

植物学报 ›› 2021, Vol. 56 ›› Issue (3): 347-362.DOI: 10.11983/CBB20160 cstr: 32102.14.CBB20160
安婷婷1,2,3, 黄帝1,2,3, 王浩1,2, 张一3, 陈应龙1,2,4,*( )
)
收稿日期:2020-09-16
接受日期:2021-02-18
出版日期:2021-05-01
发布日期:2021-04-30
通讯作者:
陈应龙
作者简介:*E-mail: yinglong.chen@uwa.edu.au基金资助:
Tingting An1,2,3, Di Huang1,2,3, Hao Wang1,2, Yi Zhang3, Yinglong Chen1,2,4,*( )
)
Received:2020-09-16
Accepted:2021-02-18
Online:2021-05-01
Published:2021-04-30
Contact:
Yinglong Chen
摘要: 镉(Cd)是一种分布广泛且污染严重的重金属; 其毒性大, 不仅影响植物的生长发育, 而且危害人类健康。该文对植物Cd胁迫的生理生化响应方面的最新研究进展进行了总结概括。从植物光合系统、活性氧、活性氮、抗氧化防御系统、激素、钙信号、蛋白和基因等方面, 概述了植物对Cd胁迫的响应及应答机制, 探讨了植物对Cd胁迫响应机制的研究方向, 旨在为今后开展植物响应Cd胁迫的生理生化及分子机制研究提供理论依据。
安婷婷, 黄帝, 王浩, 张一, 陈应龙. 植物响应镉胁迫的生理生化机制研究进展. 植物学报, 2021, 56(3): 347-362.
Tingting An, Di Huang, Hao Wang, Yi Zhang, Yinglong Chen. Research Advances in Plant Physiological and Biochemical Mechanisms in Response to Cadmium Stress. Chinese Bulletin of Botany, 2021, 56(3): 347-362.
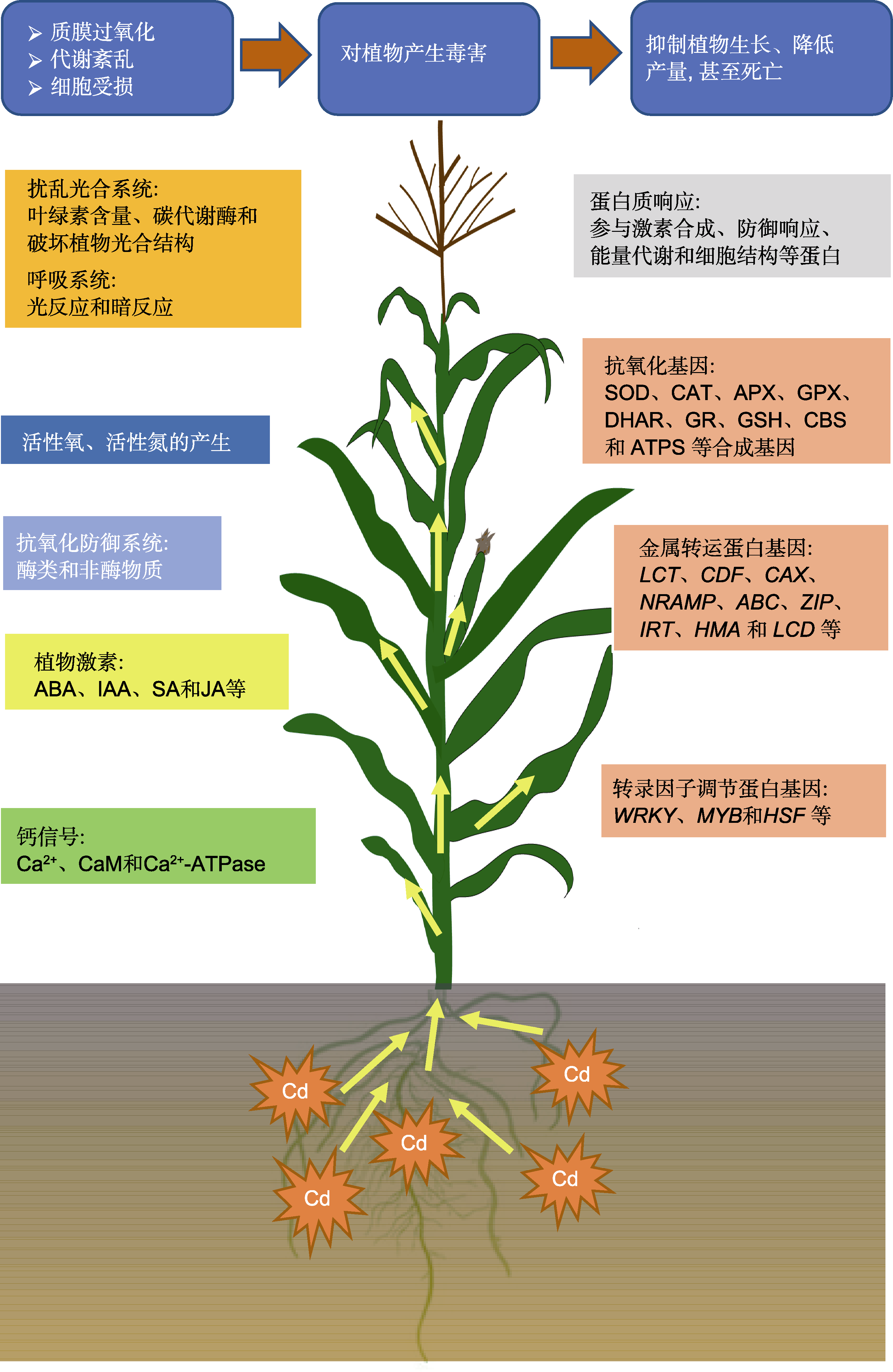
图1 Cd胁迫下植物体内主要生理生化代谢的响应机制 ABA: 脱落酸; IAA: 吲哚乙酸; SA: 水杨酸; JA: 茉莉酸; SOD: 超氧化物歧化酶; CAT: 过氧化氢酶; APX: 抗坏血酸过氧化物酶; GPX: 谷胱甘肽过氧化物酶; DHAR: 脱氢抗坏血酸还原酶; GR: 谷胱甘肽还原酶; GSH: 谷胱甘肽; CBS: 胱硫醚β-合酶; ATPS: ATP硫酸化酶
Figure 1 Response mechanism of physiological and biochemical metabolism in plants under Cd stress ABA: Abscisic acid; IAA: Indole-3-acetic acid; SA: Salicylic acid; JA: Jasmonic acid; SOD: Superoxide dismutase; CAT: Catalase; APX: Ascorbateperoxidase; GPX: Glutathione peroxidase; DHAR: Dehydroascorbate reductase; GR: Glutathione reductase; GSH: Glutathione; CBS: Cystatohinine β-synthetase; ATPS: ATP sulfatase
| 激素 | 激素合成基因 | 非酶物质 | 抗氧化酶 | 抗氧化物质基因 | 吸收和运输蛋白基因 |
|---|---|---|---|---|---|
| ABA | NCED3和Glyma17G242200 ( | AsA和GSH ( | APX、POD、SOD和CAT ( | StPCS 1 ( ski et al., 2010 | IRT1 ( |
| IAA | Glyma02G037600.1 ( | GSH ( | SOD、POD、CAT和GST ( | SODs ( | Nramp、IRT、HMA和ZIP ( |
| SA | Glyma02G063400 ( | 脯氨酸( | NR、GS和GOGAT ( et al., 2010 | SODS、CATS和APXS ( | OsLCT1、OsLCD和ZIP ( |
| JA | PtJMT1和Glyma11G007600. 1 ( | GSH ( | CAT、SOD和APX ( | SODS、APXS和CATS ( | AtIRT1、AtHMA2和At- HMA4 ( |
表1 植物激素对Cd胁迫的响应
Table 1 Responses of plant hormones to Cd stress
| 激素 | 激素合成基因 | 非酶物质 | 抗氧化酶 | 抗氧化物质基因 | 吸收和运输蛋白基因 |
|---|---|---|---|---|---|
| ABA | NCED3和Glyma17G242200 ( | AsA和GSH ( | APX、POD、SOD和CAT ( | StPCS 1 ( ski et al., 2010 | IRT1 ( |
| IAA | Glyma02G037600.1 ( | GSH ( | SOD、POD、CAT和GST ( | SODs ( | Nramp、IRT、HMA和ZIP ( |
| SA | Glyma02G063400 ( | 脯氨酸( | NR、GS和GOGAT ( et al., 2010 | SODS、CATS和APXS ( | OsLCT1、OsLCD和ZIP ( |
| JA | PtJMT1和Glyma11G007600. 1 ( | GSH ( | CAT、SOD和APX ( | SODS、APXS和CATS ( | AtIRT1、AtHMA2和At- HMA4 ( |
| 物质 | 主要因子 | 功能 | 参考文献 | |
|---|---|---|---|---|
| 非酶物质 | MT、PC、GSH和果胶 | 与Cd螯合, 固定在液泡或细胞壁中 | ||
| 抗氧化酶 | SOD、CAT、APX、GPX、DHAR、GR、GSH、CBS和ATPS | 减少ROS和RNS等物质的积累 | ||
| 金属转运蛋白基因 | LCT、CDF、CAX、NRAMP、ABC、ZIP、IRT、HMA和LCD | 吸收或运输Cd离子, 提高植物的Cd积累和对Cd的耐受性 | ||
| 转录因子调节蛋白基因 | WRKY、MYB和HSF | 与DNA结合, 参与激素和抗氧化酶等, 从而调控Cd的吸收、运输和积累 | ||
表2 植物蛋白对Cd胁迫的响应
Table 2 Responses of plant proteins to Cd stress
| 物质 | 主要因子 | 功能 | 参考文献 | |
|---|---|---|---|---|
| 非酶物质 | MT、PC、GSH和果胶 | 与Cd螯合, 固定在液泡或细胞壁中 | ||
| 抗氧化酶 | SOD、CAT、APX、GPX、DHAR、GR、GSH、CBS和ATPS | 减少ROS和RNS等物质的积累 | ||
| 金属转运蛋白基因 | LCT、CDF、CAX、NRAMP、ABC、ZIP、IRT、HMA和LCD | 吸收或运输Cd离子, 提高植物的Cd积累和对Cd的耐受性 | ||
| 转录因子调节蛋白基因 | WRKY、MYB和HSF | 与DNA结合, 参与激素和抗氧化酶等, 从而调控Cd的吸收、运输和积累 | ||
| 1 | 曹玲, 王庆成, 崔东海 (2006). 土壤镉污染对四种阔叶树苗木叶绿素荧光特性和生长的影响. 应用生态学报 17, 769-772. |
| 2 | 曹莹, 李建东, 赵天宏, 郭伟 (2007). 镉胁迫对玉米生理生化特性的影响. 农业环境科学学报 26, 8-11. |
| 3 | 陈晶, 庞思琪, 赵秀兰 (2016). 外源生长素对镉胁迫下玉米幼苗生长及抗氧化系统的影响. 植物生理学报 52, 1191-1198. |
| 4 | 陈倩, 谢旗 (2018). 内质网胁迫在植物中的研究进展. 生物技术通报 34, 15-25. |
| 5 | 付铄岚, 王昌全, 李冰, 徐强, 张敬昇, 李萌, 唐杰, 何玉亭, 沈杰, 曾杰熙, 严勋 (2017). 外源Cd在不同品种水稻组织中的细胞分布和化学形态特征研究. 中国生态农业学报 25, 903-910. |
| 6 | Gill RA (2015). 水杨酸和谷胱甘肽调控铬胁迫下油菜不同耐性品种生理生化和基因组变化的作用机理. 博士论文. 杭州: 浙江大学. pp. 1-160. |
| 7 | 郭磊 (2018). 外源硅影响镉化学形态及其生物有效性的土壤化学机制. 博士论文. 沈阳: 沈阳农业大学. pp. 1-196. |
| 8 |
郭倩倩, 周文彬 (2019). 植物响应联合胁迫机制的研究进展. 植物学报 54, 662-672.
DOI |
| 9 | 胡春霞, 王秀芹 (2010). 外源水杨酸对镉胁迫下油菜渗透调节物质和保护酶活性的影响. 鞍山师范学院学报 12(2), 40-42. |
| 10 | 黄新元, 赵方杰 (2018). 植物防御素调控水稻镉积累的新机制. 植物学报 53, 451-455. |
| 11 | 惠俊爱, 党志, 叶庆生 (2010). 镉胁迫对玉米光合特性的影响. 农业环境科学学报 29, 205-210. |
| 12 | 孔祥生, 张妙霞, 郭秀璞 (1999). Cd 2+毒害对玉米幼苗细胞膜透性及保护酶活性的影响 . 农业环境保护 18, 133-134. |
| 13 | 李佳, 刘杨, 羌维民, 王棹仁, 温晓霞, 廖允成 (2015). 镉胁迫下多胺对玉米苗期生长的影响及其机理. 农业环境科学学报 34, 1021-1027. |
| 14 | 林啸, 高素萍, 雷霆, 王成聪, 张开会 (2014). 镉胁迫下外源钙对白菜氧化应激和NO含量的影响. 农业环境科学学报 33, 1699-1705. |
| 15 | 罗琼, 葛青, 刘小京, 谢志霞, 张苹, 潘响亮, 徐进 (2015). 重金属超富集植物龙葵对镉响应的蛋白组学分析. 中国生态农业学报 23, 1429-1436. |
| 16 | 罗莎 (2017). Sasm05菌株提高东南景天锌富集的作用机制研究. 硕士论文. 杭州: 浙江大学. pp. 1-88. |
| 17 |
苗青霞, 方燕, 陈应龙 (2019). 小麦根系特征对干旱胁迫的响应. 植物学报 54, 652-661.
DOI |
| 18 |
孙瑞莲, 周启星 (2005). 高等植物重金属耐性与超积累特性及其分子机理研究. 植物生态学报 29, 497-504.
DOI |
| 19 | 汪骢跃, 王宇涛, 曾琬淋, 李韶山 (2014). Ca 2+和K +对拟南芥幼苗镉毒害的缓解作用 . 植物学报 49, 262-272. |
| 20 | 杨正婷, 刘建祥 (2016). 植物内质网胁迫应答研究进展. 生物技术通报 32, 84-96. |
| 21 | 袁连玉, 陈应娟, 魏旭, 童华荣 (2017). 茶树金属耐受蛋白基因CsMTP11的克隆及功能分析. 作物学报 43, 708-717. |
| 22 | 张标金, 张祥喜, 罗林广 (2013). 与植物镉吸收转运相关的主要基因家族. 基因组学与应用生物学 32, 127-134. |
| 23 | 张磊, 于燕玲, 张磊 (2008). 外源镉胁迫对玉米幼苗光合特性的影响. 华北农学报 23, 101-104. |
| 24 | 张瑛, 刘秀梅, 张志浩, 孟诗原, 王倩, 韦业, 王华田, 陈淑英, 丛桂芝, 唐金, 秦德明 (2019). 磁化水处理对镉胁迫下欧美杨幼苗光合及生长特性的影响. 中国生态农业学报 27, 305-313. |
| 25 | 赵士诚, 孙静文, 王秀斌, 汪洪, 梁国庆, 周卫 (2008). 镉对玉米苗中钙调蛋白含量和Ca 2+-ATPase活性的影响 . 植物营养与肥料学报 14, 264-271. |
| 26 | 赵新月, 何茂, 石辉, 屈波 (2013). 外源水杨酸对镉胁迫下玉米幼苗的叶氮素代谢和根系抗氧化酶的影响. 农业环境科学学报 32, 1950-1958. |
| 27 |
Adhikari S, Ghosh S, Azahar I, Adhikari A, Shaw AK, Konar S, Roy S, Hossain Z (2018). Sulfate improves cadmium tolerance by limiting cadmium accumulation, modulation of sulfur metabolism and antioxidant defense system in maize. Environ Exp Bot 153, 143-162.
DOI URL |
| 28 |
Agarwal P, Mitra M, Banerjee S, Roy S (2020). MYB4 transcription factor, a member of R2R3-subfamily of MYB domain protein, regulates cadmium tolerance via enhanced protection against oxidative damage and increases expression of PCS1 and MT1C in Arabidopsis. Plant Sci 297, 110501.
DOI URL |
| 29 |
Anjum SA, Tanveer M, Hussain S, Ullah E, Wang LC, Khan I, Samad RA, Tung SA, Anam M, Shahzad B (2016). Morpho-physiological growth and yield responses of two contrasting maize cultivars to cadmium exposure. Clean-Soil Air Water 44, 29-36.
DOI URL |
| 30 |
Arasimowicz-Jelonek M, Floryszak-Wieczorek J, Deckert J, Rucińska-Sobkowiak R, Gzyl J, Pawlak-Sprada S, Abramowski D, Jelonek T, Gwóźdź EA (2012). Nitric oxide implication in cadmium-induced programmed cell death in roots and signaling response of yellow lupine plants. Plant Physiol Biochem 58, 124-134.
DOI URL |
| 31 |
Bashir W, Anwar S, Zhao Q, Hussain I, Xie FT (2019). Interactive effect of drought and cadmium stress on soybean root morphology and gene expression. Ecotoxicol Environ Saf 175, 90-101.
DOI URL |
| 32 |
Beaupere C, Labunskyy VM (2019). ( Un)folding mechanisms of adaptation to ER stress: lessons from aneuploidy. Curr Genet 65, 467-471.
DOI |
| 33 |
Blaudez D, Kohler A, Martin F, Sanders D, Chalot M (2003). Poplar metal tolerance protein 1 confers zinc tolerance and is an oligomeric vacuolar zinc transporter with an essential leucine zipper motif. Plant Cell 15, 2911-2928.
PMID |
| 34 |
Bočová B, Huttová J, Mistrík I, Tamás L (2013). Auxin signaling is involved in cadmium-induced glutathione-S-transferase activity in barley root. Acta Physiol Plant 35, 2685-2690.
DOI URL |
| 35 |
Cai Z, Xian P, Wang H, Lin R, Hai N (2020). Transcription factor GmWRKY142 confers cadmium resistance by up- regulating the cadmium tolerance 1-like genes. Front Plant Sci 11, 742.
DOI URL |
| 36 |
Chao YY, Chen CY, Huang WD, Kao CH (2010). Salicylic acid-mediated hydrogen peroxide accumulation and protection against Cd toxicity in rice leaves. Plant Soil 329, 327-337.
DOI URL |
| 37 |
Chen SS, Yu M, Li H, Wang Y, Lu ZC, Zhang YX, Liu MY, Qiao GR, Wu LH, Han XJ, Zhuo RY (2020). SaHsfA4c from Sedum alfredii hance enhances cadmium tolerance by regulating ROS-scavenger activities and heat shock proteins expression. Front Plant Sci 11, 142.
DOI URL |
| 38 |
Clemens S, Aarts MGM, Thomine S, Verbruggen N (2013). Plant science: the key to preventing slow cadmium poisoning. Trends Plant Sci 18, 92-99.
DOI URL |
| 39 |
Cobbett C, Goldsbrough P (2002). Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53, 159-182.
DOI URL |
| 40 |
Curie C, Cassin G, Couch D, Divol F, Higuchi K, Le Jean M, Misson J, Schikora A, Czernic P, Mari S (2009). Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Ann Bot 103, 1-11.
DOI URL |
| 41 |
DalCorso G, Farinati S, Maistri S, Furini A (2008). How plants cope with cadmium: staking all on metabolism and gene expression. J Integr Plant Biol 50, 1268-1280.
DOI URL |
| 42 |
Danquah A, de Zelicourt A, Colcombet J, Hirt H (2014). The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol Adv 32, 40-52.
DOI URL |
| 43 | Das K, Roychoudhury A (2014). Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci 2, 53. |
| 44 |
Delledonne M, Zeier J, Marocco A, Lamb C (2001). Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA 98, 13454-13459.
DOI URL |
| 45 |
Deng Y, Srivastava R, Howell SH (2013). Protein kinase and ribonuclease domains of IRE1 confer stress tolerance, vegetative growth, and reproductive development in Arabidopsis. Proc Natl Acad Sci USA 110, 19633-19638.
DOI URL |
| 46 |
Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L (2010). MYB transcription factors in Arabidopsis. Trends Plant Sci 15, 573-581.
DOI URL |
| 47 |
Ekmekçi Y, Tanyolaç D, Ayhan B (2008). Effects of cadmium on antioxidant enzyme and photosynthetic activities in leaves of two maize cultivars. J Plant Physiol 165, 600-611.
DOI URL |
| 48 |
Erpen L, Devi HS, Grosser JW, Dutt M (2018). Potential use of the DREB/ERF, MYB, NAC and WRKY transcription factors to improve abiotic and biotic stress in transgenic plants. Plant Cell Tissue Organ Cult 132, 1-25.
DOI URL |
| 49 |
Etesami H, Jeong BR (2018). Silicon (Si): review and future prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotoxicol Environ Saf 147, 881-896.
DOI URL |
| 50 | Fan SK, Fang XZ, Guan MY, Ye YQ, Lin XY, Du ST, Jin CW (2014). Exogenous abscisic acid application decreases cadmium accumulation in Arabidopsis plants, which is associated with the inhibition of IRT1-mediated cadmium uptake. Front Plant Sci 5, 721. |
| 51 |
Feng SS, Tan JJ, Zhang YX, Liang S, Xiang SQ, Wang H, Chai TY (2017). Isolation and characterization of a novel cadmium-regulated Yellow Stripe-Like transporter (SnYSL3) in Solanum nigrum. Plant Cell Rep 36, 281-296.
DOI URL |
| 52 |
Guo JJ, Qin SY, Rengel Z, Gao W, Nie ZJ, Liu HG, Li C, Zhao P (2019). Cadmium stress increases antioxidant enzyme activities and decreases endogenous hormone concentrations more in Cd-tolerant than Cd-sensitive wheat varieties. Ecotoxicol Environ Saf 172, 380-387.
DOI URL |
| 53 |
Guo JK, Wu J, Ji Q, Wang C, Luo L, Yuan Y, Wang YH, Wang J (2008). Genome-wide analysis of heat shock transcription factor families in rice and Arabidopsis. J Genet Genomics 35, 105-118.
DOI URL |
| 54 |
Hartl FU, Hayer-Hartl M (2002). Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295, 1852-1858.
DOI URL |
| 55 |
Hong CY, Cheng D, Zhang GQ, Zhu DD, Chen YH, Tan MP (2017). The role of ZmWRKY4 in regulating maize antioxidant defense under cadmium stress. Biochem Biophys Res Commun 482, 1504-1510.
DOI URL |
| 56 |
Hu XL, Jiang MY, Zhang AY, Lu J (2005). Abscisic acid-induced apoplastic H2O2 accumulation up-regulates the activities of chloroplastic and cytosolic antioxidant enzymes in maize leaves. Planta 223, 57-68.
DOI URL |
| 57 |
Hu XL, Jiang MY, Zhang JH, Zhang AY, Lin F, Tan MP (2007). Calcium-calmodulin is required for abscisic acid- induced antioxidant defense and functions both upstream and downstream of H2O2 production in leaves of maize ( Zea mays) plants. New Phytol 173, 27-38.
DOI URL |
| 58 |
Hu YF, Zhou GY, Na XF, Yang LJ, Nan WB, Liu X, Zhang YQ, Li JL, Bi YR (2013). Cadmium interferes with maintenance of auxin homeostasis in Arabidopsis seedlings. J Plant Physiol 170, 965-975.
DOI URL |
| 59 |
Huang XY, Deng FL, Yamaji N, Pinson SRM, Fujii-Kashino M, Danku J, Douglas A, Guerinot ML, Salt DE, Ma JF (2016). A heavy metal P-type ATPase OsHMA4 prevents copper accumulation in rice grain. Nat Commun 7, 12138.
DOI URL |
| 60 |
Ismael MA, Elyamine AM, Moussa MG, Cai MM, Zhao XH, Hu CX (2019). Cadmium in plants: uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 11, 255-277.
DOI PMID |
| 61 |
Jia HL, Wang XH, Wei T, Zhou R, Muhammad H, Hua L, Ren XH, Guo JK, Ding YZ (2019). Accumulation and fixation of Cd by tomato cell wall pectin under Cd stress. Environ Exp Bot 167, 103829.
DOI URL |
| 62 |
Keunen E, Remans T, Opdenakker K, Jozefczak M, Gielen H, Guisez Y, Vangronsveld J, Cuypers A (2013). A mutant of the Arabidopsis thaliana LIPOXYGENASE1 gene shows altered signaling and oxidative stress related responses after cadmium exposure. Plant Physiol Biochem 63, 272-280.
DOI URL |
| 63 |
Khan N, You FM, Datla R, Ravichandran S, Jia BS, Cloutier S (2020). Genome-wide identification of ATP binding cassette (ABC) transporter and heavy metal associated (HMA) gene families in flax ( Linum usitatissimum L.). BMC Genomics 21, 722.
DOI URL |
| 64 |
Kleizen B, Braakman I (2004). Protein folding and quality control in the endoplasmic reticulum. Curr Opin Cell Biol 16, 343-349.
DOI URL |
| 65 |
Korenkov V, King B, Hirschi K, Wagner GJ (2009). Root-selective expression of AtCAX4 and AtCAX2 results in reduced lamina cadmium in field-grown Nicotiana tabacum L. Plant Biotechnol J 7, 219-226.
DOI URL |
| 66 |
Korshunova YO, Eide D, Clark WG, Guerinot ML, Pakrasi HB (1999). The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol Biol 40, 37-44.
PMID |
| 67 |
Krämer U, Talke IN, Hanikenne M (2007). Transition metal transport. FEBS Lett 581, 2263-2272.
PMID |
| 68 |
Krantev A, Yordanova R, Janda T, Szalai G, Popova L (2008). Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J Plant Physiol 165, 920-931.
DOI URL |
| 69 |
Lalor GC (2008). Review of cadmium transfers from soil to humans and its health effects in the Jamaican environment. Sci Total Environ 400, 162-172.
DOI URL |
| 70 |
Lei GJ, Sun L, Sun Y, Zhu XF, Li GX, Zheng SJ (2020). Jasmonic acid alleviates cadmium toxicity in Arabidopsis via suppression of cadmium uptake and translocation. J Integr Plant Biol 62, 218-227.
DOI URL |
| 71 |
Li SW, Leng Y, Feng L, Zeng XY (2014). Involvement of abscisic acid in regulating antioxidative defense systems and IAA-oxidase activity and improving adventitious rooting in mung bean (Vigna radiata(L.) Wilczek) seedlings under cadmium stress. Environ Sci Pollut Res Int 21, 525-537.
DOI URL |
| 72 |
Li ZR, Mei XY, Li T, Yang S, Qin L, Li B, Zu YQ (2021). Effects of calcium application on activities of membrane transporters in Panax notoginseng under cadmium stress. Chemosphere 262, 127905.
DOI URL |
| 73 |
Liu SL, Yang RJ, Tripathi DK, Li X, He W, Wu MX, Ali S, Ma MD, Cheng QS, Pan YZ (2018). RETRACTED: the interplay between reactive oxygen and nitrogen species contributes in the regulatory mechanism of the nitro-oxidative stress induced by cadmium in Arabidopsis. J Hazard Mater 344, 1007-1024.
DOI URL |
| 74 |
Liu XL, Li X, Dai CC, Zhou JY, Yan T, Zhang JF (2017). Improved short-term drought response of transgenic rice over-expressing maize C4 phosphoenolpyruvate carboxylase via calcium signal cascade. J Plant Physiol 218, 206-221.
DOI URL |
| 75 |
Liu ZP, Ding YF, Wang FJ, Ye YY, Zhu C (2016). Role of salicylic acid in resistance to cadmium stress in plants. Plant Cell Rep 35, 719-731.
DOI URL |
| 76 |
Mikkelsen MD, Pedas P, Schiller M, Vincze E, Mills RF, Borg S, Møller A, Schjoerring JK, Williams LE, Baekgaard L, Holm PB, Palmgren MG (2012). Barley HvHMA1 is a heavy metal pump involved in mobilizing organellar Zn and Cu and plays a role in metal loading into grains. PLoS One 7, e49027.
DOI URL |
| 77 |
Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F (2011). ROS signaling: the new wave? Trends Plant Sci 16, 300-309.
DOI URL |
| 78 |
Miyadate H, Adachi S, Hiraizumi A, Tezuka K, Nakazawa N, Kawamoto T, Katou K, Kodama I, Sakurai K, Takahashi H, Satoh-Nagasawa N, Watanabe A, Fujimura T, Akagi H (2011). OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol 189, 190-199.
DOI URL |
| 79 |
Morel M, Crouzet J, Gravot A, Auroy P, Leonhardt N, Vavasseur A, Richaud P (2009). AtHMA3, a P1B-ATPase allowing Cd/Zn/Co/Pb vacuolar storage in Arabidopsis. Plant Physiol 149, 894-904.
DOI URL |
| 80 |
Nagajyoti PC, Lee KD, Sreekanth TVM (2010). Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8, 199-216.
DOI URL |
| 81 |
Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, Morris P, Ribeiro D, Wilson I (2008a). Nitric oxide, stomatal closure, and abiotic stress. J Exp Bot 59, 165-176.
DOI URL |
| 82 |
Neill S, Bright J, Desikan R, Hancock J, Harrison J, Wilson I (2008b). Nitric oxide evolution and perception. J Exp Bot 59, 25-35.
DOI URL |
| 83 |
Nieves-Cordones M, López-Delacalle M, Ródenas R, Martínez V, Rubio F, Rivero RM (2019). Critical responses to nutrient deprivation: a comprehensive review on the role of ROS and RNS. Environ Exp Bot 161, 74-85.
DOI |
| 84 |
Noriega G, Cruz DS, Batlle A, Tomaro M, Balestrasse K (2012). Heme oxygenase is involved in the protection exerted by jasmonic acid against cadmium stress in soybean roots. J Plant Growth Regul 31, 79-89.
DOI URL |
| 85 |
Nürnberger T, Scheel D (2001). Signal transmission in the plant immune response. Trends Plant Sci 6, 372-379.
PMID |
| 86 |
Pan CL, Lu HL, Liu JC, Yu JY, Wang Q, Li JW, Yang JJ, Hong HL, Yan CL (2020). SODs involved in the hormone mediated regulation of H2O2 content in Kandelia obovata root tissues under cadmium stress. Environ Pollut 256, 113272.
DOI URL |
| 87 |
Park J, Song WY, Ko D, Eom Y, Hansen TH, Schiller M, Lee TG, Martinoia E, Lee Y (2012). The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J 69, 278-288.
DOI URL |
| 88 |
Perfus-Barbeoch L, Leonhardt N, Vavasseur A, Forestier C (2002). Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. Plant J 32, 539-548.
PMID |
| 89 |
Prasad MNV (1995). Cadmium toxicity and tolerance in vascular plants. Environ Exp Bot 35, 525-545.
DOI URL |
| 90 |
Rajakumar S, Bhanupriya N, Ravi C, Nachiappan V (2016). Endoplasmic reticulum stress and calcium imbalance are involved in cadmium-induced lipid aberrancy in Saccharomyces cerevisiae. Cell Stress Chaperones 21, 895-906.
DOI PMID |
| 91 |
Romero-Puertas MC, Sandalio LM (2016). Nitric oxide level is self-regulating and also regulates its ROS partners. Front Plant Sci 7, 316.
DOI PMID |
| 92 |
Romero-Puertas MC, Terrón-Camero LC, Peláez-Vico MÁ, Olmedilla A, Sandalio LM (2019). Reactive oxygen and nitrogen species as key indicators of plant responses to Cd stress. Environ Exp Bot 161, 107-119.
DOI |
| 93 |
Rushton PJ, Somssich IE, Ringler P, Shen QJ (2010). WRKY transcription factors. Trends Plant Sci 15, 247-258.
DOI URL |
| 94 |
Sami F, Faizan M, Faraz A, Siddiqui H, Yusuf M, Hayat S (2018). Nitric oxide-mediated integrative alterations in plant metabolism to confer abiotic stress tolerance, NO crosstalk with phytohormones and NO-mediated post translational modifications in modulating diverse plant stress. Nitric Oxide 73, 22-38.
DOI URL |
| 95 |
Sanità di Toppi L, Gabbrielli R (1999). Response to cadmium in higher plants. Environ Exp Bot 41, 105-130.
DOI URL |
| 96 |
Shahid MA, Balal RM, Khan N, Zotarelli L, Liu GD, Sarkhosh A, Fernández-Zapata JC, Nicolás JJM, Garcia-Sanchez F (2019). Selenium impedes cadmium and arsenic toxicity in potato by modulating carbohydrate and nitrogen metabolism. Ecotoxicol Environ Saf 180, 588-599.
DOI URL |
| 97 |
Shim D, Hwang JU, Lee J, Lee S, Choi Y, An G, Martinoia E, Lee Y (2009). Orthologs of the class A4 heat shock transcription factor HsfA4a confer cadmium tolerance in wheat and rice. Plant Cell 21, 4031-4043.
DOI URL |
| 98 |
Snedden WA, Fromm H (1998). Calmodulin, calmodulin- related proteins and plant responses to the environment. Trends Plant Sci 3, 299-304.
DOI URL |
| 99 |
Snedden WA, Fromm H (2001). Calmodulin as a versatile calcium signal transducer in plants. New Phytol 151, 35-66.
DOI URL |
| 100 | Sofo A, Vitti A, Nuzzaci M, Tataranni G, Scopa A, Vangronsveld J, Remans T, Falasca G, Altamura MM, Degola F, Sanità di Toppi L (2013). Correlation between hormonal homeostasis and morphogenic responses in Arabidopsis thaliana seedlings growing in a Cd/Cu/Zn multi-pollution context. Physiol Plant 149, 487-498. |
| 101 |
Song G, Yuan SX, Wen XH, Xie ZN, Lou LQ, Hu BY, Cai QS, Xu B (2018). Transcriptome analysis of Cd-treated switchgrass root revealed novel transcripts and the importance of HSF/HSP network in switchgrass Cd tolerance. Plant Cell Rep 37, 1485-1497.
DOI URL |
| 102 |
Song JY, Finnegan PM, Liu WH, Li X, Yong JWH, Xu JT, Zhang Q, Wen YX, Qin KX, Guo JZ, Li T, Zhao C, Zhang Y (2019). Mechanisms underlying enhanced Cd translocation and tolerance in roots of Populus euramericana in response to nitrogen fertilization. Plant Sci 287, 110206.
DOI URL |
| 103 |
Stroiński A, Chadzinikolau T, Giżewska K, Zielezińska M (2010). ABA or cadmium induced phytochelatin synthesis in potato tubers. Biol Plant 54, 117-120.
DOI URL |
| 104 |
Talukdar D (2012). Exogenous calcium alleviates the impact of cadmium-induced oxidative stress in Lens culinaris Medic. Seedlings through modulation of antioxidant enzyme activities. J Crop Sci Biotechnol 15, 325-334.
DOI URL |
| 105 |
Tamás L, Bočová B, Huttová J, Liptáková L, Mistrík I, Valentovičová K, Zelinová V (2012). Impact of the auxin signaling inhibitor p-chlorophenoxyisobutyric acid on short- term Cd-induced hydrogen peroxide production and growth response in barley root tip. J Plant Physiol 169, 1375-1381.
DOI URL |
| 106 |
Tamás L, Dudíková J, ĎUrčková K, HalušKová LU, Huttová J, Mistrík I, Ollé M (2008). Alterations of the gene expression, lipid peroxidation, proline and thiol content along the barley root exposed to cadmium. J Plant Physiol 165, 1193-1203.
DOI URL |
| 107 |
Ueno D, Milner MJ, Yamaji N, Yokosho K, Koyama E, Zambrano MC, Kaskie M, Ebbs S, Kochian LV, Ma JF (2011). Elevated expression of TcHMA3 plays a key role in the extreme Cd tolerance in a Cd-hyperaccumulating ecotype of Thlaspi caerulescens. Plant J 66, 852-862.
DOI URL |
| 108 |
Ülker B, Somssich IE (2004). WRKY transcription factors: from DNA binding towards biological function. Curr Opin Plant Biol 7, 491-498.
DOI URL |
| 109 | UNEP (2008). Final review of scientific information on cadmium.https://www.unep.org/resources/report/final-review-scientific-information-cadmium. |
| 110 |
Vaculík M, Pavlovic A, Lux A (2015). Silicon alleviates cadmium toxicity by enhanced photosynthetic rate and modified bundle sheath’s cell chloroplasts ultrastructure in maize. Ecotoxicol Environ Saf 120, 66-73.
DOI URL |
| 111 |
Verma S, Dubey RS (2001). Effect of cadmium on soluble sugars and enzymes of their metabolism in rice. Biol Plant 44, 117-123.
DOI URL |
| 112 |
Wang H, Chen WY, Sinumvayabo N, Li YF, Han ZX, Tian J, Ma Q, Pan ZZ, Geng ZJ, Yang SQ, Kang MM, Rahman SU, Yang GJ, Zhang Y (2020). Phosphorus deficiency induces root proliferation and Cd absorption but inhibits Cd tolerance and Cd translocation in roots of Populus × euramericana. Ecotoxicol Environ Saf 204, 111148.
DOI URL |
| 113 |
Wang P, Deng XJ, Huang Y, Fang XL, Zhang J, Wan HB, Yang CY (2016). Root morphological responses of five soybean (Glycine max(L.) Merr) cultivars to cadmium stress at young seedlings. Environ Sci Pollut Res Int 23, 1860-1872.
DOI URL |
| 114 |
Wang Y, Wang C, Liu YJ, Yu KF, Zhou YH (2018). GmHMA3 sequesters Cd to the root endoplasmic reticulum to limit translocation to the stems in soybean. Plant Sci 270, 23-29.
DOI URL |
| 115 |
Wu C (1995). Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol 11, 441-469.
PMID |
| 116 |
Wu Q, Zhu XF, Zhao XS, Shen RF (2020). Potassium affects cadmium resistance in Arabidopsis through facilitating root cell wall Cd retention in a nitric oxide dependent manner. Environ Exp Bot 178, 104175.
DOI URL |
| 117 |
Wu X, Chen JH, Yue XM, Wei XN, Zou JW, Chen YH, Su NN, Cui J (2019). The zinc-regulated protein (ZIP) family genes and glutathione S-transferase (GST) family genes play roles in Cd resistance and accumulation of pak choi ( Brassica campestris ssp. chinensis). Ecotoxicol Environ Saf 183, 109571.
DOI URL |
| 118 |
Xu H, Xu WZ, Xi HM, Ma WW, He ZY, Ma M (2013). The ER luminal binding protein (BiP) alleviates Cd 2+-induced programmed cell death through endoplasmic reticulum stress- cell death signaling pathway in tobacco cells . J Plant Physiol 170, 1434-1475.
DOI URL |
| 119 |
Xu L, Wang Y, Zhang F, Tang MJ, Chen YL, Wang J, Karanja BK, Luo XB, Zhang W, Liu LW (2017). Dissecting root proteome changes reveals new insight into cadmium stress response in radish ( Raphanus sativus L.). Plant Cell Physiol 58, 1901-1913.
DOI URL |
| 120 | Xu L, Zhang F, Tang MJ, Wang Y, Dong JH, Ying JL, Chen YL, Hu B, Li C, Liu LW (2020). Melatonin confers cadmium tolerance by modulating critical heavy metal chelators and transporters in radish plants. J Pineal Res 69, e12659. |
| 121 |
Yadav SK (2010). Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot 76, 167-179.
DOI URL |
| 122 |
Yamaji N, Xia JX, Mitani-Ueno N, Yokosho K, Feng MJ (2013). Preferential delivery of zinc to developing tissues in rice is mediated by P-type heavy metal ATPase OsHMA2. Plant Physiol 162, 927-939.
DOI URL |
| 123 |
Yang T, Poovaiah BW (2002). Hydrogen peroxide homeostasis: activation of plant catalase by calcium/calmodulin. Proc Natl Acad Sci USA 99, 4097-4102.
DOI URL |
| 124 |
Yuan LY, Yang SG, Liu BX, Zhang M, Wu KQ (2012). Molecular characterization of a rice metal tolerance protein, OsMTP1. Plant Cell Rep 31, 67-79.
DOI URL |
| 125 | Zawoznik MS, Groppa MD, Tomaro ML, Benavides MP (2007). Endogenous salicylic acid potentiates cadmium- induced oxidative stress in Arabidopsis thaliana. Plant Sci 173, 190-197. |
| 126 |
Zhang J, Zhang M, Song HY, Zhao JQ, Shabala S, Tian SK, Yang XE (2020a). A novel plasma membrane-based NRAMP transporter contributes to Cd and Zn hyperac-cumulation in Sedum alfredii Hance. Environ Exp Bot 176, 104121.
DOI URL |
| 127 | Zhang LY, Zhang HY, Guo W, Tian YL, Chen ZS, Wei XF (2011). Photosynthetic responses of energy plant maize under cadmium contamination stress. Adv Mat Res 356-360, 283-286. |
| 128 |
Zhang P, Wang RL, Ju Q, Li WQ, Tran LSP, Xu J (2019a). The R2R3-MYB transcription factor MYB49 regulates cadmium accumulation. Plant Physiol 180, 529-542.
DOI |
| 129 |
Zhang SM, Yang C, Chen MM, Chen J, Pan YH, Chen YL, Rahman SU, Fan JF, Zhang Y (2019b). Influence of nitrogen availability on Cd accumulation and acclimation strategy of Populus leaves under Cd exposure. Ecotoxicol Environ Saf 180, 439-448.
DOI URL |
| 130 |
Zhang WW, Yue SQ, Song JF, Xun M, Han MY, Yang HQ (2020b). MhNRAMP1 from Malus hupehensis exacerbates cell death by accelerating Cd uptake in tobacco and apple calli. Front Plant Sci 11, 957.
DOI URL |
| 131 |
Zhao SY, Ma QF, Xu X, Li GZ, Hao L (2016). Tomato jasmonic acid-deficient mutant spr2 seedling response to cadmium stress. J Plant Growth Regul 35, 603-610.
DOI URL |
| 132 |
Zhou M, Zheng SG, Liu R, Lu J, Lu L, Zhang CH, Liu ZH, Luo CP, Zhang L, Yant L, Wu Y (2019). Genome-wide identification, phylogenetic and expression analysis of the heat shock transcription factor family in bread wheat ( Triticum aestivum L.). BMC Genomics 20, 505.
DOI PMID |
| [1] | 惠城阳, 章巧依, 刘腾腾, 刘维勇, 周丽娜, 金鑫杰, 张永华, 刘金亮. 温州大罗山主要植被类型及物种组成特征[J]. 植物生态学报, 2025, 49(植被): 1-. |
| [2] | 曹毅 张松林 王旭峰 杨安昌 任敏慧 杨浩 韩超. 兰州市南北两山植物群落数据集[J]. 植物生态学报, 2025, 49(植被): 1-0. |
| [3] | 陈龙 郭柯 勾晓华 赵秀海 马泓若. 祁连圆柏林群落组成及特征[J]. 植物生态学报, 2025, 49(植被): 0-0. |
| [4] | 童金莲, 张博纳, 汤璐瑶, 叶琳峰, 李姝雯, 谢江波, 李彦, 王忠媛. C4植物狗尾草功能性状网络沿降水梯度带的区域分异规律[J]. 植物生态学报, 2025, 49(预发表): 1-. |
| [5] | 闫小红 胡文海. 亚热带地区3种常绿阔叶植物冬季光保护机制的差异[J]. 植物生态学报, 2025, 49(预发表): 0-0. |
| [6] | 赵常明 熊高明 申国珍 葛结林 徐文婷 徐凯 武元帅 谢宗强. 神农架常绿落叶阔叶混交林和亚高山针叶林植物群落特征数据集[J]. 植物生态学报, 2025, 49(典型生态系统数据集): 0-0. |
| [7] | 赵珮杉 高广磊 丁国栋 张英. 林龄和生态位对樟子松人工林地下真菌群落构建的影响[J]. 植物生态学报, 2025, 49(地上地下生态过程关联): 1-0. |
| [8] | 张子睿, 周静, 胡艳萍, 梁爽, 马永鹏, 陈伟乐. 极度濒危植物巧家五针松的根内和根际真菌群落特征[J]. , 2025, 49(濒危植物的保护与恢复): 0-. |
| [9] | 逯子佳, 王天瑞, 郑斯斯, 孟宏虎, 曹建国, Gregor Kozlowski, 宋以刚. 孑遗植物湖北枫杨的环境适应性遗传变异与遗传脆弱性[J]. , 2025, 49(濒危植物的保护与恢复): 0-. |
| [10] | 黄承玲, 黎荣瀚, 覃红玲, 杨胜雄, 田晓玲, 夏国威, 陈正仁, 周玮. 基于SNP分子标记的极小种群野生植物荔波杜鹃保护遗传学研究[J]. , 2025, 49(濒危植物的保护与恢复): 0-. |
| [11] | 周鑫宇, 刘会良, 高贝, 卢妤婷, 陶玲庆, 文晓虎, 张岚, 张元明. 新疆特有濒危植物雪白睡莲繁殖生物学研究[J]. , 2025, 49(濒危植物的保护与恢复): 0-. |
| [12] | 高雨轩, 苏艳军, 冯育才, 张军, 汪小全, 刘玲莉. 珍稀濒危孑遗植物银杉的研究与保护现状[J]. , 2025, 49(濒危植物的保护与恢复): 0-. |
| [13] | 朱润铖, 蔡锡安, 黄娟. 植物防御相关挥发性有机物排放及对氮沉降的响应[J]. 植物生态学报, 2025, 49(5): 681-696. |
| [14] | 平晓燕, 杜毅倩, 赖仕蓉, 孔梦桥, 余国杰. 植物应对食草动物采食的化学防御策略研究进展[J]. 植物生态学报, 2025, 49(5): 667-680. |
| [15] | 马富龙, 王雨晴, 郝瑜, 段继超, 刘霏霏, 席琳乔, 韩路. 海拔梯度对昆仑山北坡中部草原植物与土壤微生物群落结构与多样性的影响[J]. 植物生态学报, 2025, 49(5): 732-747. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||