

植物学报 ›› 2025, Vol. 60 ›› Issue (1): 90-100.DOI: 10.11983/CBB24040 cstr: 32102.14.CBB24040
陈秀秀1,2,3, 唐玲1,2,3, 胡文佳1,2,3, 杨照麟1,2,3, 邓馨1,2, 王晓华1,2,*( )
)
收稿日期:2024-03-11
接受日期:2024-05-27
出版日期:2025-01-10
发布日期:2024-05-30
通讯作者:
* 王晓华, 副研究员, 硕士生导师。主要研究方向为植物活细胞和单分子成像技术, 致力于建立适合植物细胞膜和生物大分子的单分子研究方法, 实现生物大分子运动参数的精准分析, 从而为揭示植物细胞早期响应机制提供技术支持。E-mail: wangxh@ibcas.ac.cn基金资助:
Xiuxiu Chen1,2,3, Ling Tang1,2,3, Wenjia Hu1,2,3, Zhaolin Yang1,2,3, Xin Deng1,2, Xiaohua Wang1,2,*( )
)
Received:2024-03-11
Accepted:2024-05-27
Online:2025-01-10
Published:2024-05-30
Contact:
* E-mail: 摘要: 质膜微区是细胞质膜上富含甾醇和鞘磷脂的微结构域, 参与信号转导、囊泡转运、胞吞和胞吐等众多生物学过程, 因此质膜微区动态过程是植物细胞生物学研究的重要领域之一。荧光探针结合荧光显微镜被广泛应用于检测植物活细胞状态。PA (push-pull pyrene)是一种基于芘的新型、高效且稳定的荧光探针, 但在植物活细胞成像研究中应用极少。该研究利用PA探针和激光共聚焦显微镜技术, 结合图像处理和极性归一化数值作图法对拟南芥(Arabidopsis thaliana)根中活细胞质膜的有序度进行了定量分析, 发现PA探针在拟南芥根细胞质膜中的液态有序相的发射光谱为500-550 nm, 液态无序相的发射光谱为580-700 nm。使用甾醇抽提剂MβCD处理野生型拟南芥可使质膜的有序度降低。缺乏甾醇合成关键的甲基转移酶双突变体smt2/smt3质膜的有序度与经甾醇抽提剂MβCD处理的野生型株系质膜一致。smt2/smt3突变体根毛细胞质膜的有序度低于野生型, 表明甾醇作为膜微区的关键组分在调节质膜的有序度上发挥重要作用。该研究为检测植物活细胞质膜动力学特征和质膜微区变化提供了一种直观且快速的检测手段。
陈秀秀, 唐玲, 胡文佳, 杨照麟, 邓馨, 王晓华. 植物细胞质膜有序性的活细胞定量分析. 植物学报, 2025, 60(1): 90-100.
Xiuxiu Chen, Ling Tang, Wenjia Hu, Zhaolin Yang, Xin Deng, Xiaohua Wang. Quantitative Analysis of Plasma Membrane Order in Live Plant Cells. Chinese Bulletin of Botany, 2025, 60(1): 90-100.
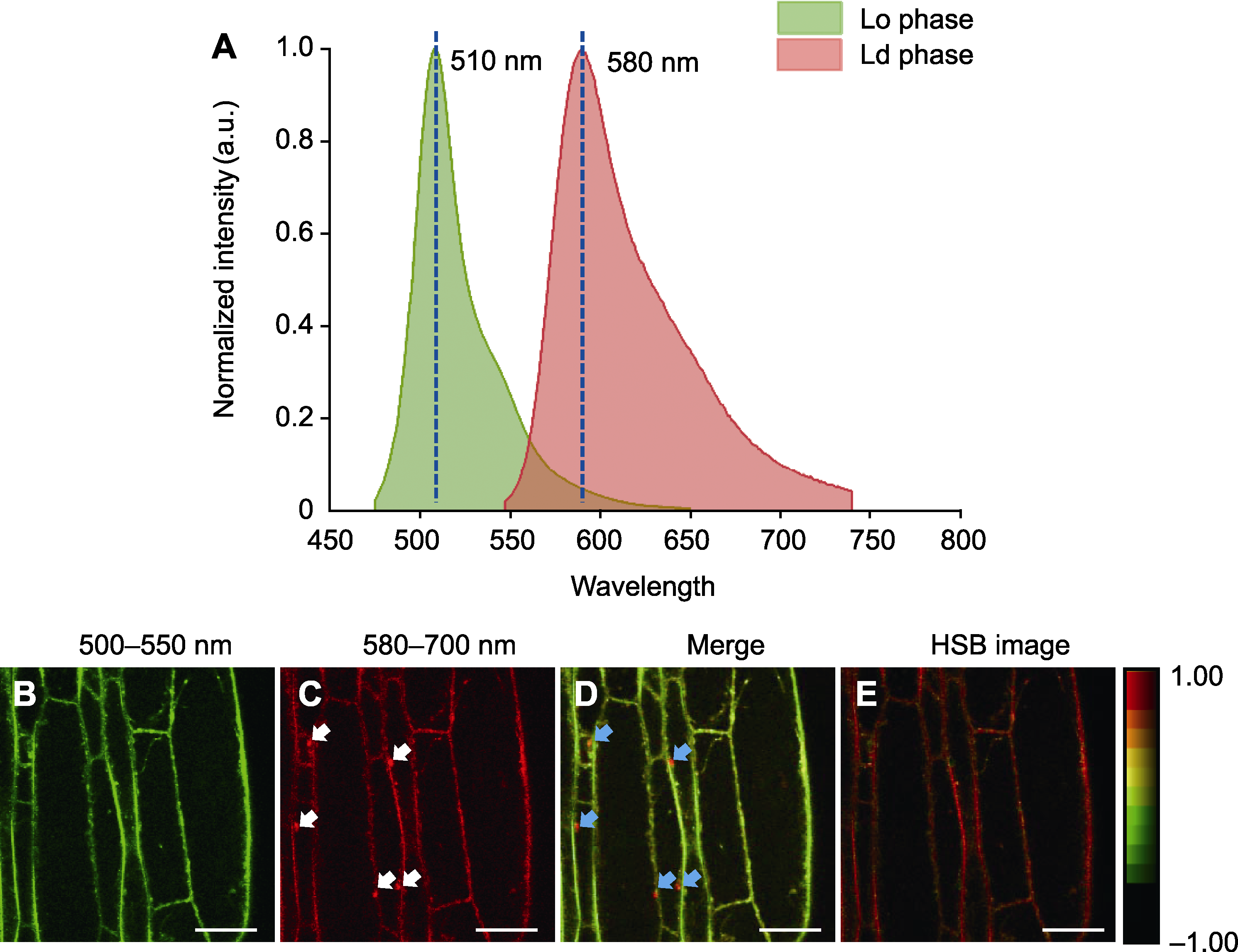
图1 PA探针标记拟南芥根细胞质膜的发射光谱分析 (A) 拟南芥根细胞中PA探针的发射光谱特征(Lo phase: 液态有序相; Ld phase: 液态无序相); (B) PA标记的拟南芥根细胞的绿色通道(500-550 nm)荧光成像(液态有序相, Lo); (C) PA标记的拟南芥根细胞的红色通道(580-700 nm)荧光成像(液态无序相, Ld) (白色箭头指示内体); (D) 绿色(B)通道与红色(C)通道的叠加图像(蓝色箭头指示内体); (E) 双通道图像对应的色相-饱和度-亮度(Hue-Saturation-Brightness, HSB)图像。Bars=10 μm
Figure 1 Emission spectrum analysis of the plasma membrane in Arabidopsis root cells labeled with PA (A) Fluorescence spectra of PA in Arabidopsis root cells (Lo phase: Liquid ordered phase; Ld phase: Liquid disordered phase); (B) Green channel (500-550 nm) imaging of PA-labeled Arabidopsis root cells (liquid ordered phase, Lo); (C) Red channel (580-700 nm) imaging of PA-labeled Arabidopsis root cells (liquid disordered phase, Ld) (white arrows indicate the presence of endosomes); (D) The merged image of green (B) and red (C) channels (blue arrows indicate the presence of endosomes); (E) Hue-Saturation-Brightness (HSB) image corresponding to the two-channel image. Bars=10 μm
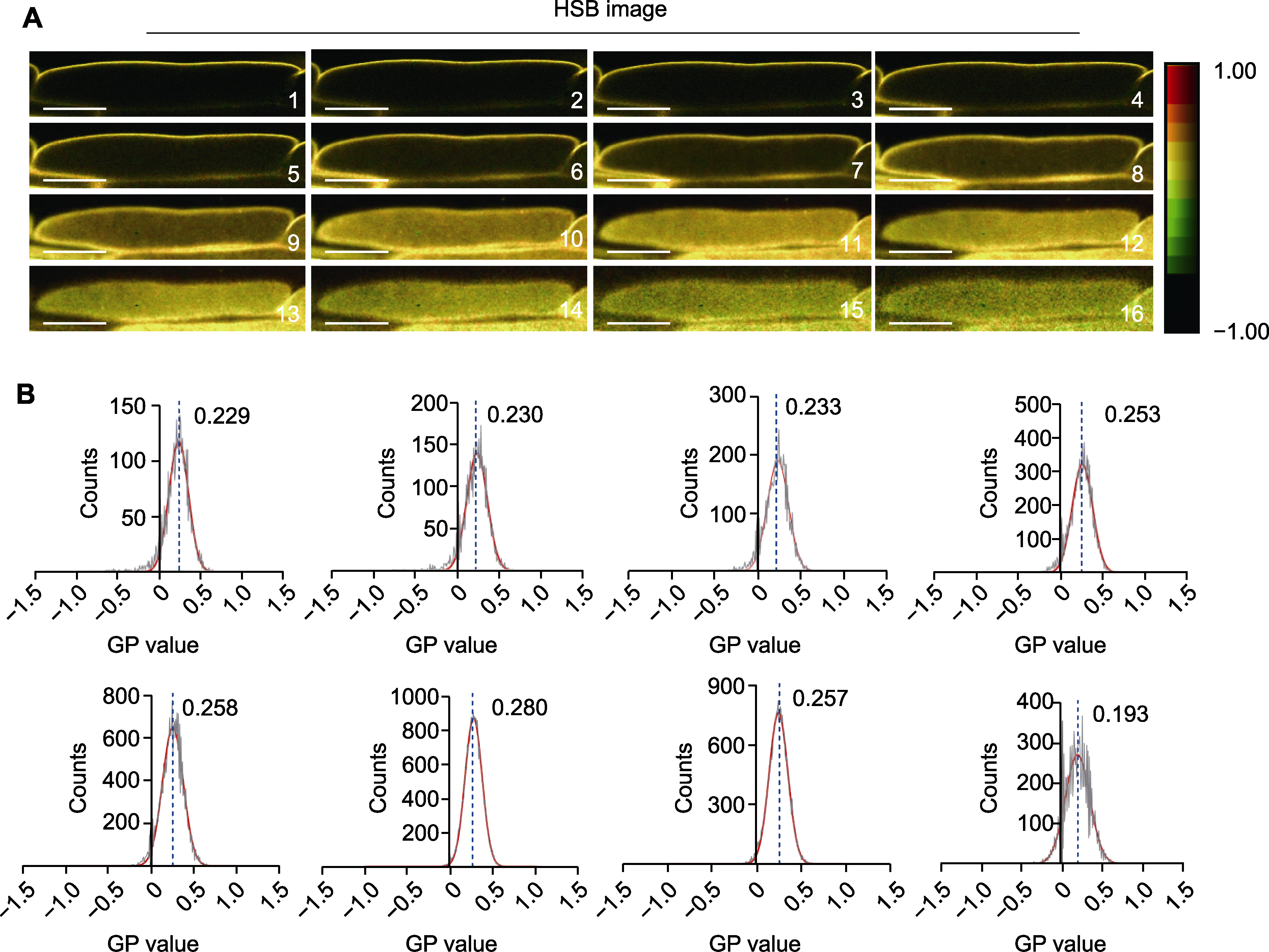
图2 拟南芥根中细胞不同切面(Z-stack)的有序度分析 (A) 拟南芥根中细胞不同切面的HSB荧光图像, 1-16所示图像代表细胞单个层面的HSB荧光图像(bars=20 μm); (B) 拟南芥根中细胞不同切面的GP值分布, 每组统计3000多个像素。
Figure 2 Analysis of membrane degree of different planes (Z-stack) in individual Arabidopsis root cells (A) HSB image of different planes (Z-stack) within individual cells of Arabidopsis roots, the images shown in 1-16 represent HSB fluorescence images of individual cell layers (bars=20 μm); (B) Distribution of GP values of different planes (Z-stack) within individual cells of Arabidopsis roots, more than 3000 pixels were analyzed for each group.
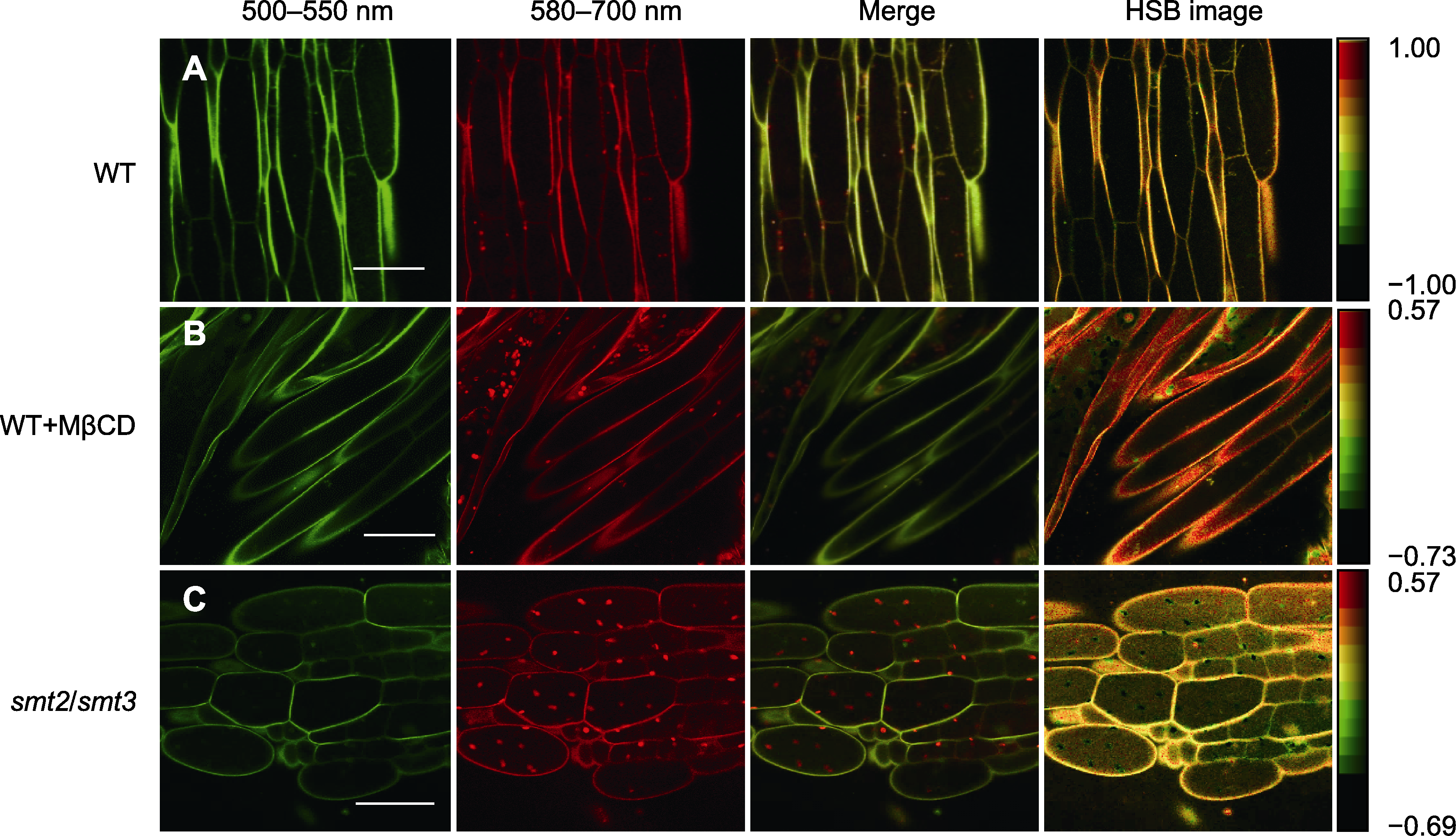
图3 PA探针标记MβCD处理及smt2/smt3双突变体拟南芥根细胞的荧光图像 (A) PA标记的野生型(WT)根细胞的荧光图像; (B) 经MβCD处理后的WT根细胞的PA标记荧光图像; (C) smt2/smt3突变体中根细胞的PA标记荧光图像。Bars=20 μm
Figure 3 Fluorescence images of Arabidopsis root cells labeled with the PA probe after MβCD treatment and in the smt2/smt3 mutant (A) Fluorescence image of wild type (WT) root cells labeled with PA; (B) Fluorescence image of WT root cells labeled with PA probe after treatment with MβCD; (C) Fluorescence image of root cells in the smt2/smt3 mutant labeled with PA probe. Bars=20 μm
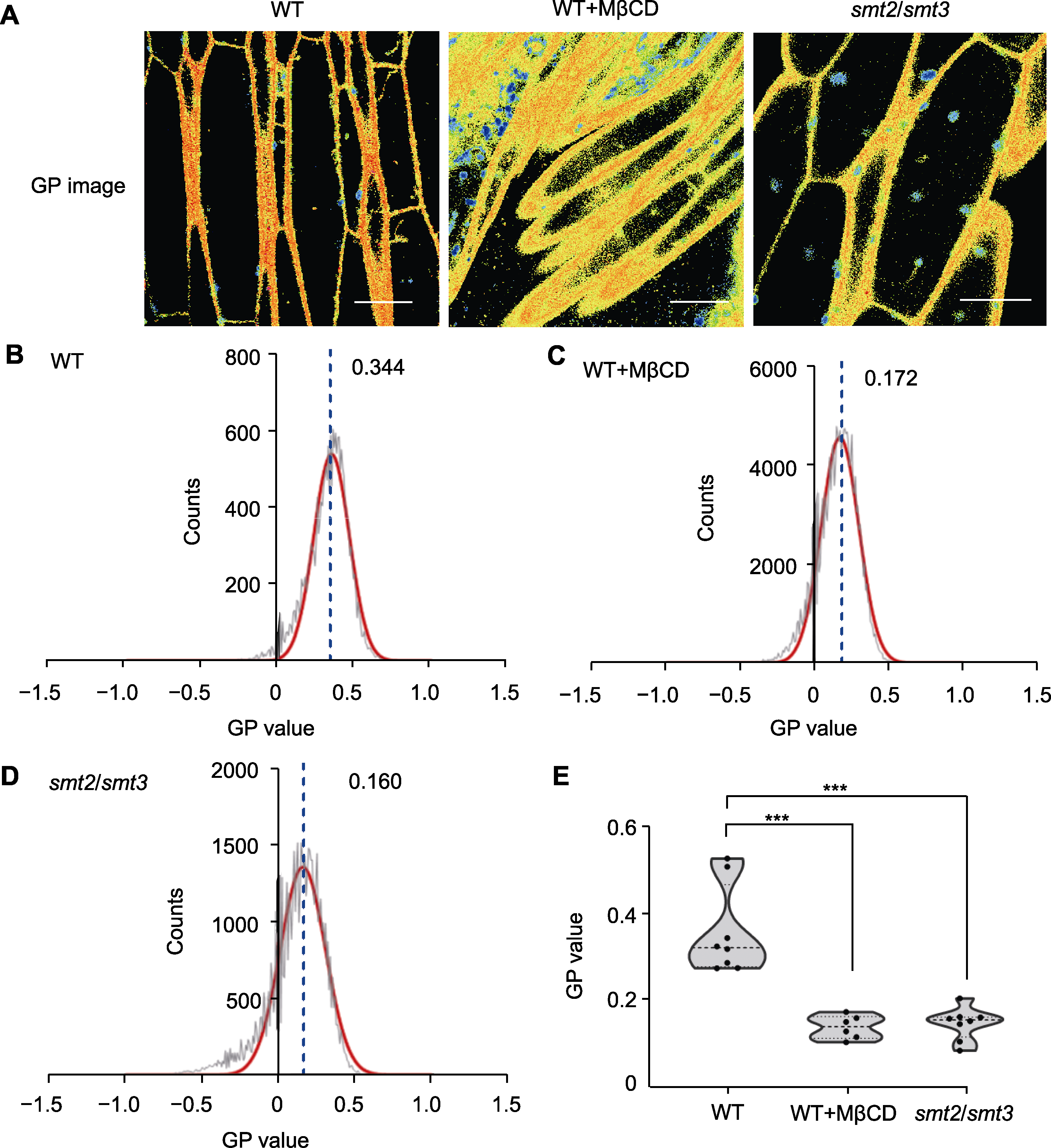
图4 甾醇缺失对拟南芥根细胞膜微区均一化极性数值定量分析和比较 (A) PA探针检测野生型(WT)、MβCD处理后的WT和smt2/smt3双突变体的GP图像(bars=10 μm); (B) 野生型拟南芥根细胞质膜有序度的GP分布曲线; (C) MβCD处理的WT根细胞质膜有序度的GP分布曲线; (D) smt2/smt3双突变体根细胞质膜有序度的GP分布曲线((B)-(D)中红色曲线指示GP值分布的高斯拟合曲线; 黑色曲线显示质膜的GP峰值分布; 不同样品中质膜GP峰值标注于曲线顶部右侧; 蓝色虚线指示GP峰值的位置); (E) WT、MβCD处理的WT和smt2/smt3双突变体的GP峰值(每组6次生物学重复; ***P<0.001, Student’s t-test)。
Figure 4 Quantitative analysis and comparison of generalized polarization values of membrane microdomains in Arabidopsis root cells after sterol depletion (A) GP image of the wild type (WT), WT treated with MβCD, and smt2/smt3 double mutant using PA probe (bars=10 μm); (B) GP distribution curve for the WT membrane; (C) GP distribution curve for the WT membrane treated with MβCD; (D) GP distribution curve for the membrane of smt2/smt3 double mutant (the red curves in (B) to (D) represent the Gaussian fitting curves for the distributions of the GP values; The black curves represent the distributions of the membrane GP values; The GP peak values are shown at the top right of each curve; The blue dotted lines indicate the locations of the GP peaks); (E) GP peak values for the WT, WT treated with MβCD, and smt2/smt3 double mutant (each group was analyzed in 6 biological repetitions; *** P<0.001, Student’s t-test).
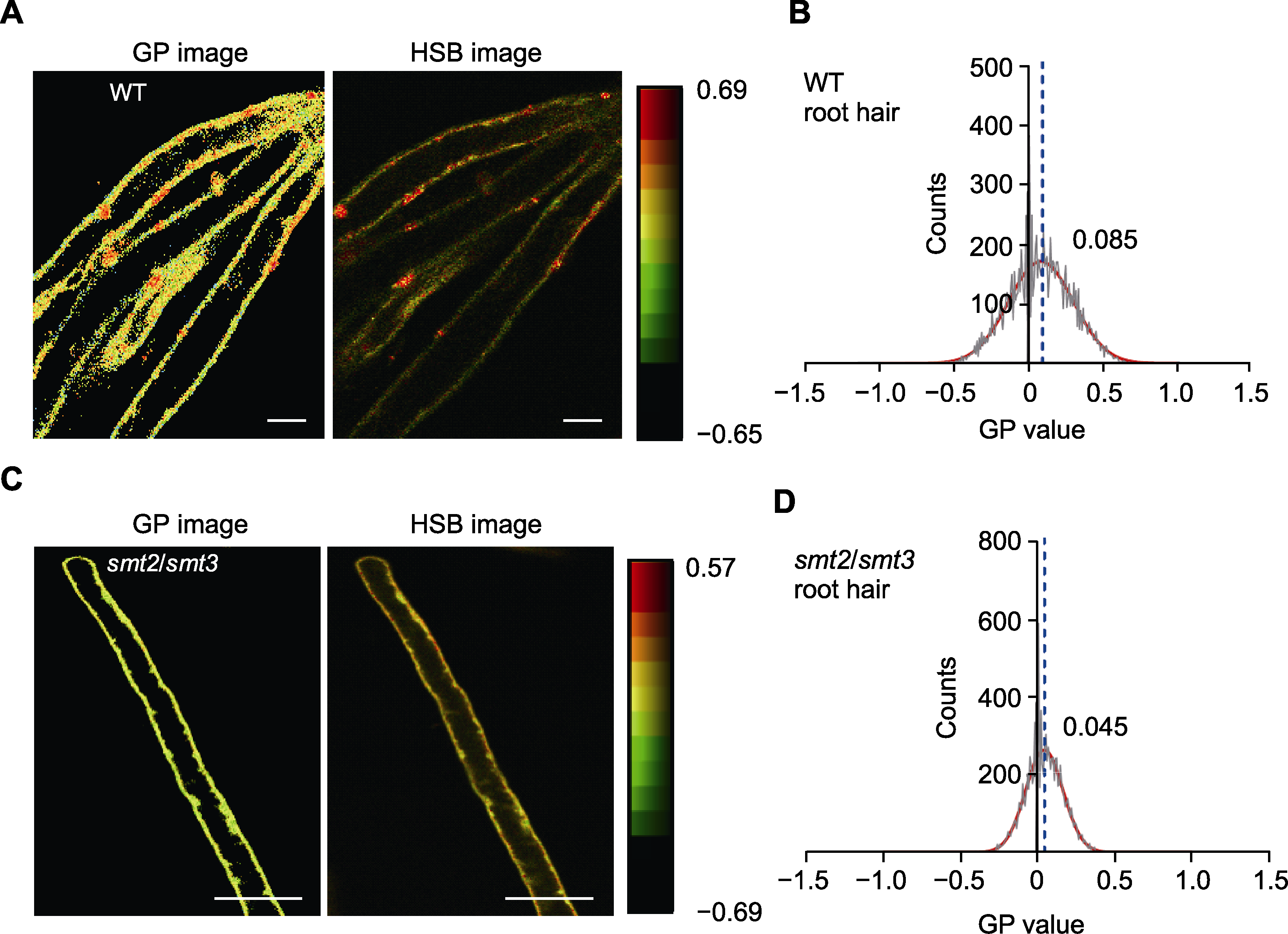
图5 野生型拟南芥和smt2/smt3双突变体根毛细胞质膜的有序度分析 (A) 野生型拟南芥根毛细胞GP和HSB荧光图像(bars=20 μm); (B) 野生型拟南芥根毛细胞质膜的GP值分布, 每组统计3000多个像素; (C) smt2/smt3突变体根毛细胞的GP和HSB荧光图像(bars=15 μm); (D) smt2/smt3突变体根毛细胞质膜的GP值分布。(B)和(D)中红色曲线为GP值分布的高斯拟合曲线; 黑色曲线代表质膜的GP值分布情况; 不同样品中质膜GP峰值标注于曲线顶部右侧; 蓝色虚线指示GP峰值的位置。
Figure 5 Quantitative analysis of the membrane order of root hair cells in wild type (WT) Arabidopsis and smt2/smt3 double mutant (A) The GP and HSB images of root hair cells in WT Arabidopsis (bars=20 μm); (B) Distribution of the GP values of the root hair cell membranes in WT Arabidopsis, more than 3000 pixels were counted for each group; (C) The GP and HSB images of root hair cells in the smt2/smt3 double mutant (bars=15 μm); (D) Distribution of the GP values of the root hair cell membranes in the smt2/smt3 double mutant. The red curves in (B) and (D) represent the Gaussian fitting curves for the distributions of the GP values; The black curves represent the distributions of the membrane GP values; The numbers indicate the GP peak values at the top right of each curve; The blue dotted lines indicate the locations of the GP peaks.
| [1] |
Brown DA (1992). Interactions between GPI-anchored proteins and membrane lipids. Trends Cell Biol 2, 338-343.
PMID |
| [2] |
Brown DA, London E (2000). Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem 275, 17221-17224.
DOI PMID |
| [3] | Carland F, Fujioka S, Nelson T (2010). The sterol methyltransferases SMT1, SMT2, and SMT3 influence Arabidopsis development through nonbrassinosteroid products. Plant Physiol 153, 741-756. |
| [4] |
Carland FM, Fujioka S, Takatsuto S, Yoshida S, Nelson T (2002). The identification of CVP1 reveals a role for sterols in vascular patterning. Plant Cell 14, 2045-2058.
DOI PMID |
| [5] | Chiantia S, Ries J, Kahya N, Schwille P (2006). Combined AFM and two-focus SFCS study of raft-exhibiting model membranes. Chem Phys Chem 7, 2409-2418. |
| [6] | Dong ZY, Song CW, Cui YN, Yu M, Li RL, Lin JX (2019). Structural models of membrane microdomains and sterol imaging technology. J Chin Electron Microsc Soc 38, 542-549. (in Chinese) |
| 董紫怡, 宋程威, 崔亚宁, 玉猛, 李瑞丽, 林金星 (2019). 膜微区相关结构模型及甾醇成像技术的研究进展. 电子显微学报 38, 542-549. | |
| [7] |
Filippov A, Orädd G, Lindblom G (2003). The effect of cholesterol on the lateral diffusion of phospholipids in oriented bilayers. Biophys J 84, 3079-3086.
PMID |
| [8] | Gerke V, Gavins FNE, Geisow M, Grewal T, Jaiswal JK, Nylandsted J, Rescher U (2024). Annexins—a family of proteins with distinctive tastes for cell signaling and mem-brane dynamics. Nat Commun 15, 1574. |
| [9] |
Goksu EI, Vanegas JM, Blanchette CD, Lin WC, Longo ML (2009). AFM for structure and dynamics of biomembranes. Biochim Biophys Acta (BBA) Biomembr 1788,254-266.
DOI PMID |
| [10] |
Hoppe T, Rape M, Jentsch S (2001). Membrane-bound transcription factors: regulated release by RIP or RUP. Curr Opin Cell Biol 13, 344-348.
PMID |
| [11] | Huang XH, Liu W, Tian SP, Chen T (2023). Advances in the regulation of protein liquid-liquid phase separation on development and stress responses in plants. Chin Bull Bot 58, 946-955. (in Chinese) |
|
黄鑫华, 刘伟, 田世平, 陈彤 (2023). 蛋白液-液相分离调控植物发育及胁迫应答研究进展. 植物学报 58, 946-955.
DOI |
|
| [12] |
Ivanov S, Harrison MJ (2024). Receptor-associated kinases control the lipid provisioning program in plant-fungal symbiosis. Science 383, 443-448.
DOI PMID |
| [13] |
Jin L, Millard AC, Wuskell JP, Clark HA, Loew LM (2005). Cholesterol-enriched lipid domains can be visualized by di-4-ANEPPDHQ with linear and nonlinear optics. Biophys J 89, L04-L06.
DOI PMID |
| [14] |
Kusumi A, Fujiwara TK, Chadda R, Xie M, Tsunoyama TA, Kalay Z, Kasai RS, Suzuki KGN (2012). Dynamic organizing principles of the plasma membrane that regulate signal transduction: commemorating the fortieth anniversary of singer and nicolson's fluid-mosaic model. Annu Rev Cell Dev Biol 28, 215-250.
DOI PMID |
| [15] |
Lingwood D, Simons K (2010). Lipid rafts as a membrane-organizing principle. Science 327, 46-50.
DOI PMID |
| [16] | Luo PY, Qian HP, Liu Y, Xu CW, Cui YN (2023). Regulation of plasma membrane protein dynamics and its research methods. Chin Bull Bot 58, 590-601. (in Chinese) |
|
罗鹏云, 钱虹萍, 刘艳, 徐昌文, 崔亚宁 (2023). 质膜蛋白动力学的调控及其研究方法. 植物学报 58, 590-601.
DOI |
|
| [17] | Lv XQ, Jin K, Liu JH, Cui SX, Li JH, Du GC, Liu L (2021). Quantitative analysis of membrane ordering of living industrial model microorganisms. China Biotechnol 41, 20-29. (in Chinese) |
| 吕雪芹, 金柯, 刘家恒, 崔世修, 李江华, 堵国成, 刘龙 (2021). 工业模式微生物膜有序性的活细胞定量分析. 中国生物工程杂志 41, 20-29. | |
| [18] |
Mesmin B, Bigay J, Polidori J, Jamecna D, Lacas-Gervais S, Antonny B (2017). Sterol transfer, PI4P consumption, and control of membrane lipid order by endogenous OS- BP. EMBO J 36, 3156-3174.
DOI PMID |
| [19] |
Misteli T (2001). Protein dynamics: implications for nuclear architecture and gene expression. Science 291, 843-847.
DOI PMID |
| [20] |
Munro S (2003). Lipid rafts: elusive or illusive? Cell 115, 377-388.
DOI PMID |
| [21] |
Niko Y, Didier P, Mely Y, Konishi GI, Klymchenko AS (2016). Bright and photostable push-pull pyrene dye visualizes lipid order variation between plasma and intracellular membranes. Sci Rep 6, 18870.
DOI PMID |
| [22] | Niko Y, Kawauchi S, Konishi GI (2013). Solvatochromic pyrene analogues of prodan exhibiting extremely high fluorescence quantum yields in apolar and polar solvents. Chem Eur J 19, 9760-9765. |
| [23] |
Parasassi T, Gratton E, Yu WM, Wilson P, Levi M (1997). Two-photon fluorescence microscopy of laurdan generalized polarization domains in model and natural membranes. Biophys J 72, 2413-2429.
PMID |
| [24] |
Roche Y, Gerbeau-Pissot P, Buhot B, Thomas D, Bonneau L, Gresti J, Mongrand S, Perrier-Cornet JM, Simon-Plas F (2008). Depletion of phytosterols from the plant plasma membrane provides evidence for disruption of lipid rafts. FASEB J 22, 3980-3991.
DOI PMID |
| [25] |
Sezgin E, Sadowski T, Simons K (2014). Measuring lipid packing of model and cellular membranes with environment sensitive probes. Langmuir 30, 8160-8166.
DOI PMID |
| [26] |
Shaw JE, Epand RF, Epand RM, Li ZG, Bittman R, Yip CM (2006). Correlated fluorescence-atomic force microscopy of membrane domains: structure of fluorescence probes determines lipid localization. Biophys J 90, 2170-2178.
PMID |
| [27] | Simons K, Ikonen E (1997). Functional rafts in cell membranes. Nature 387, 569-572. |
| [28] | Simons K, Toomre D (2000). Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1, 31-39. |
| [29] |
Singer SJ, Nicolson GL (1972). The fluid mosaic model of the structure of cell membranes. Science 175, 720-731.
DOI PMID |
| [30] | Tang L, Li Y, Zhong C, Deng X, Wang XH (2021). Plant sterol clustering correlates with membrane microdomains as revealed by optical and computational microscopy. Mem- branes 11, 747. |
| [31] | Yan X, Xu M, Wang YT, Pan WH, Pan JW, Shou JX, Wang C (2022). Coupling regulation of endocytosis and exocytosis in plants. Chin Bull Bot 57, 375-387. (in Chinese) |
|
严旭, 徐梅, 王玉同, 潘伟槐, 潘建伟, 寿建昕, 王超 (2022). 植物胞吞和胞吐的耦合调控. 植物学报 57, 375-387.
DOI |
|
| [32] |
Zhang L, Xing JJ, Lin JX (2019). At the intersection of exocytosis and endocytosis in plants. New Phytol 224, 1479-1489.
DOI PMID |
| [33] | Zhao XY (2014). Application of di-4-ANEPPDHQ as a Novel Fluorescent Probe for Visualization and Detection of Membrane Microdomains in Living Cells in Arabidopsis thaliana. Master’s thesis. Beijing: Beijing Forestry University. pp. 1-82. (in Chinese) |
| [34] | 赵晓玉 (2014). 新型荧光探针di-4-ANEPPDHQ在拟南芥质膜微区显微成像和定量检测中的应用. 硕士论文. 北京: 北京林业大学. pp. 1-82. |
| [35] | Zuo CS, Liu DY, Xu QJ, Shi WZ, Niu J, Chass GC (2013). Research progress on structure and function of phytosterols. J Henan Sci Technol 32, 211-213. (in Chinese) |
| 左春山, 刘大勇, 徐启杰, 时文中, 牛静, Chass GC (2013). 植物甾醇的结构与功能的研究进展. 河南科技 32, 211-213. |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||