

植物学报 ›› 2023, Vol. 58 ›› Issue (4): 638-655.DOI: 10.11983/CBB22109 cstr: 32102.14.CBB22109
收稿日期:2022-05-25
接受日期:2022-09-19
出版日期:2023-07-01
发布日期:2022-09-27
通讯作者:
*E-mail: haitao-hu@zjnu.cn;guolongbiao@caas.cn
基金资助:Received:2022-05-25
Accepted:2022-09-19
Online:2023-07-01
Published:2022-09-27
Contact:
*E-mail: haitao-hu@zjnu.cn;guolongbiao@caas.cn
摘要: 核黄素是生物体维持正常代谢所必需的辅酶因子FMN和FAD的合成前体, 其在线粒体电子传递链、三羧酸循环、脂肪酸β氧化、支链氨基酸分解代谢、氧化还原稳态、染色质重塑、DNA修复、细胞凋亡和次生代谢产物合成中发挥关键作用。核黄素缺乏会引发机体代谢紊乱和一系列表型缺陷, 严重时甚至导致生物体死亡。自然界生命体中仅微生物和植物可以从头合成核黄素, 而人和动物需从食物中获取核黄素。目前, 微生物中核黄素的合成及其调控机制已研究得比较清晰, 而核黄素在植物体内转运和代谢的调控机制尚不清楚。因此, 挖掘核黄素缺乏相关突变体对解析植物核黄素生物合成、转运和代谢的分子机制以及其对植物生长发育的调控机理具有重要意义。该文综述了核黄素的生物合成途径及其关键限速酶, 重点阐述了核黄素参与的植物生长发育过程, 并展望了植物核黄素的研究前景。
胡海涛, 郭龙彪. 植物核黄素的生物合成及其功能研究进展. 植物学报, 2023, 58(4): 638-655.
Haitao Hu, Longbiao Guo. Progress in the Research on Riboflavin Biosynthesis and Function in Plants. Chinese Bulletin of Botany, 2023, 58(4): 638-655.
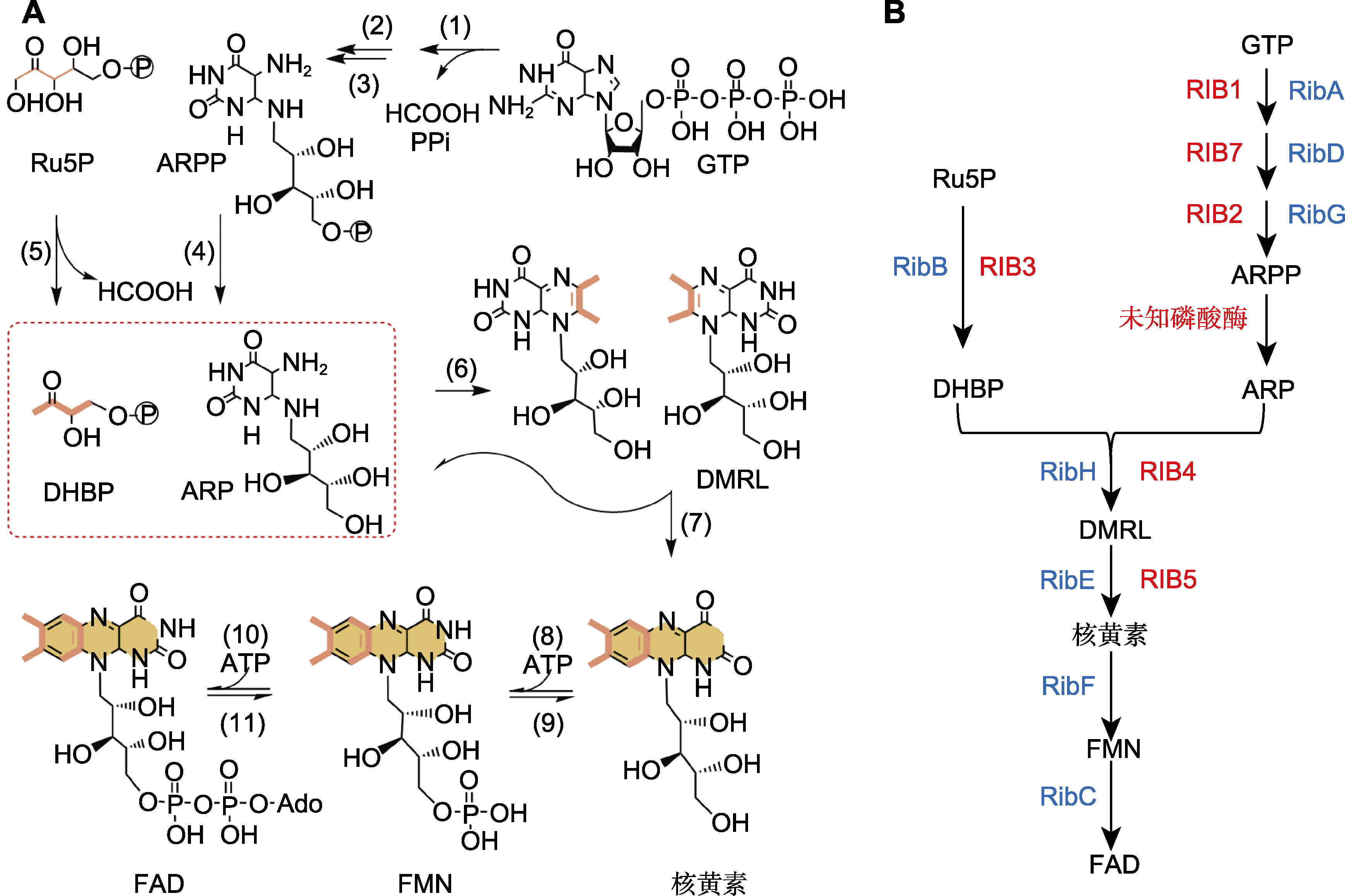
图2 植物和微生物中核黄素的合成途径(Sa et al., 2016; Averianova et al., 2020) (A) 植物中核黄素的合成途径, (1) GTP环水解酶II; (2) 嘧啶脱氨酶; (3) 嘧啶还原酶; (4) 嘧啶磷酸酶; (5) 3,4-二羟基-2-丁酮-4-磷酸合酶; (6) 二氧四氢蝶啶合成酶; (7) 核黄素合成酶; (8) 核黄素激酶; (9) FMN水解酶; (10) FAD合成酶; (11) FAD焦磷酸酶; (B) 枯草芽孢杆菌(蓝色)和棉囊阿舒氏酵母(红色)中核黄素的合成路线。GTP: 鸟苷三磷酸; Ru5P: 核酮糖-5-磷酸; DHBP: 3,4-二羟基- 2-丁酮-4-磷酸; ARPP: 5-氨基-6-核糖醇氨基-2,4(1H,3H)-嘧啶酮-5′-磷酸; ARP: 5-氨基-6-核糖醇氨基-2,4(1H,3H)-嘧啶酮; DMRL: 6,7-二甲基-8-核糖醇基二氧四氢蝶啶; FAD: 黄素腺嘌呤二核苷酸; FMN: 黄素单核苷酸; RibAB: 双功能酶GTP环水解酶II (A)/3,4-二羟基-2-丁酮-4-磷酸合酶(B); RibDG: 双功能嘧啶脱氨酶(D)/嘧啶还原酶(G); RibH: 二氧四氢蝶啶合成酶; RibE: 核黄素合成酶; RibFC: 双功能黄素激酶(F)/FAD合成酶(C); ARPP转化为ARP的磷酸酶尚不清楚; RIB1: GTP环水解酶II; RIB7: 嘧啶还原酶; RIB2: 嘧啶脱氨酶; RIB3: 3,4-二羟基-2-丁酮-4-磷酸合酶合成酶; RIB4: 二氧四氢蝶啶合成酶; RIB5: 核黄素合成酶
Figure 2 Riboflavin biosynthesis pathway in plants and microorganisms (Sa et al., 2016; Averianova et al., 2020) (A) Riboflavin biosynthesis pathway in plants, (1) GTP cyclohydrolase II; (2) 2,5-diamino-6-ribosylamino-4-(3H) pyrimidinone 5′-phosphate deaminase; (3) 5-amino-6-ribosylamino-2,4(1H,3H) pyrimidinedione 5′-phosphate reductase; (4) 5-amino-6- ribitylamino-2,4(1H,3H) pyrimidinedione 5′-phosphate phosphatase; (5) 3,4-dihydroxy-2-butanone 4-phosphate synthase; (6) 6,7-dimethyl-8-ribityllumazine synthase; (7) Riboflavin synthase; (8) Riboflavin kinase; (9) FMN hydrolase; (10) FAD synthase; (11) FAD pyrophosphatase; (B) Enzyme of riboflavin biosynthesis pathway in Bacillus subtilis (blue) and Ashbya gossypii (red). GTP: Guanosine triphosphate; Ru5P: Ribulose 5-phosphate; DHBP: 3,4-dihydroxy-2-butanone 4-phosphate; ARPP: 5-amino- 6-ribitylamino-2,4(1H,3H) pyrimidinedione 5'-phosphate; ARP: 5-amino-6-ribitylamino-2,4(1H,3H) pyrimidinedione; DMRL: 6,7- dimethyl-8-ribityllumazine; FAD: Flavin adenine dinucleotide; FMN: Flavin mononucleotide; RibAB: Bifunctional enzyme GTP cyclohydrolase II(A)/3,4-dihydroxy-2-butanone 4-phosphate synthase (B); RibDG: Bifunctional deaminase(D)/reductase(G); RibH: Lumazine synthase; RibE: Riboflavin synthase; RibFC: Bifunctional flavokinase(F)/FAD synthtase(C); The phosphatase converting ARPP to ARP is unknown; RIB1: GTP cyclohydrolase II; RIB7: 5-amino-6-(5-phosphoribosylamino) uracil reductase; RIB2: 2,5-diamino-6-ribitylamino-4(3H) pyrimidinedione 5′-phosphate; RIB3: 3,4-dihydroxy-2-butanone 4-phosphate synthase synthase; RIB4: Lumazine synthase; RIB5: Riboflavin synthase
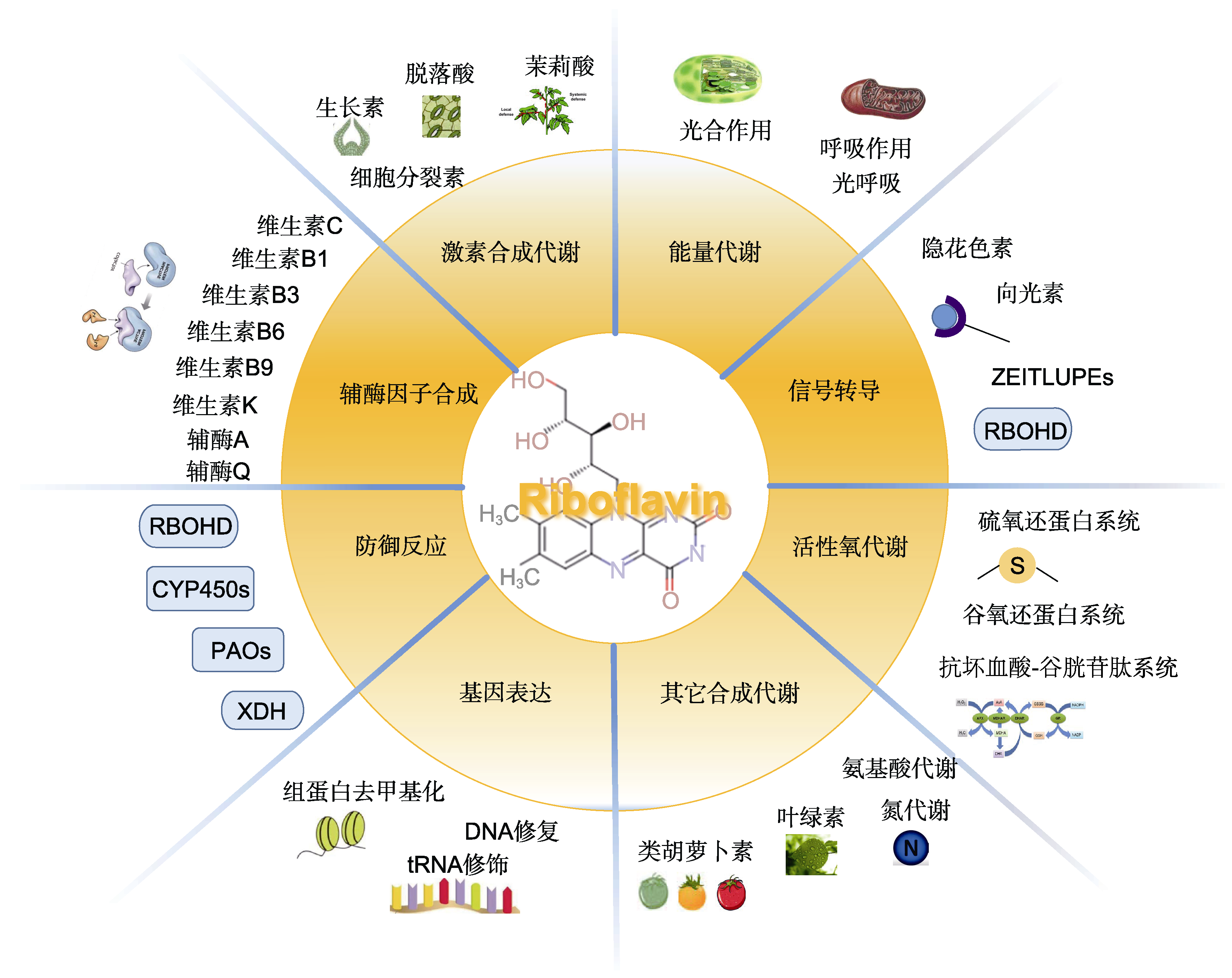
图3 黄素蛋白参与的植物生物学反应 黄素蛋白参与植物的发育、能量代谢、激素合成代谢、信号转导、活性氧代谢、基因表达、防御反应和辅酶因子合成等过程。RBOHD: 呼吸爆发氧化酶同源物D; CYP450s: 细胞色素P450氧化还原酶系统; PAOs: 多胺氧化酶; XDH: 黄嘌呤脱氢酶
Figure 3 Flavin protein are involved in many aspects of plant biology Flavin protein participates in development, energy metabolism, hormone metabolism, signaling, reactive oxygen species metabolism, gene expression, immunity, and cofactor biosynthesis. RBOHD: Respiratory burst oxidase homologue D; CYP450s: Cytochrome P450 oxidoreductase system: PAOs: Polyamine oxidase; XDH: Xanthine dehydrogenase
| 代谢途径 | 黄素酶 | 参考文献 |
|---|---|---|
| 光合电子传递链 | Ferredoxin-NADP氧化还原酶 | Mulo, |
| 线粒体电子传递链 | NADH脱氢酶和琥珀酸脱氢酶 | Rasmusson et al., |
| 三羧酸循环 | 琥珀酸脱氢酶和二氢硫辛酰胺脱氢酶 | Huang and Millar, |
| 脂肪酸氧化 | 脂酰辅酶A脱氢酶 | Pedersen and Henriksen, |
| 脯氨酸分解代谢 | 脯氨酸脱氢酶 | Schertl et al., |
| 赖氨酸分解代谢 | D-2-羟基戊二酸脱氢酶 | Engqvist et al., |
| 四吡咯生物合成 | 原卟啉原IX氧化酶 | Zhang et al., |
| 抗坏血酸合成 | L-半乳糖-1,4-内酯脱氢酶和L-古洛糖-1,4-内酯氧化酶 | Wheeler et al., |
| 抗坏血酸-谷胱甘肽循环 | 单氢抗坏血酸还原酶和谷胱甘肽还原酶 | Liao et al., |
| 类胡萝卜素合成代谢 | 八氢番茄红素脱氢酶、胡萝卜素顺反异构酶、番茄红素环化酶和玉米黄素环氧酶 | Nisar et al., |
| 生长素合成 | YUCCA单氧化酶 | Yamamoto et al., |
| 脱落酸合成 | 醛氧化酶 | Seo et al., |
| 细胞分裂素降解 | 细胞分裂素氧化酶 | Jones and Schreiber, |
| 氮代谢 | 硝酸还原酶和谷氨酸合酶 | Masclaux-Daubresse et al., |
| 组蛋白去甲基化 | 赖氨酸特异性去甲基化酶 | Yang et al., |
表1 植物中黄素酶催化的部分反应
Table 1 Partial reactions catalyzed by flavoenzyme in plants
| 代谢途径 | 黄素酶 | 参考文献 |
|---|---|---|
| 光合电子传递链 | Ferredoxin-NADP氧化还原酶 | Mulo, |
| 线粒体电子传递链 | NADH脱氢酶和琥珀酸脱氢酶 | Rasmusson et al., |
| 三羧酸循环 | 琥珀酸脱氢酶和二氢硫辛酰胺脱氢酶 | Huang and Millar, |
| 脂肪酸氧化 | 脂酰辅酶A脱氢酶 | Pedersen and Henriksen, |
| 脯氨酸分解代谢 | 脯氨酸脱氢酶 | Schertl et al., |
| 赖氨酸分解代谢 | D-2-羟基戊二酸脱氢酶 | Engqvist et al., |
| 四吡咯生物合成 | 原卟啉原IX氧化酶 | Zhang et al., |
| 抗坏血酸合成 | L-半乳糖-1,4-内酯脱氢酶和L-古洛糖-1,4-内酯氧化酶 | Wheeler et al., |
| 抗坏血酸-谷胱甘肽循环 | 单氢抗坏血酸还原酶和谷胱甘肽还原酶 | Liao et al., |
| 类胡萝卜素合成代谢 | 八氢番茄红素脱氢酶、胡萝卜素顺反异构酶、番茄红素环化酶和玉米黄素环氧酶 | Nisar et al., |
| 生长素合成 | YUCCA单氧化酶 | Yamamoto et al., |
| 脱落酸合成 | 醛氧化酶 | Seo et al., |
| 细胞分裂素降解 | 细胞分裂素氧化酶 | Jones and Schreiber, |
| 氮代谢 | 硝酸还原酶和谷氨酸合酶 | Masclaux-Daubresse et al., |
| 组蛋白去甲基化 | 赖氨酸特异性去甲基化酶 | Yang et al., |
| [1] |
朱丽, 钱前 (2019). 虾青素功能米: 生物强化新思路, 优质米培育新资源. 植物学报 54, 4-8.
DOI |
| [2] |
Agrawal GK, Yamazaki M, Kobayashi M, Hirochika R, Miyao A, Hirochika H (2001). Screening of the rice viviparous mutants generated by endogenous retrotransposon Tos17 insertion. Tagging of a zeaxanthin epoxidase gene and a novel OsTATC gene. Plant Physiol 125, 1248-1257.
DOI PMID |
| [3] |
Averianova LA, Balabanova LA, Son OM, Podvolotskaya AB, Tekutyeva LA (2020). Production of vitamin B2 (Riboflavin) by microorganisms: an overview. Front Bioeng Biotechnol 8, 570828.
DOI URL |
| [4] |
Balasubramaniam S, Christodoulou J, Rahman S (2019). Disorders of riboflavin metabolism. J Inherit Metab Dis 42, 608-619.
DOI PMID |
| [5] |
Barrero JM, Rodríguez PL, Quesada V, Alabadí D, Blázquez MA, Boutin JP, Marion-Poll A, Ponce MR, Micol JL (2008). The ABA1 gene and carotenoid biosynthesis are required for late skotomorphogenic growth in Arabidopsis thaliana. Plant Cell Environ 31, 227-234.
PMID |
| [6] |
Blancquaert D, Storozhenko S, Loizeau K, De Steur H, De Brouwer V, Viaene J, Ravanel S, Rébeillé F, Lambert W, Van Der Straeten D (2010). Folates and folic acid: from fundamental research toward sustainable health. Crit Rev Plant Sci 29, 14-35.
DOI URL |
| [7] |
Böttcher C, Chapman A, Fellermeier F, Choudhary M, Scheel D, Glawischnig E (2014). The biosynthetic pathway of indole-3-carbaldehyde and indole-3-carboxylic acid derivatives in Arabidopsis. Plant Physiol 165, 841-853.
DOI URL |
| [8] |
Brito DS, Quinhones CGS, Neri-Silva R, Heinemann B, Schertl P, Cavalcanti JHF, Eubel H, Hildebrandt T, Nunes-Nesi A, Braun HP, Araújo WL (2022). The role of the electron-transfer flavoprotein: ubiquinone oxidoreductase following carbohydrate starvation in Arabidopsis cell cultures. Plant Cell Rep 41, 431-446.
DOI |
| [9] |
Bulley S, Laing W (2016). The regulation of ascorbate biosynthesis. Curr Opin Plant Biol 33, 15-22.
DOI PMID |
| [10] |
Cao X, Yang HL, Shang CQ, Ma S, Liu L, Cheng JL (2019). The roles of auxin biosynthesis YUCCA gene family in plants. Int J Mol Sci 20, 6343.
DOI URL |
| [11] |
Chapman JM, Muhlemann JK, Gayomba SR, Muday GK (2019). RBOH-dependent ROS synthesis and ROS scavenging by plant specialized metabolites to modulate plant development and stress responses. Chem Res Toxicol 32, 370-396.
DOI PMID |
| [12] |
Chen K, Li GJ, Bressan RA, Song CP, Zhu JK, Zhao Y (2020). Abscisic acid dynamics, signaling, and functions in plants. J Integr Plant Biol 62, 25-54.
DOI |
| [13] |
Chen P, Jäger G, Zheng B (2010). Transfer RNA modifications and genes for modifying enzymes in Arabidopsis thaliana. BMC Plant Biol 10, 201.
DOI |
| [14] |
Christie JM, Blackwood L, Petersen J, Sullivan S (2015). Plant flavoprotein photoreceptors. Plant Cell Physiol 56, 401-413.
DOI PMID |
| [15] |
Dai DW, Tong HY, Cheng LJ, Peng F, Zhang TT, Qi WW, Song RT (2019). Maize Dek33 encodes a pyrimidine reductase in riboflavin biosynthesis that is essential for oil- body formation and ABA biosynthesis during seed development. J Exp Bot 70, 5173-5187.
DOI URL |
| [16] |
Delker C, Zolman BK, Miersch O, Wasternack C (2007). Jasmonate biosynthesis in Arabidopsis thaliana requires peroxisomal β-oxidation enzymes—additional proof by properties of pex6 and aim1. Phytochemistry 68, 1642-1650.
DOI URL |
| [17] |
Dellero Y, Jossier M, Schmitz J, Maurino VG, Hodges M (2016). Photorespiratory glycolate-glyoxylate metabolism. J Exp Bot 67, 3041-3052.
DOI PMID |
| [18] |
Demarsy E, Fankhauser C (2009). Higher plants use LOV to perceive blue light. Curr Opin Plant Biol 12, 69-74.
DOI PMID |
| [19] |
Di Salvo ML, Contestabile R, Safo MK (2011). Vitamin B6 salvage enzymes: mechanism, structure and regulation. BBA-Proteins Proteom 1814, 1597-1608.
DOI URL |
| [20] |
Ding HY, Wang BP, Han Y, Li SC (2020). The pivotal function of dehydroascorbate reductase in glutathione homeostasis in plants. J Exp Bot 71, 3405-3416.
DOI PMID |
| [21] |
Engqvist MKM, Kuhn A, Wienstroer J, Weber K, Jansen EEW, Jakobs C, Weber APM, Maurino VG (2011). Plant d-2-hydroxyglutarate dehydrogenase participates in the catabolism of lysine especially during senescence. J Biol Chem 286, 11382-11390.
DOI PMID |
| [22] |
Fang J, Chai CL, Qian Q, Li CL, Tang JY, Sun L, Huang ZJ, Guo XL, Sun CH, Liu M, Zhang Y, Lu QT, Wang YQ, Lu CM, Han B, Chen F, Cheng ZK, Chu CC (2008). Mutations of genes in synthesis of the carotenoid precursors of ABA lead to pre-harvest sprouting and photo- oxidation in rice. Plant J 54, 177-189.
DOI URL |
| [23] |
Fatihi A, Latimer S, Schmollinger S, Block A, Dussault PH, Vermaas WFJ, Merchant SS, Basset GJ (2015). A dedicated type II NADPH dehydrogenase performs the penultimate step in the biosynthesis of Vitamin K1 in Synechocystis and Arabidopsis. Plant Cell 27, 1730-1741.
DOI URL |
| [24] |
Fernie AR, Carrari F, Sweetlove LJ (2004). Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Curr Opin Plant Biol 7, 254-261.
DOI PMID |
| [25] |
Grabsztunowicz M, Rantala M, Ivanauskaite A, Blomster T, Koskela MM, Vuorinen K, Tyystjärvi E, Burow M, Overmyer K, Mähönen AP, Mulo P (2021). Root-type ferredoxin-NADP+ oxidoreductase isoforms in Arabidopsis thaliana: expression patterns, location and stress responses. Plant Cell Environ 44, 548-558.
DOI URL |
| [26] |
Hall M (2020). Flavoenzymes for biocatalysis. Enzymes 47, 37-62.
DOI PMID |
| [27] |
Han ML, Lv QY, Zhang J, Wang T, Zhang CX, Tan RJ, Wang YL, Zhong LY, Gao YQ, Chao ZF, Li QQ, Chen GY, Shi Z, Lin HX, Chao DY (2022). Decreasing nitrogen assimilation under drought stress by suppressing DST- mediated activation of Nitrate reductase 1.2 in rice. Mol Plant 15, 167-178.
DOI URL |
| [28] |
Han RC, He XF, Pan XH, Shi QH, Wu ZM (2020). Enhancing xanthine dehydrogenase activity is an effective way to delay leaf senescence and increase rice yield. Rice 13, 16.
DOI PMID |
| [29] |
Hanson AD, Gregory III JF (2011). Folate biosynthesis, turnover, and transport in plants. Annu Rev Plant Biol 62, 105-125.
DOI PMID |
| [30] |
Hao JF, Pétriacq P, De Bont L, Hodges M, Gakière B (2018). Characterization of L-aspartate oxidase from Arabidopsis thaliana. Plant Sci 271, 133-142.
DOI URL |
| [31] |
Hashida SN, Takahashi H, Uchimiya H (2009). The role of NAD biosynthesis in plant development and stress responses. Ann Bot 103, 819-824.
DOI URL |
| [32] |
He KX, Cao XF, Deng X (2021). Histone methylation in epigenetic regulation and temperature responses. Curr Opin Plant Biol 61, 102001.
DOI URL |
| [33] |
Hedtke B, Alawady A, Albacete A, Kobayashi K, Melzer M, Roitsch T, Masuda T, Grimm B (2012). Deficiency in riboflavin biosynthesis affects tetrapyrrole biosynthesis in etiolated Arabidopsis tissue. Plant Mol Biol 78, 77-93.
DOI PMID |
| [34] |
Herrero S, González E, Gillikin JW, Vélëz H, Daub ME (2011). Identification and characterization of a pyridoxal reductase involved in the vitamin B6 salvage pathway in Arabidopsis. Plant Mol Biol 76, 157-169.
DOI PMID |
| [35] |
Higuchi-Takeuchi M, Ichikawa T, Kondou Y, Matsui K, Hasegawa Y, Kawashima M, Sonoike K, Mori M, Hirochika H, Matsui M (2011). Functional analysis of two isoforms of leaf-type ferredoxin-NADP+-oxidoreductase in rice using the heterologous expression system of Arabidopsis. Plant Physiol 157, 96-108.
DOI PMID |
| [36] |
Hino S, Sakamoto A, Nagaoka K, Anan K, Wang YQ, Mimasu S, Umehara T, Yokoyama S, Kosai KI, Nakao M (2012). FAD-dependent lysine-specific demethylase-1 regulates cellular energy expenditure. Nat Commun 3, 758.
DOI PMID |
| [37] |
Hu HT, Ren DY, Hu J, Jiang HZ, Chen P, Zeng DL, Qian Q, Guo LB (2021). WHITE AND LESION-MIMIC LEAF1, encoding a lumazine synthase, affects reactive oxygen species balance and chloroplast development in rice. Plant J 108, 1690-1703.
DOI URL |
| [38] |
Huang SB, Millar AH (2013). Succinate dehydrogenase: the complex roles of a simple enzyme. Curr Opin Plant Biol 16, 344-349.
DOI PMID |
| [39] |
Hwang OJ, Back K (2021). Suppression of rice cryptochrome 1b decreases both melatonin and expression of brassinosteroid biosynthetic genes resulting in salt tolerance. Molecules 26, 1075.
DOI URL |
| [40] |
Ishizaki K, Schauer N, Larson TR, Graham IA, Fernie AR, Leaver CJ (2006). The mitochondrial electron transfer flavoprotein complex is essential for survival of Arabidopsis in extended darkness. Plant J 47, 751-760.
DOI URL |
| [41] |
Jiang DH, Yang WN, He YH, Amasino RM (2007). Arabidopsis relatives of the human lysine-specific demethylase1 repress the expression of FWA and FLOWERING LOCUS C and thus promote the floral transition. Plant Cell 19, 2975-2987.
DOI URL |
| [42] |
Jones RJ, Schreiber BMN (1997). Role and function of cytokinin oxidase in plants. Plant Growth Regul 23, 123-134.
DOI URL |
| [43] | Joosten V, Van Berkel WJH (2007). Flavoenzymes. Curr O- pin Chem Biol 11, 195-202. |
| [44] |
Jurca M, Sjölander J, Ibáñez C, Matrosova A, Johansson M, Kozarewa I, Takata N, Bakó L, Webb AAR, Israelsson-Nordström M, Eriksson ME (2022). ZEITLUPE promotes ABA-induced stomatal closure in Arabidopsis and Populus. Front Plant Sci 13, 829121.
DOI URL |
| [45] |
Kampire MG, Sanglou RK, Wang HM, Kazeem BB, Wu JL, Zhang XB (2021). A novel allele encoding 7-hydroxymethyl chlorophyll a reductase confers bacterial blight resistance in rice. Int J Mol Sci 22, 7585.
DOI URL |
| [46] |
Kang ZH, Qin T, Zhao ZP (2019). Thioredoxins and thioredoxin reductase in chloroplasts: a review. Gene 706, 32-42.
DOI PMID |
| [47] |
Kong WW, Li J, Yu QY, Cang W, Xu R, Wang Y, Ji W (2016). Two novel flavin-containing monooxygenases involved in biosynthesis of aliphatic glucosinolates. Front Plant Sci 7, 1292.
DOI PMID |
| [48] |
Konishi N, Ishiyama K, Matsuoka K, Maru I, Hayakawa T, Yamaya T, Kojima S (2014). NADH-dependent glutamate synthase plays a crucial role in assimilating ammonium in the Arabidopsis root. Physiol Plant 152, 138-151.
DOI URL |
| [49] | Kramer M, Rodriguez-Heredia M, Saccon F, Mosebach L, Twachtmann M, Krieger-Liszkay A, Duffy C, Knell RJ, Finazzi G, Hanke GT (2021). Regulation of photosynthetic electron flow on dark to light transition by ferredoxin:NADP(H) oxidoreductase interactions. eLife 10, e56-088. |
| [50] |
Kumar R, Wallis JG, Skidmore C, Browse J (2006). A mutation in Arabidopsis cytochrome b5 reductase identified by high-throughput screening differentially affects hydroxylation and desaturation. Plant J 48, 920-932.
DOI URL |
| [51] |
Kupke T, Hernández-Acosta P, Steinbacher S, Culiáñez- Macià FA (2001). Arabidopsis thaliana flavoprotein AtHAL3a catalyzes the decarboxylation of 4'-phosphopantothenoylcysteine to 4'-phosphopantetheine, a key step in coenzyme A biosynthesis. J Biol Chem 276, 19190-19196.
DOI PMID |
| [52] |
Lan F, Nottke AC, Shi Y (2008). Mechanisms involved in the regulation of histone lysine demethylases. Curr Opin Cell Biol 20, 316-325.
DOI PMID |
| [53] |
Lermontova I, Grimm B (2006). Reduced activity of plastid protoporphyrinogen oxidase causes attenuated photodynamic damage during high-light compared to low-light exposure. Plant J 48, 499-510.
PMID |
| [54] |
Li C, Wang X, Zhang LY, Zhang CY, Yu CS, Zhao T, Liu B, Li HY, Liu J (2022). OsBIC1 directly interacts with OsCRYs to regulate leaf sheath length through mediating GA-responsive pathway. Int J Mol Sci 23, 287.
DOI URL |
| [55] |
Li SY, Du L, Bernhardt R (2020). Redox partners: function modulators of bacterial P450 enzymes. Trends Microbiol 28, 445-454.
DOI PMID |
| [56] |
Liao GL, Chen L, He YQ, Li XS, Lv ZX, Yi SY, Zhong M, Huang CH, Jia DF, Qu XY, Xu XB (2021). Three metabolic pathways are responsible for the accumulation and maintenance of high AsA content in kiwifruit (Actinidia eriantha). BMC Genom 22, 13.
DOI |
| [57] | Liscombe DK, Kamiyoshihara Y, Ghironzi J, Kempthorne CJ, Hooton K, Bulot B, Kanellis V, McNulty J, Lam NB, Nadeau LF, Pautler M, Tieman DM, Klee HJ, Goulet C (2022). A flavin-dependent monooxygenase produces nitrogenous tomato aroma volatiles using cysteine as a nitrogen source. Proc Natl Acad Sci USA 119, e2118676119. |
| [58] |
Liu S, Hu WY, Wang ZW, Chen T (2020). Production of riboflavin and related cofactors by biotechnological processes. Microb Cell Fact 19, 31.
DOI PMID |
| [59] |
Liu XJ, Hu B, Chu CC (2022). Nitrogen assimilation in plants: current status and future prospects. J Genet Genomics 49, 394-404.
DOI URL |
| [60] |
Liu YH, Yu L, Tong JH, Ding JH, Wang RZ, Lu YS, Xiao LT (2013). Tiller number is altered in the ascorbic acid- deficient rice suppressed for L-galactono-1,4-lactone dehydrogenase. J Plant Physiol 170, 389-396.
DOI URL |
| [61] |
Maoka T (2020). Carotenoids as natural functional pigments. J Nat Med 74, 1-16.
DOI |
| [62] |
Marty L, Bausewein D, Müller C, Bangash SAK, Moseler A, Schwarzländer M, Müller-Schüssele SJ, Zechmann B, Riondet C, Balk J, Wirtz M, Hell R, Reichheld JP, Meyer AJ (2019). Arabidopsis glutathione reductase 2 is indispensable in plastids, while mitochondrial glutathione is safeguarded by additional reduction and transport systems. New Phytol 224, 1569-1584.
DOI URL |
| [63] |
Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A (2010). Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann Bot 105, 1141-1157.
DOI URL |
| [64] |
Meguro M, Ito H, Takabayashi A, Tanaka R, Tanaka A (2011). Identification of the 7-hydroxymethyl chlorophyll a reductase of the chlorophyll cycle in Arabidopsis. Plant Cell 23, 3442-3453.
DOI URL |
| [65] |
Meyer Y, Belin C, Delorme-Hinoux V, Reichheld JP, Riondet C (2012). Thioredoxin and glutaredoxin systems in plants: molecular mechanisms, crosstalks, and functional significance. Antioxid Redox Signal 17, 1124-1160.
DOI URL |
| [66] | Miyazaki Y, Takase T, Kiyosue T (2015). ZEITLUPE positively regulates hypocotyl elongation at warm temperature under light in Arabidopsis thaliana. Plant Signal Behav 10, e998540. |
| [67] |
Mizutani M, Sato F (2011). Unusual P450 reactions in plant secondary metabolism. Arch Biochem Biophys 507, 194-203.
DOI PMID |
| [68] |
Möglich A, Yang XJ, Ayers RA, Moffat K (2010). Structure and function of plant photoreceptors. Annu Rev Plant Biol 61, 21-47.
DOI PMID |
| [69] |
Mosegaard S, Dipace G, Bross P, Carlsen J, Gregersen N, Olsen RKJ (2020). Riboflavin deficiency-implications for general human health and inborn errors of metabolism. Int J Mol Sci 21, 3847.
DOI URL |
| [70] |
Mulo P (2011). Chloroplast-targeted ferredoxin-NADP+ oxidoreductase (FNR): structure, function and location. BBA- Bioenergetics 1807, 927-934.
DOI URL |
| [71] |
Nisar N, Li L, Lu S, Khin NC, Pogson B (2015). Carotenoid metabolism in plants. Mol Plant 8, 68-82.
DOI PMID |
| [72] |
Niu GQ, Zhao S, Wang L, Dong W, Liu L, He YK (2017). Structure of the Arabidopsis thaliana NADPH-cytochrome P450 reductase 2 (ATR2) provides insight into its function. FEBS J 284, 754-765.
DOI URL |
| [73] | Noh SW, Seo RR, Park HJ, Jung HW (2021). Two Arabidopsis homologs of human lysine-specific demethylase function in epigenetic regulation of plant defense responses. Front Plant Sci 12, 688003. |
| [74] |
Oh YJ, Kim H, Seo SH, Hwang BG, Chang YS, Lee J, Lee DW, Sohn EJ, Lee SJ, Lee Y, Hwang I (2016). Cytochrome b5 reductase 1 triggers serial reactions that lead to iron uptake in plants. Mol Plant 9, 501-513.
DOI PMID |
| [75] |
Pandian BA, Sathishraj R, Djanaguiraman M, Prasad PVV, Jugulam M (2020). Role of cytochrome P450 enzymes in plant stress response. Antioxidants (Basel) 9, 454.
DOI URL |
| [76] |
Patel MS, Nemeria NS, Furey W, Jordan F (2014). The pyruvate dehydrogenase complexes: structure-based func- tion and regulation. J Biol Chem 289, 16615-16623.
DOI URL |
| [77] |
Pedersen L, Henriksen A (2005). Acyl-CoA oxidase 1 from Arabidopsis thaliana. Structure of a key enzyme in plant lipid metabolism. J Mol Biol 345, 487-500.
PMID |
| [78] | Piao WL, Han SH, Sakuraba Y, Paek NC (2017). Rice 7-hydroxymethyl chlorophyll a reductase is involved in the promotion of chlorophyll degradation and modulates cell death signaling. Mol Cells 40, 773-786. |
| [79] |
Plantone D, Pardini M, Rinaldi G (2021). Riboflavin in neurological diseases: a narrative review. Clin Drug Investig 41, 513-527.
DOI |
| [80] |
Rasmusson AG, Geisler DA, Møller IM (2008). The multiplicity of dehydrogenases in the electron transport chain of plant mitochondria. Mitochondrion 8, 47-60.
DOI PMID |
| [81] | Rébeillé F, Ravanel S, Jabrin S, Douce R, Storozhenko S, Van Der Straeten DVD (2006). Folates in plants: biosynthesis, distribution, and enhancement. Physiol Plant 126, 330-342. |
| [82] |
Rong CY, Liu YX, Chang ZY, Liu ZY, Ding YF, Ding CQ (2022). Cytokinin oxidase/dehydrogenase family genes exhibit functional divergence and overlap in rice growth and development, especially in control of tillering. J Exp Bot 73, 3552-3568.
DOI PMID |
| [83] |
Ruan JJ, Zhou YX, Zhou ML, Yan J, Khurshid M, Weng WF, Cheng JP, Zhang KX (2019). Jasmonic acid signaling pathway in plants. Int J Mol Sci 20, 2479.
DOI URL |
| [84] |
Sa N, Rawat R, Thornburg C, Walker KD, Roje S (2016). Identification and characterization of the missing phosphatase on the riboflavin biosynthesis pathway in Arabidopsis thaliana. Plant J 88, 705-716.
DOI URL |
| [85] |
Sagi M, Fluhr R (2006). Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol 141, 336-340.
DOI PMID |
| [86] |
Schertl P, Cabassa C, Saadallah K, Bordenave M, Savouré A, Braun HP (2014). Biochemical characterization of proline dehydrogenase in Arabidopsis mitochondria. FEBS J 281, 2794-2804.
DOI PMID |
| [87] |
Schilmiller AL, Koo AJK, Howe GA (2007). Functional diversification of acyl-coenzyme A oxidases in jasmonic acid biosynthesis and action. Plant Physiol 143, 812-824.
DOI PMID |
| [88] |
Schlaich NL (2007). Flavin-containing monooxygenases in plants: looking beyond detox. Trends Plant Sci 12, 412-418.
PMID |
| [89] |
Schmülling T, Werner T, Riefler M, Krupková E, Manns IBY (2003). Structure and function of cytokinin oxidase/ dehydrogenase genes of maize, rice, Arabidopsis and other species. J Plant Res 116, 241-252.
DOI PMID |
| [90] |
Schwechheimer SK, Park EY, Revuelta JL, Becker J, Wittmann C (2016). Biotechnology of riboflavin. Appl Microbiol Biotechnol 100, 2107-2119.
DOI PMID |
| [91] |
Seo M, Koiwai H, Akaba S, Komano T, Oritani T, Kamiya Y, Koshiba T (2000a). Abscisic aldehyde oxidase in leaves of Arabidopsis thaliana. Plant J 23, 481-488.
DOI URL |
| [92] |
Seo M, Peeters AJM, Koiwai H, Oritani T, Marion-Poll A, Zeevaart JAD, Koornneef M, Kamiya Y, Koshiba T (2000b). The Arabidopsis aldehyde oxidase 3 (AAO3) gene product catalyzes the final step in abscisic acid biosynthesis in leaves. Proc Natl Acad Sci USA 97, 12908-12913.
DOI URL |
| [93] |
Shen SQ, Peng M, Fang H, Wang ZX, Zhou S, Jing XY, Zhang M, Yang CK, Guo H, Li YF, Lei L, Shi YH, Sun YY, Liu XQ, Xu CP, Tohge T, Yuan M, Fernie AR, Ning YS, Wang GL, Luo J (2021). An Oryza-specific hydroxycinnamoyl tyramine gene cluster contributes to enhanced disease resistance. Sci Bull 66, 2369-2380.
DOI URL |
| [94] |
Shibaya T, Hori K, Ogiso-Tanaka E, Yamanouchi U, Shu K, Kitazawa N, Shomura A, Ando T, Ebana K, Wu JZ, Yamazaki T, Yano M (2016). Hd18, encoding histone acetylase related to Arabidopsis FLOWERING LOCUS D, is involved in the control of flowering time in rice. Plant Cell Physiol 57, 1828-1838.
DOI PMID |
| [95] |
Smith EN, Schwarzländer M, Ratcliffe RG, Kruger NJ (2021). Shining a light on NAD- and NADP-based metabolism in plants. Trends Plant Sci 26, 1072-1086.
DOI PMID |
| [96] |
Sundin L, Vanholme R, Geerinck J, Goeminne G, Höfer R, Kim H, Ralph J, Boerjan W (2014). Mutation of the inducible ARABIDOPSIS THALIANA CYTOCHROME P450 REDUCTASE 2 alters lignin composition and improves saccharification. Plant Physiol 166, 1956-1971.
DOI PMID |
| [97] |
Suwannasom N, Kao I, Pruß A, Georgieva R, Bäumler H (2020). Riboflavin: the health benefits of a forgotten natural vitamin. Int J Mol Sci 21, 950.
DOI URL |
| [98] |
Tanaka R, Tanaka A (2007). Tetrapyrrole biosynthesis in higher plants. Annu Rev Plant Biol 58, 321-346.
PMID |
| [99] |
Tani T, Sobajima H, Okada K, Chujo T, Arimura SI, Tsutsumi N, Nishimura M, Seto H, Nojiri H, Yamane H (2008). Identification of the OsOPR7 gene encoding 12- oxophytodienoate reductase involved in the biosynthesis of jasmonic acid in rice. Planta 227, 517-526.
DOI URL |
| [100] |
Thodberg S, Neilson EHJ (2020). The “Green” FMOs: diversity, functionality and application of plant flavoproteins. Catalysts 10, 329.
DOI URL |
| [101] |
Tian QZ, Wang G, Ma XX, Shen QW, Ding ML, Yang XY, Luo XL, Li RR, Wang ZH, Wang XY, Fu ZY, Yang QH, Tang JH, Wang GF (2022). Riboflavin integrates cellular energetics and cell cycle to regulate maize seed development. Plant Biotechnol J 20, 1487-1501.
DOI URL |
| [102] |
Tian YS, Xu J, Wang B, Fu XY, Gao JJ, Han HJ, Li ZJ, Wang LJ, Zhang FJ, Zhang WH, Deng YD, Wang Y, Peng RH, Yao QH (2021). Riboflavin fortification of rice endosperm by metabolic engineering. Plant Biotechnol J 19, 1483-1485.
DOI URL |
| [103] |
Tu B, Zhang T, Wang SG, Zhou L, Zheng L, Zhang C, Li XZ, Zhang XY, Yin JJ, Zhu XB, Yuan H, Li T, Chen WL, Qin P, Ma BT, Wang YP, Li SG (2022). Loss of Gn1a/ OsCKX2 confers heavy-panicle rice with excellent lodging resistance. J Integr Plant Biol 64, 23-38.
DOI URL |
| [104] |
Vercellino I, Sazanov LA (2022). The assembly, regulation and function of the mitochondrial respiratory chain. Nat Rev Mol Cell Biol 23, 141-161.
DOI |
| [105] |
Vorbach C, Harrison R, Capecchi MR (2003). Xanthine oxidoreductase is central to the evolution and function of the innate immune system. Trends Immunol 24, 512-517.
PMID |
| [106] |
Wang Q, Lin CT (2020). Mechanisms of cryptochrome- mediated photoresponses in plants. Annu Rev Plant Biol 71, 103-129.
DOI PMID |
| [107] |
Wang RY, He F, Ning YS, Wang GL (2020). Fine-tuning of RBOH-mediated ROS signaling in plant immunity. Trends Plant Sci 25, 1060-1062.
DOI PMID |
| [108] |
Wang X, Jiang BC, Gu LF, Chen YD, Mora M, Zhu MLM, Noory E, Wang Q, Lin CT (2021). A photoregulatory mechanism of the circadian clock in Arabidopsis. Nat Plants 7, 1397-1408.
DOI PMID |
| [109] |
Wayne LL, Wallis JG, Kumar R, Markham JE, Browse J (2013). Cytochrome b5 reductase encoded by CBR1 is essential for a functional male gametophyte in Arabidopsis. Plant Cell 25, 3052-3066.
DOI URL |
| [110] |
Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T (2003). Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15, 2532-2550.
DOI PMID |
| [111] |
Wheeler GL, Jones MA, Smirnoff N (1998). The biosynthetic pathway of vitamin C in higher plants. Nature 393, 365-369.
DOI URL |
| [112] | Wu J, Kamanga BM, Zhang WY, Xu YH, Xu L (2022). Research progress of aldehyde oxidases in plants. PeerJ 10, e13119. |
| [113] |
Wu ZY, Ren H, Xiong WD, Roje S, Liu YC, Su KL, Fu CX (2018). Methylenetetrahydrofolate reductase modulates methyl metabolism and lignin monomer methylation in maize. J Exp Bot 69, 3963-3973.
DOI PMID |
| [114] |
Xiao S, Dai LY, Liu FQ, Wang ZL, Peng W, Xie DX (2004). COS1: an Arabidopsis coronatine insensitive 1 suppressor essential for regulation of jasmonate-mediated plant defense and senescence. Plant Cell 16, 1132-1142.
PMID |
| [115] | Xu JJ, Zhang XF, Jiang Y, Fan H, Li JX, Li CY, Zhao Q, Yang L, Hu YH, Martin C, Chen XY (2021). A unique flavoenzyme operates in ubiquinone biosynthesis in photosynthesis-related eukaryotes. Sci Adv 7, eabl3594. |
| [116] |
Yamamoto Y, Kamiya N, Morinaka Y, Matsuoka M, Sazuka T (2007). Auxin biosynthesis by the YUCCA genes in rice. Plant Physiol 143, 1362-1371.
DOI PMID |
| [117] |
Yamauchi T, Yoshioka M, Fukazawa A, Mori H, Nishizawa NK, Tsutsumi N, Yoshioka H, Nakazono M (2017). An NADPH oxidase RBOH functions in rice roots during lysigenous aerenchyma formation under oxygen- deficient conditions. Plant Cell 29, 775-790.
DOI URL |
| [118] |
Yamaya T, Kusano M (2014). Evidence supporting distinct functions of three cytosolic glutamine synthetases and two NADH-glutamate synthases in rice. J Exp Bot 65, 5519-5525.
DOI PMID |
| [119] |
Yang H, Li YF, Cao YW, Shi WQ, Xie E, Mu N, Du GJ, Shen Y, Tang D, Cheng ZK (2022). Nitrogen nutrition contributes to plant fertility by affecting meiosis initiation. Nat Commun 13, 485.
DOI PMID |
| [120] |
Yang WN, Jiang DH, Jiang JF, He YH (2010). A plant- specific histone H3 lysine 4 demethylase represses the floral transition in Arabidopsis. Plant J 62, 663-673.
DOI URL |
| [121] | Yokochi Y, Fukushi Y, Wakabayashi KI, Yoshida K, Hisabori T (2021). Oxidative regulation of chloroplast enzymes by thioredoxin and thioredoxin-like proteins in Arabidopsis thaliana. Proc Natl Acad Sci USA 118, e2114952118. |
| [122] |
Yoshimoto N, Onuma M, Mizuno S, Sugino Y, Nakabayashi R, Imai S, Tsuneyoshi T, Sumi SI, Saito K (2015). Identification of a flavin-containing S-oxygenating monooxygenase involved in alliin biosynthesis in garlic. Plant J 83, 941-951.
DOI URL |
| [123] |
Yu Z, Jia DY, Liu TB (2019). Polyamine oxidases play various roles in plant development and abiotic stress tolerance. Plants(Basel) 8, 184.
DOI URL |
| [124] |
Zeng DD, Qin R, Li M, Alamin M, Jin XL, Liu Y, Shi CH (2017). The ferredoxin-dependent glutamate synthase (OsFd-GOGAT) participates in leaf senescence and the nitrogen remobilization in rice. Mol Genet Genom 292, 385-395.
DOI |
| [125] |
Zhang F, Tang WJ, Hedtke B, Zhong LL, Liu L, Peng LW, Lu CM, Grimm B, Lin RC (2014). Tetrapyrrole biosynthetic enzyme protoporphyrinogen IX oxidase 1 is required for plastid RNA editing. Proc Natl Acad Sci USA 111, 2023-2028.
DOI PMID |
| [126] |
Zhang JR, Ge YY, Liu PH, Wu DT, Liu HY, Li HB, Corke H, Gan RY (2022). Biotechnological strategies of riboflavin biosynthesis in microbes. Engineering 12, 115-127.
DOI URL |
| [127] | Zhao D, Wang H, Li ZY, Han SN, Han C, Liu AX (2022). LC_Glucose-inhibited division protein is required for motility, biofilm formation, and stress response in Lysobacter capsici X2-3. Front Microbiol 13, 840792. |
| [128] |
Zhao YD, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J (2001). A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291, 306-309.
DOI PMID |
| [1] | 惠城阳, 章巧依, 刘腾腾, 刘维勇, 周丽娜, 金鑫杰, 张永华, 刘金亮. 温州大罗山主要植被类型及物种组成特征[J]. 植物生态学报, 2025, 49(植被): 1-. |
| [2] | 曹毅 张松林 王旭峰 杨安昌 任敏慧 杨浩 韩超. 兰州市南北两山植物群落数据集[J]. 植物生态学报, 2025, 49(植被): 1-0. |
| [3] | 陈龙 郭柯 勾晓华 赵秀海 马泓若. 祁连圆柏林群落组成及特征[J]. 植物生态学报, 2025, 49(植被): 0-0. |
| [4] | 童金莲, 张博纳, 汤璐瑶, 叶琳峰, 李姝雯, 谢江波, 李彦, 王忠媛. C4植物狗尾草功能性状网络沿降水梯度带的区域分异规律[J]. 植物生态学报, 2025, 49(预发表): 1-. |
| [5] | 闫小红 胡文海. 亚热带地区3种常绿阔叶植物冬季光保护机制的差异[J]. 植物生态学报, 2025, 49(预发表): 0-0. |
| [6] | 赵常明 熊高明 申国珍 葛结林 徐文婷 徐凯 武元帅 谢宗强. 神农架常绿落叶阔叶混交林和亚高山针叶林植物群落特征数据集[J]. 植物生态学报, 2025, 49(典型生态系统数据集): 0-0. |
| [7] | 赵珮杉 高广磊 丁国栋 张英. 林龄和生态位对樟子松人工林地下真菌群落构建的影响[J]. 植物生态学报, 2025, 49(地上地下生态过程关联): 1-0. |
| [8] | 张子睿, 周静, 胡艳萍, 梁爽, 马永鹏, 陈伟乐. 极度濒危植物巧家五针松的根内和根际真菌群落特征[J]. , 2025, 49(濒危植物的保护与恢复): 0-. |
| [9] | 逯子佳, 王天瑞, 郑斯斯, 孟宏虎, 曹建国, Gregor Kozlowski, 宋以刚. 孑遗植物湖北枫杨的环境适应性遗传变异与遗传脆弱性[J]. , 2025, 49(濒危植物的保护与恢复): 0-. |
| [10] | 黄承玲, 黎荣瀚, 覃红玲, 杨胜雄, 田晓玲, 夏国威, 陈正仁, 周玮. 基于SNP分子标记的极小种群野生植物荔波杜鹃保护遗传学研究[J]. , 2025, 49(濒危植物的保护与恢复): 0-. |
| [11] | 周鑫宇, 刘会良, 高贝, 卢妤婷, 陶玲庆, 文晓虎, 张岚, 张元明. 新疆特有濒危植物雪白睡莲繁殖生物学研究[J]. , 2025, 49(濒危植物的保护与恢复): 0-. |
| [12] | 高雨轩, 苏艳军, 冯育才, 张军, 汪小全, 刘玲莉. 珍稀濒危孑遗植物银杉的研究与保护现状[J]. , 2025, 49(濒危植物的保护与恢复): 0-. |
| [13] | 朱润铖, 蔡锡安, 黄娟. 植物防御相关挥发性有机物排放及对氮沉降的响应[J]. 植物生态学报, 2025, 49(5): 681-696. |
| [14] | 贾妍妍, 柳华清, 解欣然, 王博, 张维, 杨允菲. 珍稀濒危植物天山梣林龄结构及种群动态[J]. 植物生态学报, 2025, 49(5): 760-772. |
| [15] | 平晓燕, 杜毅倩, 赖仕蓉, 孔梦桥, 余国杰. 植物应对食草动物采食的化学防御策略研究进展[J]. 植物生态学报, 2025, 49(5): 667-680. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
