

植物学报 ›› 2022, Vol. 57 ›› Issue (3): 340-349.DOI: 10.11983/CBB21206 cstr: 32102.14.CBB21206
张玉琴1,2, 吴嘉诚1,2, 何萌2, 刘仁义3, 朱晓玥2,*( )
)
收稿日期:2021-11-24
接受日期:2022-02-07
出版日期:2022-05-01
发布日期:2022-05-18
通讯作者:
朱晓玥
作者简介:* E-mail: xiaoyuezhu@fafu.edu.cn基金资助:
Yuqin Zhang1,2, Jiacheng Wu1,2, Meng He2, Renyi Liu3, Xiaoyue Zhu2,*( )
)
Received:2021-11-24
Accepted:2022-02-07
Online:2022-05-01
Published:2022-05-18
Contact:
Xiaoyue Zhu
摘要: 近年来, 茶树基因组测序的完成为茶树在分子和基因水平的研究奠定了基础。但由于转基因技术尚不成熟且茶树生长周期较长, 茶树的基因功能研究依然不能有效开展。采用铁观音(Camellia sinensis var. sinensis cv. ‘Tieguanyin’)实生幼苗叶片, 通过筛选多种纤维素酶、果胶酶、离析酶和甘露醇的浓度组合, 并结合原生质体的数量、活性和杂质含量综合确定了最佳配方, 成功建立了铁观音茶苗叶片原生质体提取和PEG介导的高效瞬时转化体系, 转化率达56.25%。利用该系统探索了茶氨酸代谢通路中2个重要合成酶(茶氨酸合成酶(TSI)和谷氨酰胺合成酶(GSII-1.1))的亚细胞定位。研究发现, 这2种酶均定位于铁观音原生质体细胞质中。茶苗叶片原生质体提取和瞬时转化体系的建立为茶树基因组功能研究奠定了技术基础。
张玉琴, 吴嘉诚, 何萌, 刘仁义, 朱晓玥. 铁观音原生质体高效瞬时转化方法的建立. 植物学报, 2022, 57(3): 340-349.
Yuqin Zhang, Jiacheng Wu, Meng He, Renyi Liu, Xiaoyue Zhu. An Efficient Protoplast Transient Expression System in Camellia sinensis var. sinensis cv. ‘Tieguanyin’. Chinese Bulletin of Botany, 2022, 57(3): 340-349.
| Treatment number | Cellulase (%) | Pectinase (%) | Macerozy-me (%) | Mannitol (mol·L-1) |
|---|---|---|---|---|
| 1 | 1.4 | 0.3 | 0.1 | 0.3 |
| 2 | 1.4 | 0.4 | 0.2 | 0.4 |
| 3 | 1.4 | 0.5 | 0.3 | 0.5 |
| 4 | 1.5 | 0.3 | 0.2 | 0.5 |
| 5 | 1.5 | 0.4 | 0.3 | 0.3 |
| 6 | 1.5 | 0.5 | 0.1 | 0.4 |
| 7 | 1.6 | 0.3 | 0.3 | 0.4 |
| 8 | 1.6 | 0.4 | 0.1 | 0.5 |
| 9 | 1.6 | 0.5 | 0.2 | 0.3 |
表1 铁观音幼叶原生质体提取试剂配比
Table 1 Protoplast extraction reagent for Tieguanyin young leaves
| Treatment number | Cellulase (%) | Pectinase (%) | Macerozy-me (%) | Mannitol (mol·L-1) |
|---|---|---|---|---|
| 1 | 1.4 | 0.3 | 0.1 | 0.3 |
| 2 | 1.4 | 0.4 | 0.2 | 0.4 |
| 3 | 1.4 | 0.5 | 0.3 | 0.5 |
| 4 | 1.5 | 0.3 | 0.2 | 0.5 |
| 5 | 1.5 | 0.4 | 0.3 | 0.3 |
| 6 | 1.5 | 0.5 | 0.1 | 0.4 |
| 7 | 1.6 | 0.3 | 0.3 | 0.4 |
| 8 | 1.6 | 0.4 | 0.1 | 0.5 |
| 9 | 1.6 | 0.5 | 0.2 | 0.3 |
| Reagent name | Amount (10 mL) |
|---|---|
| PEG-4000 | 4 g |
| 1 mol·L-1 CaCl2 | 2 mL |
| 0.8 mol·L-1 D-mannitol | 2.5 mL |
| ddH2O | 2 mL |
表2 聚乙二醇(PEG)诱导铁观音原生质体转化试剂用量
Table 2 Reagent for polyethylene glycol (PEG)-induced Tieguanyin protoplast transformation
| Reagent name | Amount (10 mL) |
|---|---|
| PEG-4000 | 4 g |
| 1 mol·L-1 CaCl2 | 2 mL |
| 0.8 mol·L-1 D-mannitol | 2.5 mL |
| ddH2O | 2 mL |
| Primer name | Primer sequence | Tm (°C ) |
|---|---|---|
| GS-F | TCCCCCGGGTCTCTTCTTTCCGATCTTTGCA | 66.3 |
| GS-R | TCCCCCGGGTTACGGTTTCCAGAGGATGG | 68.1 |
| TS-F | TCCCCCGGGGAGAAATTTGCAGAGCTGAGAG | 69.9 |
| TS-R | TCCCCCGGGTCAATAGCGATGTATAAGTTGCTT | 64.4 |
表3 GSII-1.1和TSI基因引物序列
Table 3 Primer sequences of GSII-1.1 and TSI genes
| Primer name | Primer sequence | Tm (°C ) |
|---|---|---|
| GS-F | TCCCCCGGGTCTCTTCTTTCCGATCTTTGCA | 66.3 |
| GS-R | TCCCCCGGGTTACGGTTTCCAGAGGATGG | 68.1 |
| TS-F | TCCCCCGGGGAGAAATTTGCAGAGCTGAGAG | 69.9 |
| TS-R | TCCCCCGGGTCAATAGCGATGTATAAGTTGCTT | 64.4 |
| Treatment number | Yield (106·g-1 FW) | Viability (%) |
|---|---|---|
| 1 | 1.58±1.22 | 40.69±29.05 |
| 2 | 1.95±0.77 | 80.53±1.74 |
| 3 | 0.38±0.04 | 65.31±14.40 |
| 4 | 0.66±0.33 | 65.49±10.50 |
| 5 | 1.54±0.76 | 64.16±6.39 |
| 6 | 2.04±1.01 | 75.38±2.56 |
| 7 | 0.60±0.31 | 71.06±15.20 |
| 8 | 2.09±0.70 | 72.01±12.55 |
| 9 | 1.83±0.51 | 70.19±2.09 |
表4 不同酶解液配方下铁观音幼叶原生质体产量与活性(平均值±标准差)
Table 4 The number and viability of protoplasts extracted from young leaves of Tieguanyin under different enzymatic hydrolysis solutions (means±SD)
| Treatment number | Yield (106·g-1 FW) | Viability (%) |
|---|---|---|
| 1 | 1.58±1.22 | 40.69±29.05 |
| 2 | 1.95±0.77 | 80.53±1.74 |
| 3 | 0.38±0.04 | 65.31±14.40 |
| 4 | 0.66±0.33 | 65.49±10.50 |
| 5 | 1.54±0.76 | 64.16±6.39 |
| 6 | 2.04±1.01 | 75.38±2.56 |
| 7 | 0.60±0.31 | 71.06±15.20 |
| 8 | 2.09±0.70 | 72.01±12.55 |
| 9 | 1.83±0.51 | 70.19±2.09 |
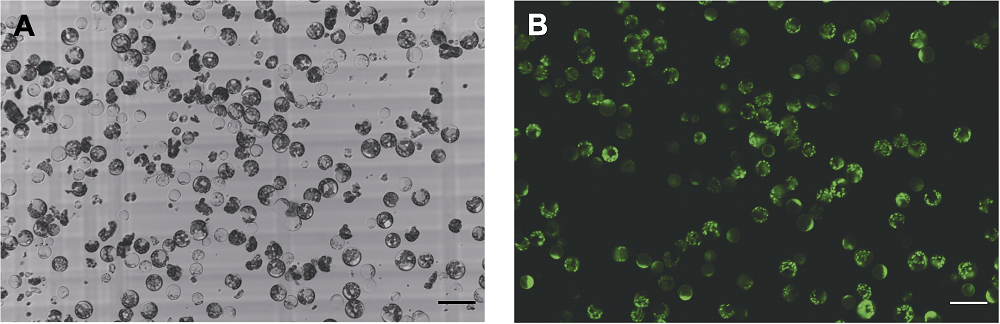
图1 采用9号酶解液配方提取的铁观音幼叶原生质体 (A) 原生质体明场图; (B) 原生质体二乙酸荧光素染色图。Bars=50 μm
Figure 1 Tieguanyin young leaves protoplasts extracted using No. 9 enzymatic hydrolysis solution (A) Bright field image of protoplasts; (B) Fluorescein diacetate staining image. Bars=50 μm
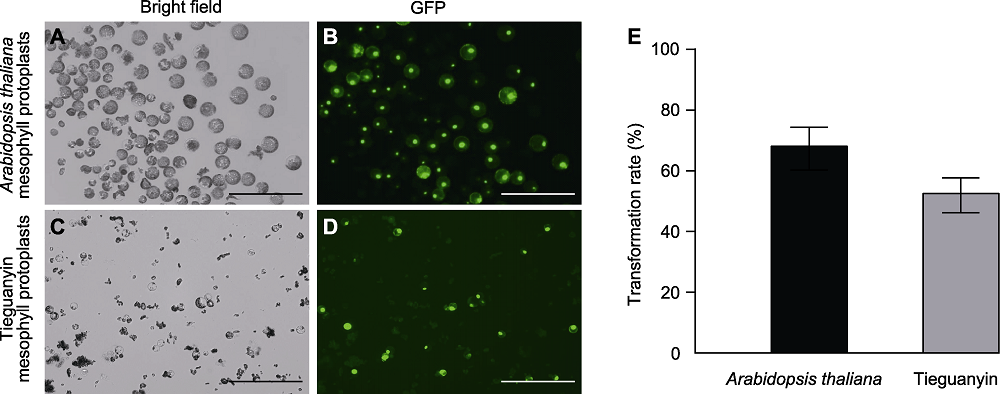
图2 采用聚乙二醇(PEG)诱导HBT-TCP20-GFP质粒在拟南芥和铁观音叶肉原生质体中转化 (A)-(D) Bars=200 μm
Figure 2 Polyethylene glycol (PEG) induced transformation of HBT-TCP20-GFP plasmid into Arabidopsis thaliana and Tieguanyin mesophyll protoplasts (A)-(D) Bars=200 μm
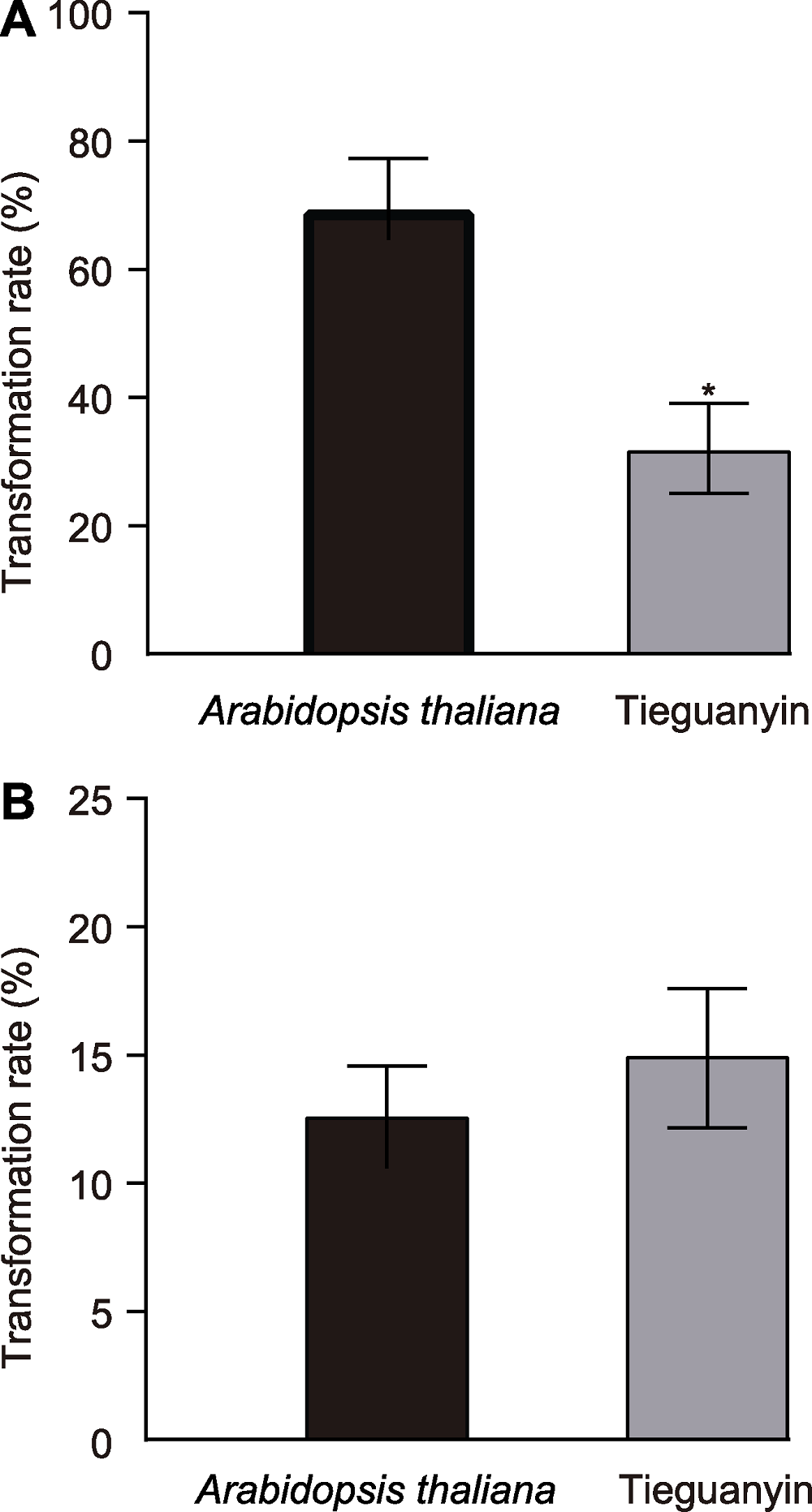
图3 拟南芥和铁观音叶肉原生质体转化率 (A) HBT-GFP-GSII-1.1的转化率; (B) HBT-GFP-TSI的转化率。 *代表显著性差异水平(P<0.05, 卡方检验)。误差线表示平均值±标准差。
Figure 3 Transformation rate in Arabidopsis thaliana and Tieguanyin mesophyll protoplasts (A) Transformation rate of HBT-GFP-GSII-1.1; (B) Transformation rate of HBT-GFP-TSI. * represents significant difference (P<0.05, X2 test). Error bars represent means ± SD.
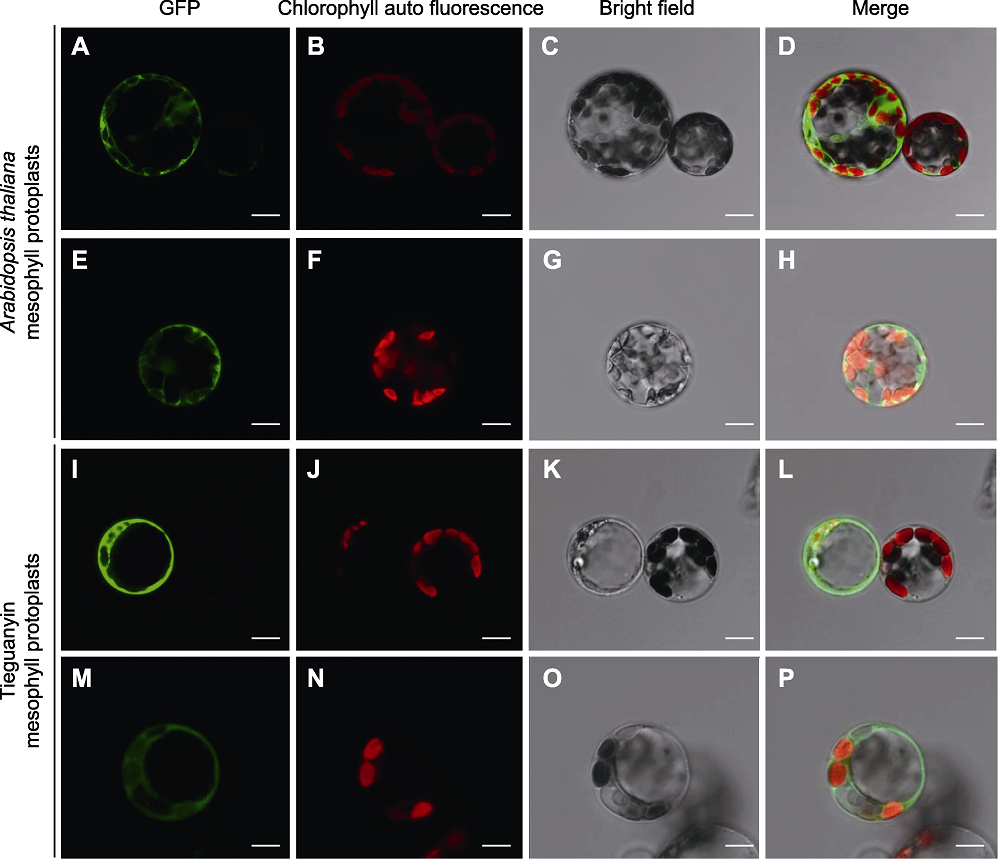
图4 拟南芥和铁观音叶肉原生质体中GSII-1.1和TSI蛋白亚细胞定位 (A)-(D) 拟南芥原生质体中转化HBT-GFP-GSII-1.1; (E)-(H) 拟南芥原生质体中转化HBT-GFP-TSI; (I)-(L) 铁观音叶肉原生质体中转化HBT-GFP-GSII-1.1; (M)-(P) 铁观音叶肉原生质体中转化HBT-GFP-TSI。Bars=10 μm
Figure 4 GSII-1.1 and TSI subcellular localization in Arabidopsis thaliana and Tieguanyin mesophyll protoplasts (A)-(D) Transformation of HBT-GFP-GSII-1.1 in Arabidopsis thaliana protoplasts; (E)-(H) Transformation of HBT-GFP-TSI in Arabidopsis thaliana protoplasts; (I)-(L) Transformation of HBT-GFP-GSII-1.1 in Tieguanyin protoplasts; (M)-(P) Transformation of HBT-GFP-TSI in Tieguanyin protoplasts. Bars=10 μm
| [1] | 陈琪, 江雪梅, 孟祥宇, 张正竹, 宛晓春 (2015). 茶树茶氨酸合成酶基因的酶活性验证与蛋白三维结构分析. 广西植物 35, 384-392, 377. |
| [2] | 陈瑛 (1999). 茶氨酸在茶树体内的分布规律和年变化的研究. 绍兴文理学院学报 19(5), 70-73. |
| [3] | 郭颖, 陈琦, 黄峻榕, 吴雪原, 吴琼 (2015). 茶叶滋味与其品质成分的关系. 茶叶通讯 42(3), 13-15, 28. |
| [4] | 韩庆芬, 陈海飞, 张振华 (2019). 不同生态型拟南芥耐铵毒害差异的生理机制. 植物营养与肥料学报 25, 1185-1193. |
| [5] |
胡添源, 王睿, 陈上, 马宝伟, 高伟, 黄璐琦 (2017). 雷公藤悬浮细胞原生质体的制备及瞬时转化体系的建立. 植物学报 52, 774-782.
DOI |
| [6] | 李春芳 (2016). 茶树类黄酮等次生代谢产物的合成及基因的表达分析. 博士后论文. 杭州: 中国农业科学院. pp. 16- 18. |
| [7] | 刘婉如, 徐海滨 (2006). 茶氨酸的生理活性及药理作用研究进展. 国外医学(卫生学分册) 33, 287-291. |
| [8] | 刘艳丽, 金孝芳, 马林龙, 曹丹, 龚自明, 韦朝领 (2017). 茶树叶肉原生质体的分离与纯化. 植物科学学报 35, 908- 911. |
| [9] | 裴祎, 李玉环, 朱宏波, 胡雪琼, 钟敏, 李雁群 (2018). 圆红冬孢酵母高效电转化条件的优化. 广东海洋大学学报 38 (6), 48-54. |
| [10] | 彭章, 童华荣, 梁国鲁, 石艺琦, 袁连玉 (2018). 茶树叶片和胚根原生质体的分离及PEG诱导融合. 作物学报 44, 463- 470. |
| [11] | 舒英杰, 黄丽燕, 陈明, 陶源, 王占奎, 麻浩 (2017). 基于亚细胞定位的大豆和鹰嘴豆原生质体分离体系的建立与优化. 生物工程学报 33, 976-985. |
| [12] |
宋爱华, 张文斌, 孙姝兰, 李凌飞, 王小菁 (2017). 非洲菊原生质体制备及瞬时转化系统的建立. 植物学报 52, 511- 519.
DOI |
| [13] | 汪滢, 尚俊军, 茅文俊, 王莹, 鲍大鹏, 李燕 (2019). 不同氮源对刺芹侧耳谷氨酰胺合成酶的酶活力及基因表达影响. 生物学杂志 36(2), 33-36. |
| [14] | 魏晓惠, 谢颖颖, 杨玉玲, 何春雷 (2018). 表征不同季节蒙顶甘露茶的特征指标研究. 安徽农业科学 46(11), 155-156, 201. |
| [15] | 徐超 (2016). 脂质体介导的遗传转化体系的构建及漆酶在金针菇生长发育过程中的研究. 硕士论文. 南京: 南京农业大学. pp. 10-11. |
| [16] | 张文锦, 陈常颂, 杨如兴 (2001). 铁观音肥料主效因子的研究. 福建茶叶 (3), 7-9. |
| [17] |
Ashihara H (2015). Occurrence, biosynthesis and metabolism of theanine (γ-glutamyl-L-ethylamide) in plants: a comprehensive review. Nat Prod Commun 10, 803-810.
PMID |
| [18] |
Bai PX, Wei K, Wang LY, Zhang F, Ruan L, Li HL, Wu LY, Cheng H (2019). Identification of a novel gene encoding the specialized alanine decarboxylase in tea (Camellia sinensis) plants. Molecules 24, 540.
DOI URL |
| [19] |
Bernard SM, Møller ALB, Dionisio G, Kichey T, Jahn TP, Dubois F, Baudo M, Lopes MS, Tercé-Laforgue T, Foyer CH, Parry MAJ, Forde BG, Araus JL, Hirel B, Schjoerring JK, Habash DZ (2008). Gene expression, cellular localisation and function of glutamine synthetase isozymes in wheat (Triticum aestivum L.). Plant Mol Biol 67, 89-105.
DOI URL |
| [20] |
Berthold F, Roujol D, Hemmer C, Jamet E, Ritzenthaler C, Hoffmann L, Schmitt-Keichinger C (2019). Inside or outside? A new collection of Gateway vectors allowing plant protein subcellular localization or over-expression. Plasmid 105, 102436.
DOI URL |
| [21] |
Breitsamer M, Stulz A, Heerklotz HH, Winter G (2019). Do interactions between protein and phospholipids influence the release behavior from lipid-based exenatide depot systems? Eur J Pharm Biopharm 142, 61-69.
DOI PMID |
| [22] |
Buschhaus C, Jetter R (2011). Composition differences between epicuticular and intracuticular wax substructures: how do plants seal their epidermal surfaces? J Exp Bot 62, 841-853.
DOI URL |
| [23] |
Dong CX, Li F, Yang TY, Feng L, Zhang SP, Li FD, Li WH, Xu GH, Bao SL, Wan XC, Lucas WJ, Zhang ZL (2020). Theanine transporters identified in tea plants (Camellia sinensis L.). Plant J 101, 57-70.
DOI URL |
| [24] |
Fu XM, Liao YY, Cheng SH, Xu XL, Grierson D, Yang ZY (2021). Nonaqueous fractionation and overexpression of fluorescent-tagged enzymes reveals the subcellular sites of L-theanine biosynthesis in tea. Plant Biotechnol 19, 98- 108.
DOI URL |
| [25] |
Hirel B, McNally SF, Gadal P, Sumar N, Stewart GR (1984). Cytosolic glutamine synthetase in higher plants: a comparative immunological study. Eur J Biochem 138, 63-66.
PMID |
| [26] |
Martín-Trillo M, Cubas P (2010). TCP genes: a family snapshot ten years later. Trends Plant Sci 15, 31-39.
DOI PMID |
| [27] |
Reddy SSS, Singh B, Peter AJ, Rao TV (2019). Genetic transformation of indica rice varieties involving Am-SOD gene for improved abiotic stress tolerance. Saudi J Biol Sci 26, 294-300.
DOI URL |
| [28] | She GB, Yu SW, Li ZG, Peng AQ, Li PH, Li YY, Chang MM, Liu LL, Chen Q, Shi CY, Sun J, Zhao J, Wan XC (2022). Characterization of CsTSI in the biosynthesis of theanine in tea plants (Camellia sinensis). J Agric Food Chem 70, 826-836. |
| [29] |
Sheen J (2001). Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiol 127, 1466- 1475.
PMID |
| [30] |
Türközü D, Şanlier N (2017). L-theanine, unique amino acid of tea, and its metabolism, health effects, and safety. Crit Rev Food Sci Nutr 57, 1681-1687.
DOI PMID |
| [31] | Wei CL, Yang H, Wang SB, Zhao J, Liu C, Gao LP, Xia EH, Lu Y, Tai YL, She GB, Sun J, Cao HS, Tong W, Gao Q, Li YY, Deng WW, Jiang XL, Wang WZ, Chen Q, Zhang SH, Li HJ, Wu JL, Wang P, Li PH, Shi CY, Zheng FY, Jian JB, Huang B, Shan D, Shi MM, Fang CB, Yue Y, Li FD, Li DX, Wei S, Han B, Jiang CJ, Yin Y, Xia T, Zhang ZZ, Bennetzen JL, Zhao SC, Wan XC (2018). Draft genome sequence of Camellia sinensis var. sinensis provides insights into the evolution of the tea genome and tea quality. Proc Natl Acad Sci USA 115, E4151-E4158. |
| [32] |
Xia EH, Zhang HB, Sheng J, Li K, Zhang QJ, Kim C, Zhang Y, Liu Y, Zhu T, Li W, Huang H, Tong Y, Nan H, Shi C, Shi C, Jiang JJ, Mao SY, Jiao JY, Zhang D, Zhao Y, Zhao YJ, Zhang LP, Liu YL, Liu BY, Yu Y, Shao SF, Ni DJ, Eichler EE, Gao LZ (2017). The tea tree genome provides insights into tea flavor and independent evolution of caffeine biosynthesis. Mol Plant 10, 866-877.
DOI URL |
| [33] |
Xiong L, Li C, Li HY, Lyu X, Zhao T, Liu J, Zuo ZC, Liu B (2019). A transient expression system in soybean mesophyll protoplasts reveals the formation of cytoplasmic GmCRY1 photobody-like structures. Sci China Life Sci 62, 1070-1077.
DOI PMID |
| [34] |
Xiong Y, Sheen J (2012). Rapamycin and glucose-target of rapamycin (TOR) protein signaling in plants. J Biol Chem 287, 2836-2842.
DOI PMID |
| [35] |
Xu XF, Zhu HY, Ren YF, Feng C, Ye ZH, Cai HM, Wan XC, Peng CY (2021). Efficient isolation and purification of tissue-specific protoplasts from tea plants (Camellia sinensis (L.) O. Kuntze). Plant Methods 17, 84.
DOI URL |
| [36] |
Yoneda Y, Kuramoto N, Kawada K (2019). The role of glutamine in neurogenesis promoted by the green tea amino acid theanine in neural progenitor cells for brain health. Neurochem Int 129, 104505.
DOI URL |
| [37] |
Yoo SD, Cho YH, Sheen J (2007). Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2, 1565-1572.
DOI URL |
| [38] |
Zhang C, Zong H, Zhuge B, Lu XY, Fang HY, Zhu JL, Zhuge J (2016). Protoplast preparation and polyethylene glycol (PEG)-mediated transformation of Candida glycerinogenes. Biotechnol Bioprocess Eng 21, 95-102.
DOI URL |
| [39] |
Zhang XT, Chen S, Shi LQ, Gong DP, Zhang SC, Zhao Q, Zhan DL, Vasseur L, Wang YB, Yu JX, Liao ZY, Xu XD, Qi R, Wang WL, Ma YR, Wang PJ, Ye NX, Ma DN, Shi Y, Wang HF, Ma XK, Kong XR, Lin J, Wei LF, Ma YY, Li RY, Hu GP, He HF, Zhang L, Ming R, Wang G, Tang HB, You MS (2021a). Haplotype-resolved genome assembly provides insights into evolutionary history of the tea plant Camellia sinensis. Nat Genet 53, 1250-1259.
DOI URL |
| [40] |
Zhang Y, Chen XB, Du ZH, Zhang WJ, Devkota AR, Chen ZJ, Chen CS, Sun WJ, Chen MJ (2020). A proposed method for simultaneous measurement of cuticular transpiration from different leaf surfaces in Camellia sinensis. Front Plant Sci 11, 420.
DOI PMID |
| [41] |
Zhang Y, Du ZH, Han YT, Chen XB, Kong XR, Sun WJ, Chen CS, Chen MJ (2021b). Plasticity of the cuticular transpiration barrier in response to water shortage and resupply in Camellia sinensis: a role of cuticular waxes. Front Plant Sci 11, 600069.
DOI URL |
| [42] | Zhou Y, Deng RF, Xu XL, Yang ZY (2021). Isolation of mesophyll protoplasts from tea (Camellia sinensis) and localization analysis of enzymes involved in the biosynthesis of specialized metabolites. Beverage Plant Res 1, 2. |
| [43] |
Zhu XF, Zhang Y, Du ZH, Chen XB, Zhou X, Kong XR, Sun WJ, Chen ZJ, Chen CS, Chen MJ (2018). Tender leaf and fully-expanded leaf exhibited distinct cuticle structure and wax lipid composition in Camellia sinensis cv. ‘Fuyun 6’. Sci Rep 8, 14944.
DOI URL |
| [1] | 张智猛, 万书波, 宁堂原, 戴良香. 氮素水平对花生氮素代谢及相关酶活性的影响[J]. 植物生态学报, 2008, 32(6): 1407-1416. |
| [2] | 黄其满, 刘伟华, 孙辉, 邓新, 苏金. 农杆菌介导的转谷氨酰胺合成酶基因小麦的抗除草剂特性研究 (英文)[J]. 植物生态学报, 2005, 29(2): 338-344. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||