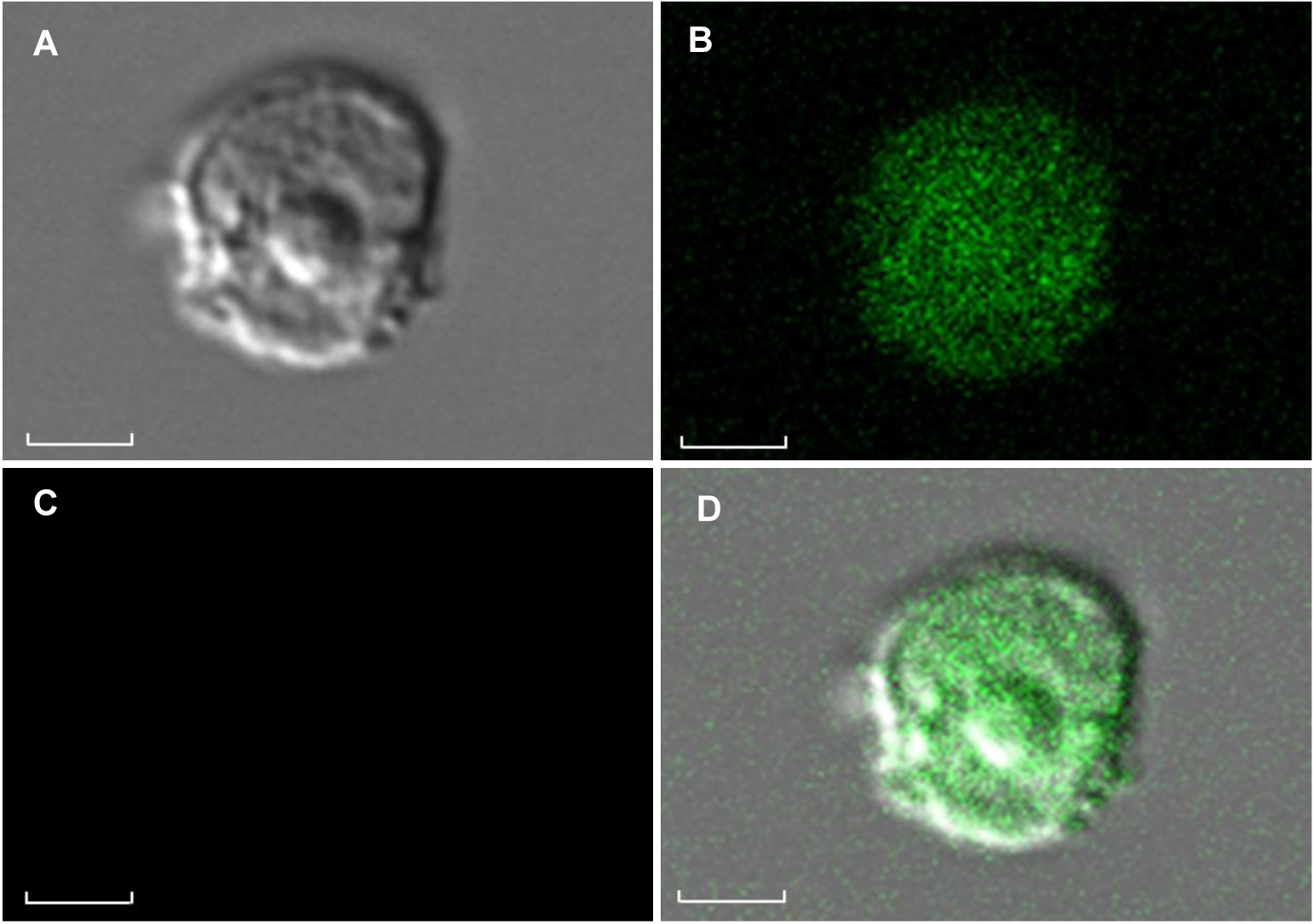
图6 激光共聚焦扫描显微镜下观察雷公藤悬浮细胞原生质体(A) 明场下的原生质体; (B) 488 nm激发光下GFP荧光; (C) 原生质体无自发荧光; (D) 叠加效果图。Bars=1 μm
Figure 6 Protoplast of Tripterygium wilfordii suspension cells under laser scanning confocal microscop(A) Protoplasts under the laser confocal microscope in bright field; (B) GFP fluorescence of protoplast under the laser confocal microscope in excitation 488 nm; (C) There is no red chloroplast spontaneous fluorescence of protoplast under the laser confocal microscope in excitation 488 nm; (D) Red chlorophyll fluorescence signals and GFP signals from protoplast. Bars=1 μm
| [1] | 段炼, 钱君, 郭小雨, 朱英 (2014). 一种快速高效的水稻原生质体制备和转化方法的建立. 植物生理学报 50, 351-357. |
| [2] |
李妮娜, 丁林云, 张志远, 郭旺珍 (2014). 棉花叶肉原生质体分离及目标基因瞬时表达体系的建立. 作物学报 40, 231-239.
DOI URL |
| [3] |
刘凡, 赵泓, 秦帆 (2006). 结球白菜下胚轴原生质体培养及其体细胞胚植株再生. 植物学通报 23, 275-280.
DOI URL |
| [4] | 刘继红, 邓秀新 (1999). 植物原生质体非对称融合及其在育种上的应用. 生命科学 11, 88-91. |
| [5] |
张良波, 李培旺, 黄振, 李昌珠 (2011). 木本植物原生质体制备体系的研究进展. 中南林业科技大学学报 31(8), 102-107.
DOI URL |
| [6] |
朱楠, 刘俊, 张馨宇, 董娟娥 (2014). 丹参悬浮培养细胞原生质体的制备和活力检测. 生物工程学报 30, 1612-1621.
DOI URL |
| [7] | Chugh R, Sangwan V, Patil SP, Dudeja V, Dawra RK, Banerjee S, Schumacher RJ, Blazar BR, Georg GI, Vickers SM, Saluja AK (2012). A preclinical evaluation of minnelide as a therapeutic agent against pancreatic cancer. Sci Transl Med 4, 156ra139. |
| [8] |
Cocking EC (1960). A method for the isolation of plant protoplasts and vacuoles.Nature 187, 927-929.
DOI URL PMID |
| [9] |
Duarte P, Ribeiro D, Carqueijeiro I, Bettencourt S, Sottomayor M (2016). Protoplast transformation as a plant- transferable transient expression system.Methods Mol Biol 1405, 137.
DOI URL |
| [10] |
Gilroy S, Jones RL (1992). Gibberellic acid and abscisic acid coordinately regulate cytoplasmic calcium and secre- tory activity in barley aleurone protoplasts.Proc Natl Acad Sci USA 89, 3591-3595.
DOI URL |
| [11] | Guo ZJ, Kallus S, Akiyoshi K, Sunamoto J (2006). Artificial cell wall for plant protoplast. Coating of plasma membrane with hydrophobized polysaccharides.Chem Lett 31, 415-416. |
| [12] |
Knight MR, Campbell AK, Smith SM, Trewavas AJ (1991). Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium.Nature 352, 524-526.
DOI URL |
| [13] |
Liu JL, Lee J, Salazar Hernandez MA, Mazitschek R, Ozcan U (2015). Treatment of obesity with celastrol.Cell 161, 999-1011.
DOI URL |
| [14] |
Lu L, Li FQ, Wang XM (2010). Novel anti-inflammatory and neuroprotective agents for Parkinson’s disease.CNS Neu- rol Disord Drug Targets 9, 232-240.
DOI URL |
| [15] |
Maas C, Werr W (1989). Mechanism and optimized conditions for PEG mediated DNA transfection into plant protoplasts.Plant Cell Rep 8, 148-151.
DOI URL PMID |
| [16] |
Nagata T, Takebe I (1970). Cell wall regeneration and cell division in isolated tobacco mesophyll protoplasts.Planta 92, 301-308.
DOI URL |
| [17] |
Sheen J (2001). Signal transduction in maize and Arabidopsis mesophyll protoplasts.Plant Physiol 127, 1466-1475.
DOI URL PMID |
| [18] | Su P, Tong YR, Cheng QQ, Hu YT, Zhang M, Yang J, Teng QZ, Gao W, Huang LQ (2016). Functional characterization of ent-copalyl diphosphate synthase, kaurene synthase and kaurene oxidase in the Salvia miltiorrhiza gibberellin biosynthetic pathway. Sci Rep 6, 23057. |
| [19] |
Titov DV, Gilman B, He QL, Bhat S, Low WK, Dang YJ, Smeaton M, Demain AL, Miller PS, Kugel JF, Goodrich JA, Liu JO (2011). XPB, a subunit of TFIIH, is a target of the natural product triptolide.Nat Chem Biol 7, 182-188.
DOI URL PMID |
| [20] | Tudses N, Premjet S, Premjet D (2015). Establishment of method for protoplast fusion with peg-mediated between jatropha curcas l. and ricinus communis l.Int J Life Sci Biotech Pharm Res 4, 50-56. |
| [21] |
Wang X, Liang XB, Li FQ, Zhou HF, Liu XY, Wang JJ, Wang XM (2008). Therapeutic strategies for Parkinson’s disease: the ancient meets the future-traditional Chinese herbal medicine, electroacupuncture, gene therapy and stem cells.Neurochem Res 33, 1956-1963.
DOI URL |
| [22] |
Woo JW, Kim J, Kwon SI, Corvalán C, Cho SW, Kim H, Kim SG, Kim ST, Choe S, Kim JS (2015). DNA-free genome editing in plants with preassembled CRISPR- Cas9 ribonucleoproteins. Nat Biotechnol 33, 1162-1164.
DOI URL PMID |
| [23] | Zhang M, Su P, Zhou YJ, Wang XJ, Zhao YJ, Liu YJ, Tong YR, Hu TY, Huang LQ, Gao W (2015). Identification of geranylgeranyl diphosphate synthase genes from Tripterygium wilfordii. Plant Cell Rep 34, 2179-2188. |
| [24] | Zhao YJ, Chen X, Zhang M, Su P, Liu YJ, Tong YR, Wang XJ, Huang LQ, Gao W (2015). Molecular cloning and characterisation of farnesyl pyrophosphate synthase from Tripterygium wilfordii. PLoS One 10, r0125415. |
| [25] |
Zhou ZL, Yang YX, Ding J, Li YC, Miao ZH (2012). Triptolide: structural modifications, structure-activity relations- hips, bioactivities, clinical development and mechanisms.Nat Prod Rep 29, 457-475.
DOI URL PMID |
| [1] | 张玉琴, 吴嘉诚, 何萌, 刘仁义, 朱晓玥. 铁观音原生质体高效瞬时转化方法的建立[J]. 植物学报, 2022, 57(3): 340-349. |
| [2] | 宋爱华, 张文斌, 孙姝兰, 李凌飞, 王小菁. 非洲菊原生质体制备及瞬时转化系统的建立[J]. 植物学报, 2017, 52(4): 511-519. |
| [3] | 乔妹, 孙嘉炜, 陈琰, 韩胜芳, 侯春燕, 刘刚, 王冬梅. 小麦悬浮细胞应答激发子刺激的过敏性反应中Ca2+和 NO的动态变化及其相互作用[J]. 植物学报, 2015, 50(1): 1-11. |
| [4] | 杜玮炜, 姚小洪, 黄宏文. 环境胁迫对雷公藤中雷公藤红素含量的影响[J]. 植物生态学报, 2009, 33(1): 180-185. |
| [5] | 张改娜;贾敬芬*. 草木樨状黄芪和木本霸王的科间体细胞杂交[J]. 植物学报, 2009, 44(04): 442-450. |
| [6] | 聂志刚;王艳;李韶山. 重金属诱导拟南芥原生质体DNA 损伤的单细胞凝胶电泳检测[J]. 植物学报, 2009, 44(01): 117-123. |
| [7] | 杜玮炜;黄宏文;. 雷公藤次生代谢产物雷公藤红素含量与环境因子相关性分析[J]. 植物学报, 2008, 25(06): 707-713. |
| [8] | 王文霞;李曙光;赵小明;林炳承;杜昱光. 壳寡糖对烟草悬浮细胞茉莉酸合成基因转录的影响[J]. 植物学报, 2008, 25(05): 526-532. |
| [9] | 熊丹;陈发菊;梁宏伟;王玉兵. 珍稀濒危植物香果树胚性细胞悬浮系的建立和植株再生[J]. 植物学报, 2008, 25(03): 337-343. |
| [10] | 詹宝 徐文忠 麻密. 砷超富集植物蜈蚣草原生质体的分离及其抗砷性分析[J]. 植物学报, 2006, 23(4): 363-367. |
| [11] | 刘凡 赵泓 秦帆. 结球白菜下胚轴原生质体培养及其体细胞胚植株再生[J]. 植物学报, 2006, 23(3): 275-280. |
| [12] | 付春华 余龙江. 细胞融合与植物细胞质雄性不育性状改良[J]. 植物学报, 2005, 22(增刊): 99-107. |
| [13] | 张贵友 李萍 戴尧仁. 低温胁迫下褪黑激素对烟草悬浮细胞精氨酸脱羧酶活性的影响[J]. 植物学报, 2005, 22(05): 555-559. |
| [14] | 张国增 安国勇 宋纯鹏. 不同培养条件对拟南芥根细胞膜片钳记录的影响[J]. 植物学报, 2005, 22(01): 27-31. |
| [15] | 王晶珊 孙世孟 王维华 毕英娜 徐丽娟 孟祥霞. 甘薯同一不亲和群内品种间体细胞杂种[J]. 植物学报, 2004, 21(03): 306-311. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||