

植物学报 ›› 2022, Vol. 57 ›› Issue (2): 197-208.DOI: 10.11983/CBB21074 cstr: 32102.14.CBB21074
王建武1, 王文娟2, 相微微1, 代惠萍3, 王海庆2, 屈香香1, 亢福仁1,*( )
)
收稿日期:2021-05-02
接受日期:2021-10-13
出版日期:2022-03-01
发布日期:2022-03-24
通讯作者:
亢福仁
作者简介:*E-mail: kangfuren1987@163.com基金资助:
Jianwu Wang1, Wenjuan Wang2, Weiwei Xiang1, Huiping Dai3, Haiqing Wang2, Xiangxiang Qu1, Furen Kang1,*( )
)
Received:2021-05-02
Accepted:2021-10-13
Online:2022-03-01
Published:2022-03-24
Contact:
Furen Kang
摘要: I型H+-PPase参与糖异生和蔗糖分解代谢, 利用不同的糖(蔗糖、葡萄糖和果糖)饲喂拟南芥(Arabidopsis thaliana) I型H+-PPase基因不同类型的突变体, 产生的表型不一致, 因此, 推测I型H+-PPase可能存在其它影响糖代谢的机制。为进一步明确该酶对糖代谢的影响, 以过表达MtVP1的马铃薯(Solanum tuberosum)渭薯4号为研究对象, 观察不同培养条件下的表型, 监测糖含量变化, 并利用转录组测序分析转录谱。结果表明, 过表达MtVP1马铃薯表现出红色茎、紫色花和表皮毛更发达, 单株块茎数减少, 块茎变大, 块茎皱缩速度加快; 转基因马铃薯块茎中淀粉、葡萄糖和果糖含量显著下降, 芽中葡萄糖和果糖含量也显著下降。果糖饲喂导致转基因马铃薯花青素含量显著降低; 转基因马铃薯体内果糖-1,6-二磷酸酶和果糖- 2,6-二磷酸酶基因表达上调3-7倍。研究结果为进一步从糖代谢角度探究I型H+-PPase的生理功能提供参考。
王建武, 王文娟, 相微微, 代惠萍, 王海庆, 屈香香, 亢福仁. 过表达MtVP1对马铃薯表型及糖代谢的影响. 植物学报, 2022, 57(2): 197-208.
Jianwu Wang, Wenjuan Wang, Weiwei Xiang, Huiping Dai, Haiqing Wang, Xiangxiang Qu, Furen Kang. Effects of Overexpression of MtVP1 on Potato Phenotypes and Sugar Metabolism. Chinese Bulletin of Botany, 2022, 57(2): 197-208.
| Primer name | Sequences (5′-3′) |
|---|---|
| 10788-F | TCTTGTACGCTTGTCTTGAGCACTG |
| 10788-R | CTTCCCTTTCTTCGGAATCTTGATA |
| 19189-F | CACTCCAACTGATGATTGCCTCC |
| 19189-R | CCTGCTGCTAGAAGATTGTTCCCT |
| 20363-F | ATACAGCCCGAATGATGAGTGC |
| 20363-R | GCTGCAAGAAGGTTTGTCCCT |
| 19188-F | ACCACTTAAAGCAACCAGGTCC |
| 19188-R | TCCCGCCATACAACAACATTC |
| 24109-F | GAATGAGCAGACGAAGCACCCT |
| 24109-R | ACGAACTTGCAGCCAAGAACAA |
| 30370-F | TTGGGAAGTACCGCCGACTC |
| 30370-R | TGCAAGGGCAGCTACCTCATT |
| ef1α-F | ATTGGAAACGGATATGCTCCA |
| ef1α-R | TCCTTACCTGAACGCCTGTCA |
表1 qRT-PCR所用引物序列
Table 1 Primer sequences for qRT-PCR
| Primer name | Sequences (5′-3′) |
|---|---|
| 10788-F | TCTTGTACGCTTGTCTTGAGCACTG |
| 10788-R | CTTCCCTTTCTTCGGAATCTTGATA |
| 19189-F | CACTCCAACTGATGATTGCCTCC |
| 19189-R | CCTGCTGCTAGAAGATTGTTCCCT |
| 20363-F | ATACAGCCCGAATGATGAGTGC |
| 20363-R | GCTGCAAGAAGGTTTGTCCCT |
| 19188-F | ACCACTTAAAGCAACCAGGTCC |
| 19188-R | TCCCGCCATACAACAACATTC |
| 24109-F | GAATGAGCAGACGAAGCACCCT |
| 24109-R | ACGAACTTGCAGCCAAGAACAA |
| 30370-F | TTGGGAAGTACCGCCGACTC |
| 30370-R | TGCAAGGGCAGCTACCTCATT |
| ef1α-F | ATTGGAAACGGATATGCTCCA |
| ef1α-R | TCCTTACCTGAACGCCTGTCA |
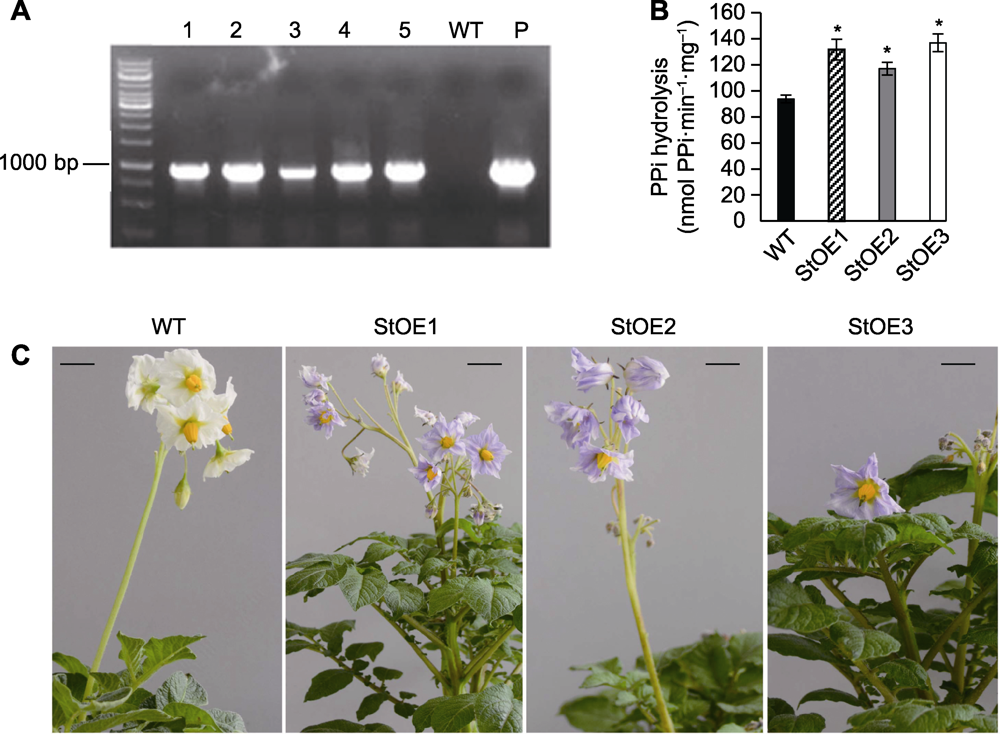
图1 过表达MtVP1马铃薯转基因株系分子鉴定及花色变化 (A) DNA水平的PCR检测结果; (B) I型H+-PPase的底物水解活性; (C) 花色变化。WT: 野生型马铃薯株系(阴性对照); P: 转基因表达载体(阳性对照); 1-5表示不同的转基因马铃薯株系。数据为3个重复的平均值±标准误(student’s t检验)。* P<0.05; Bars=1 cm
Figure 1 Molecular identification and changes of flower color in MtVP1 overexpressed potato (A) PCR result at DNA level; (B) Substrate hydrolysis activity of type I H+-PPase; (C) Changes of flower color. WT: Wild type potato (negative control); P: The expression vector for transgene (positive control); 1-5 indicate different transgenic potato lines. Data are reported as means±SE of three replicates (student’s t-test). * P<0.05; Bars=1 cm
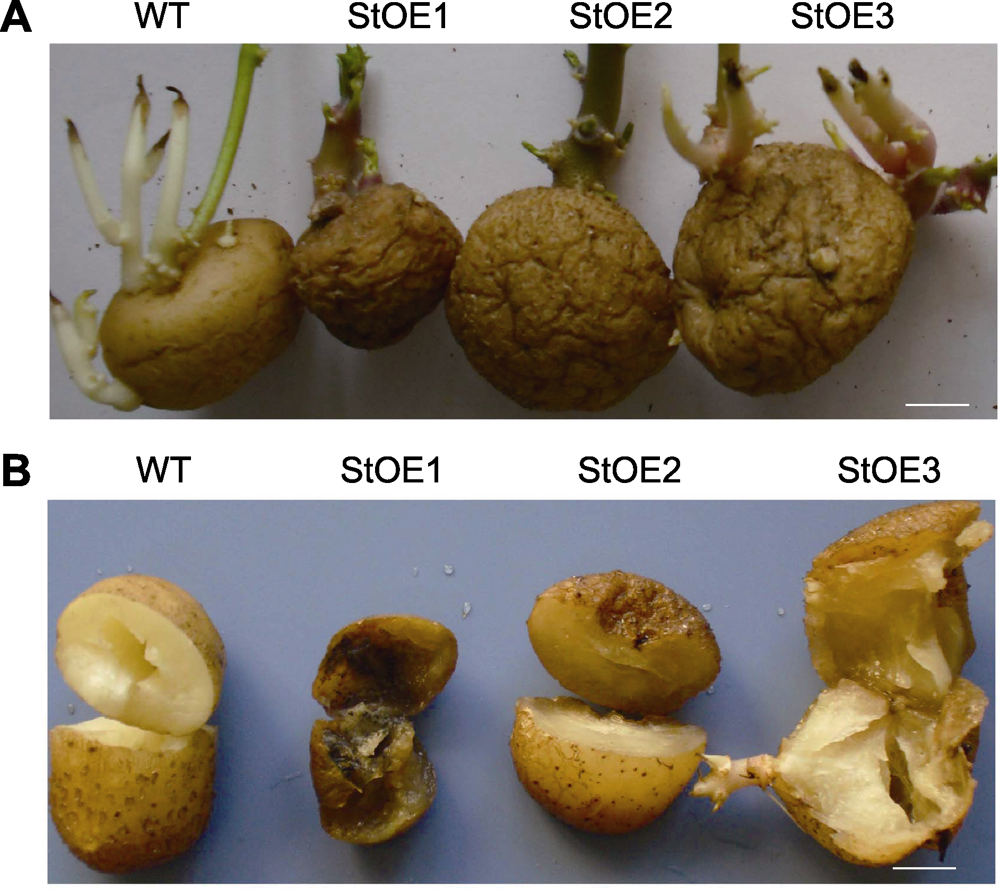
图3 过表达MtVP1马铃薯块茎的表型 (A) 贮藏5个月的块茎; (B) 种植后现蕾期的块茎(作种薯)。WT: 野生型。Bars=1 cm
Figure 3 Phenotype of tubers in MtVP1 overexpressed potato (A) Tubers after 5 months storage; (B) Tubers (as seed) at the beginning of the budding stage. WT: Wild type. Bars=1 cm
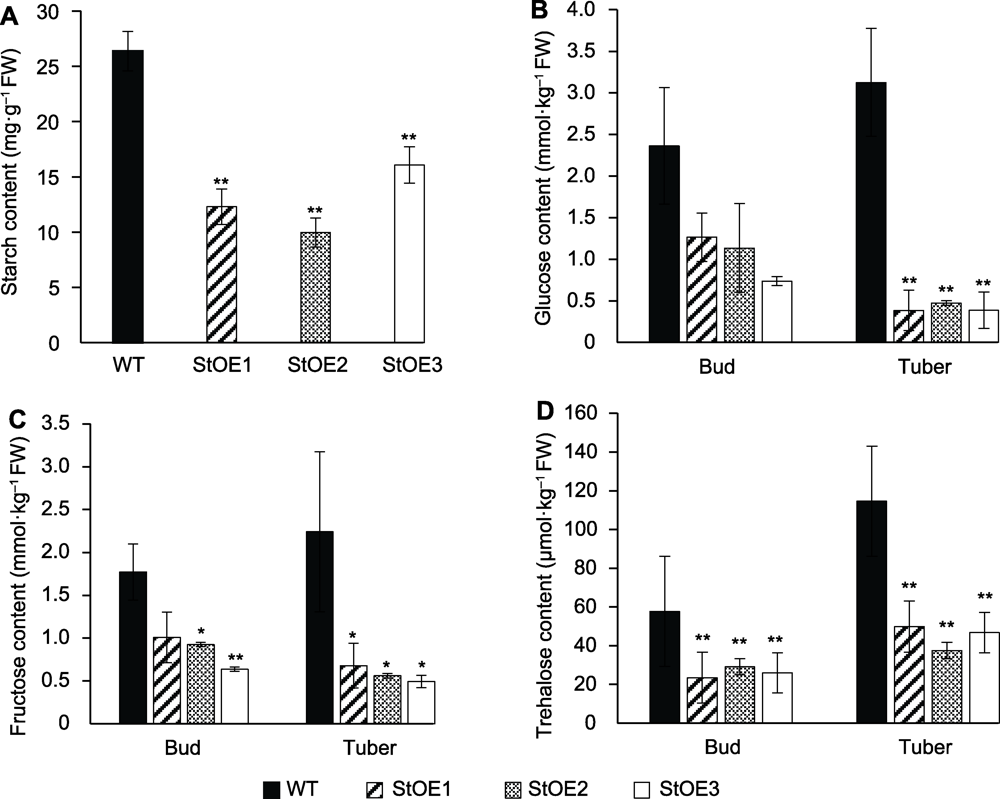
图4 过表达MtVP1马铃薯块茎和芽中不同糖类含量 (A) 块茎淀粉含量; (B) 芽和块茎的葡萄糖含量; (C) 芽和块茎的果糖含量; (D) 芽和块茎的海藻糖含量。数据为3个重复的平均值±标准误(student’s t检验)。WT: 野生型。* P<0.05, ** P<0.01
Figure 4 Contents of different sugars in tuber and bud of MtVP1 overexpressed potato (A) Starch contents of tubers; (B) Glucose contents of bud and tuber; (C) Fructose contents of bud and tuber; (D) Trehalose contents of bud and tuber. Data are reported as means±SE of three replicates (student’s t-test). WT: Wild type. * P<0.05, ** P<0.01
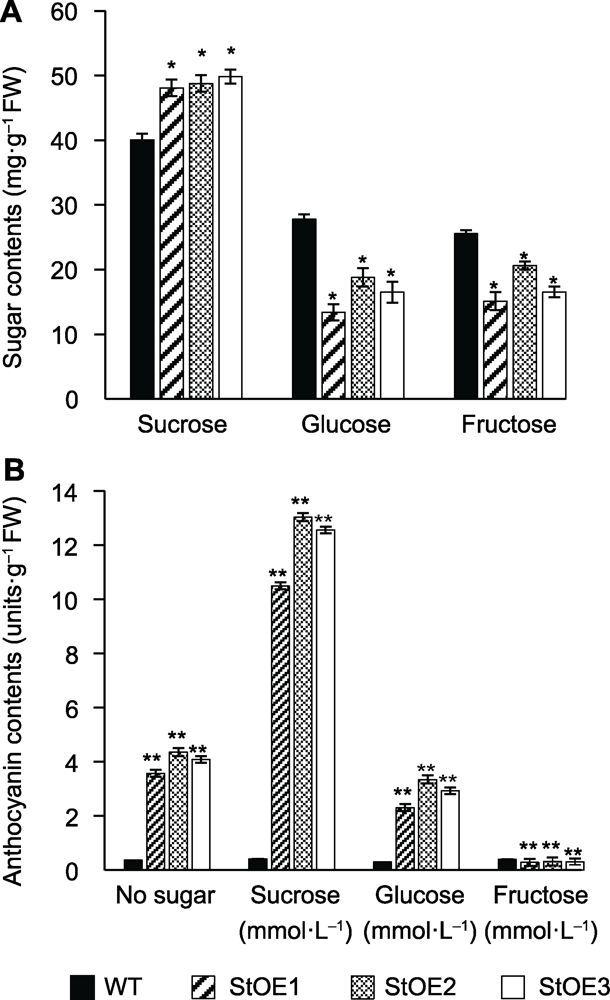
图5 常规MS培养基上马铃薯无菌苗糖含量(A)及不同糖饲喂下马铃薯无菌苗茎叶花青素含量(B) 数据为3个重复的平均值±标准误(student’s t检验)。WT: 野生型。* P<0.05, ** P<0.01
Figure 5 Sugar contents in potato sterile seedlings cultured by normal MS medium (A) and anthocyanin content in shoots by various sugar feeding in MS medium (B) Data are reported as means±SE of three replicates (student’s t-test). WT: Wild type. * P<0.05, ** P<0.01
| Lines | Differentially expressed genes | ||
|---|---|---|---|
| Total | Up | Down | |
| StOE1 vs WT | 8943 | 4397 | 4546 |
| StOE2 vs WT | 14121 | 7045 | 7076 |
| StOE3 vs WT | 14654 | 7310 | 7335 |
| StOE1 vs Favorita | 5489 | 3091 | 2398 |
| StOE2 vs Favorita | 8058 | 4251 | 3707 |
| StOE3 vs Favorita | 12305 | 6485 | 5820 |
表2 转录组测序分析得到的马铃薯差异表达基因数目
Table 2 Number of differentially expressed genes in potato determined by transcriptome sequencing
| Lines | Differentially expressed genes | ||
|---|---|---|---|
| Total | Up | Down | |
| StOE1 vs WT | 8943 | 4397 | 4546 |
| StOE2 vs WT | 14121 | 7045 | 7076 |
| StOE3 vs WT | 14654 | 7310 | 7335 |
| StOE1 vs Favorita | 5489 | 3091 | 2398 |
| StOE2 vs Favorita | 8058 | 4251 | 3707 |
| StOE3 vs Favorita | 12305 | 6485 | 5820 |
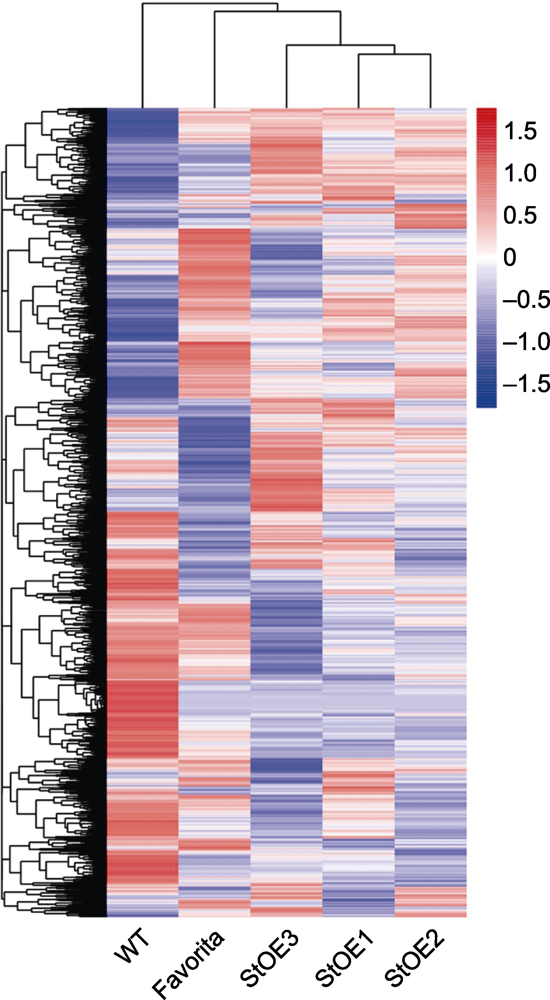
图6 过表达MtVP1马铃薯、野生型对照(WT)和商业品种Favorita成对比较热图
Figure 6 Heat map of pairwise comparison among MtVP1 overexpressed potato, wild type (WT) control and commercial cultivar Favorita
| Lines | Fructose-1,6-bisphosphatase | Fructose-2,6- bisphosphatase | ||||
|---|---|---|---|---|---|---|
| 10788 | 19189 | 20363 | 19188 | 24109 | 30370 | |
| StOE1 vs WT | 0.39262 | 1.1159 | 0.8341 | 0.91068 | 1.2916 | 0.77498 |
| StOE2 vs WT | 0.28647 | 1.0103 | 0.9656 | 0.66701 | 1.0852 | 0.91597 |
| StOE3 vs WT | 0.1806 | 0.83961 | 1.0418 | 0.87353 | 1.3994 | 0.80856 |
| StOE1 vs Favorita | NSC | 0.64089 | NSC | 0.81127 | 0.37061 | NSC |
| StOE2 vs Favorita | NSC | 0.55679 | 0.44266 | 0.58928 | 0.18557 | 0.35543 |
| StOE3 vs Favorita | NSC | 0.40857 | 0.54143 | 0.8185 | 0.52253 | 0.27085 |
表3 转录组分析得到的马铃薯果糖-1,6-二磷酸酶和果糖-2,6-二磷酸酶转录本log2FoldChange值
Table 3 log2FoldChange value of fructose-1,6-bisphosphatase and fructose-2,6-bisphosphatase transcripts of potato determined by transcriptome sequencing
| Lines | Fructose-1,6-bisphosphatase | Fructose-2,6- bisphosphatase | ||||
|---|---|---|---|---|---|---|
| 10788 | 19189 | 20363 | 19188 | 24109 | 30370 | |
| StOE1 vs WT | 0.39262 | 1.1159 | 0.8341 | 0.91068 | 1.2916 | 0.77498 |
| StOE2 vs WT | 0.28647 | 1.0103 | 0.9656 | 0.66701 | 1.0852 | 0.91597 |
| StOE3 vs WT | 0.1806 | 0.83961 | 1.0418 | 0.87353 | 1.3994 | 0.80856 |
| StOE1 vs Favorita | NSC | 0.64089 | NSC | 0.81127 | 0.37061 | NSC |
| StOE2 vs Favorita | NSC | 0.55679 | 0.44266 | 0.58928 | 0.18557 | 0.35543 |
| StOE3 vs Favorita | NSC | 0.40857 | 0.54143 | 0.8185 | 0.52253 | 0.27085 |
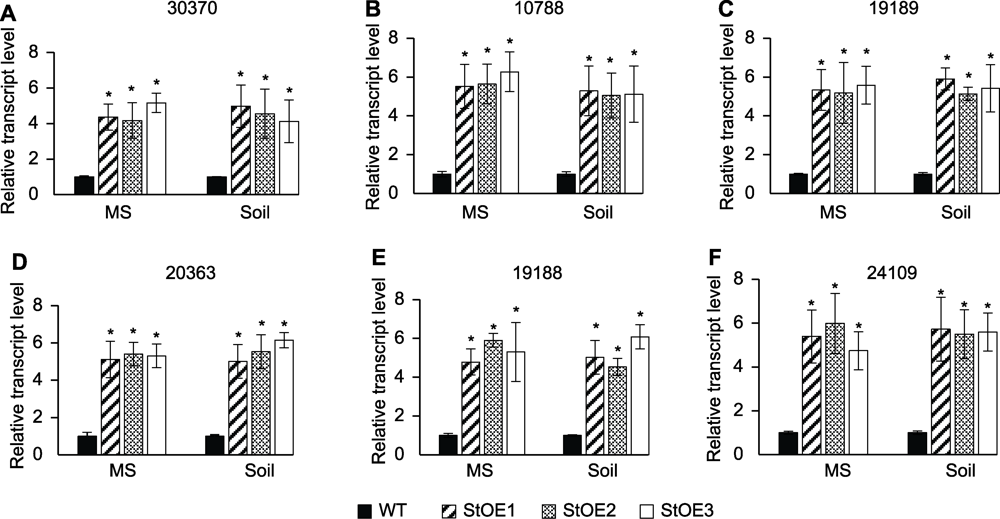
图7 马铃薯体内果糖-2,6-二磷酸酶基因(A)和果糖-1,6-二磷酸酶基因(B-F)的表达谱 数据为3个重复的平均值±标准误(student’s t检验)。* P<0.05
Figure 7 Expression profiles of fructose-2,6-bisphosphatase gene (A) and fructose-1,6-bisphosphatase genes (B-F) transcripts in potato Data are reported as means±SE of three replicates (student’s t-test). * P<0.05
| [1] | 柳俊, 谢从华 (2001). 马铃薯块茎发育机理及其基因表达. 植物学通报 18, 531-539. |
| [2] | 王建武, 相微微, 亢福仁 (2016). 过表达截形苜蓿液泡膜H+-PPase基因提高马铃薯的抗旱性. 分子植物育种 14, 1500-1506. |
| [3] | 卫生部食品卫生监督检验所 (2003). 食品中淀粉的测定标准:GB/T 5009.9-2003. 北京: 中国标准出版社. pp. 1-3. |
| [4] |
Baysal C, He WS, Drapal M, Villorbina G, Medina V, Capell T, Khush GS, Zhu CF, Fraser PD, Christou P (2020). Inactivation of rice starch branching enzyme IIb triggers broad and unexpected changes in metabolism by transcriptional reprogramming. Proc Natl Acad Sci USA 117, 26503-26512.
DOI URL |
| [5] | Bussard A, Lopez PJ (2014). Evolution of vacuolar pyrophosphatases and vacuolar H+-ATPases in diatoms. J Marine Sci Technol 22, 50-59. |
| [6] |
Drozdowicz YM, Rea PA (2001). Vacuolar H+ pyrophosphatases: from the evolutionary backwaters into the mainstream. Trends Plant Sci 6, 206-211.
PMID |
| [7] |
Duan XG, Yang AF, Gao F, Zhang SL, Zhang JR (2007). Heterologous expression of vacuolar H+-PPase enhances the electrochemical gradient across the vacuolar membrane and improves tobacco cell salt tolerance. Protoplasma 232, 87-95.
DOI URL |
| [8] |
Farré EM, Geigenberger P, Willmitzer L, Trethewey RN (2000). A possible role for pyrophosphate in the coordination of cytosolic and plastidial carbon metabolism within the potato tuber. Plant Physiol 123, 681-688.
PMID |
| [9] |
Ferjani A, Segami S, Horiguchi G, Muto Y, Maeshima M, Tsukaya H (2011). Keep an eye on PPi: the vacuolar-type H+-pyrophosphatase regulates postgerminative development in Arabidopsis. Plant Cell 23, 2895-2908.
DOI URL |
| [10] | Fuglsang AT, Paez-Valencia J, Gaxiola RA (2011). Plant proton pumps:regulatory circuits involving H+-ATPase and H+-PPase. In: Geisler M, Venema K, eds. Transporters and Pumps in Plant Signaling. Heidelberg: Springer. pp. 39-64. |
| [11] |
Gao F, Gao Q, Duan XG, Yue GD, Yang AF, Zhang JR (2006). Cloning of an H+-PPase gene from Thellungiella halophila and its heterologous expression to improve tobacco salt tolerance. J Exp Bot 57, 3259-3270.
DOI URL |
| [12] |
Gaxiola RA, Li JS, Undurraga S, Dang LM, Allen GJ, Alper SL, Fink GR (2001). Drought- and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc Natl Acad Sci USA 98, 11444-11449.
DOI URL |
| [13] |
Gaxiola RA, Rao R, Sherman A, Grisafi P, Alper SL, Fink GR (1999). The Arabidopsis thaliana proton transporters, AtNhx1 and Avp1, can function in cation detoxification in yeast. Proc Natl Acad Sci USA 96, 1480-1485.
DOI URL |
| [14] |
Gaxiola RA, Regmi K, Paez-Valencia J, Pizzio G, Zhang SJ (2016). Plant H+-PPases: reversible enzymes with contrasting functions dependent on membrane environment. Mol Plant 9, 317-319.
DOI URL |
| [15] |
Gaxiola RA, Sanchez CA, Paez-Valencia J, Ayre BG, Elser JJ (2012). Genetic manipulation of a ‘vacuolar’ H+-PPase: from salt tolerance to yield enhancement under phosphorus-deficient soils. Plant Physiol 159, 3-11.
DOI URL |
| [16] |
Graus D, Konrad KR, Bemm F, Nebioglu MGP, Lorey C, Duscha K, Güthoff T, Herrmann J, Ferjani A, Cuin TA, Roelfsema MRG, Schumacher K, Neuhaus HE, Marten I, Hedrich R (2018). High V-PPase activity is beneficial under high salt loads, but detrimental without salinity. New Phytol 219, 1421-1432.
DOI URL |
| [17] |
Guo S, Yin H, Zhang X, Zhao F, Li P, Chen S, Zhao Y, Zhang H (2006). Molecular cloning and characterization of a vacuolar H+-pyrophos-phatase gene, SsVP, from the halophyte Suaeda salsa and its overexpression increases salt and drought tolerance of Arabidopsis. Plant Mol Biol 60, 41-50.
DOI URL |
| [18] |
Khuri S, Moorby J (1995). Investigations into the role of sucrose in potato cv. Estima microtuber production in vitro. Ann of Bot 75, 295-303
DOI URL |
| [19] |
Lee CH, Pan YJ, Huang YT, Liu TH, Hsu SH, Lee CH, Chen YW, Lin SM, Huang LK, Pan RL (2011). Identification of essential lysines involved in substrate binding of vacuolar H+-pyrophosphatase. J Biol Chem 286, 11970- 11976.
DOI URL |
| [20] |
Li B, Wei AY, Song CX, Li N, Zhang JR (2008). Heterologous expression of the TsVP gene improves the drought resistance of maize. Plant Biotechnol J 6, 146-159.
DOI URL |
| [21] |
Li JS, Yang HB, Peer WA, Richter G, Blakeslee J, Bandyo padhyay A, Titapiwantakun B, Undurraga S, Khodakovskaya M, Richards EL, Krizek B, Murphy AS, Gilroy S, Gaxiola RA (2005). Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science 310, 121-125.
DOI URL |
| [22] |
Lv SL, Zhang KW, Gao Q, Lian LJ, Song YJ, Zhang JR (2008). Overexpression of an H+-PPase gene from Thellungiella halophila in cotton enhances salt tolerance and improves growth and photosynthetic performance. Plant Cell Physiol 49, 1150-1164.
DOI URL |
| [23] |
Maeshima M (2000). Vacuolar H+-pyrophosphatase. Biochim Biophys Acta 1465, 37-51.
PMID |
| [24] |
Maeshima M, Yoshida S (1989). Purification and properties of vacuolar membrane proton-translocating inorganic pyrophosphatase from mung bean. J Biol Chem 264, 20068- 20073.
PMID |
| [25] |
Nakanishi Y, Saijo T, Wada Y, Maeshima M (2001). Mutagenic analysis of functional residues in putative substrate- binding site and acidic domains of vacuolar H+-pyrophosphatase. J Biol Chem 276, 7654-7660.
DOI PMID |
| [26] |
Nel W, Terblanche SE (1992). Plant fructose-1,6-bisphosphatases: characteristics and properties. Int J Biochem 24, 1267-1283.
DOI URL |
| [27] |
Nielsen TH, Rung JH, Villadsen D (2004). Fructose-2,6- bisphosphate: a traffic signal in plant metabolism. Trends Plant Sci 9, 556-563.
DOI URL |
| [28] |
Paez-Valencia J, Patron-Soberano A, Rodriguez-Leviz A, Sanchez-Lares J, Sanchez-Gomez C, Valencia-Mayoral P, Diaz-Rosas G, Gaxiola RA (2011). Plasma membrane localization of the type I H+-PPase AVP1 in sieve element-companion cell complexes from Arabidopsis thaliana. Plant Sci 181, 23-30.
DOI PMID |
| [29] |
Pizzio GA, Paez-Valencia J, Khadilkar AS, Regmi K, Patron-Soberano A, Zhang SJ, Sanchez-Lares J, Furstenau T, Li JS, Sanchez-Gomez C, Valencia-Mayoral P, Yadav UP, Ayre BG, Gaxiola RA (2015). Arabidopsis type I proton-pumping pyrophosphatase expresses strongly in phloem, where it is required for pyrophosphate metabolism and photosynthate partitioning. Plant Physiol 167, 1541-1553.
DOI URL |
| [30] |
Rea PA, Poole RJ (1993). Vacuolar H+-translocating pyrophosphatase. Annu Rev Plant Physiol Plant Mol Biol 44, 157-180.
DOI URL |
| [31] |
Ruan YL (2014). Sucrose metabolism: gateway to diverse carbon use and sugar signaling. Annu Rev Plant Biol 65, 33-67.
DOI URL |
| [32] |
Rufty TW Jr, Huber SC (1983). Changes in starch formation and activities of sucrose phosphate synthase and cytoplasmic fructose-1,6-bisphosphatase in response to source-sink alterations. Plant Physiol 72, 474-480.
DOI PMID |
| [33] |
Schilling RK, Tester M, Marschner P, Plett DC, Roy SJ (2017). AVP1: one protein, many roles. Trends Plant Sci 22, 154-162.
DOI PMID |
| [34] |
Scott P, Kruger NJ (1995). Influence of elevated fructose- 2,6-bisphosphate levels on starch mobilization in transgenic tobacco leaves in the dark. Plant Physiol 108, 1569-1577.
PMID |
| [35] |
Segami S, Tomoyama T, Sakamoto S, Gunji S, Fukuda M, Kinoshita S, Mitsuda N, Ferjani A, Maeshima M (2018). Vacuolar H+-pyrophosphatase and cytosolic soluble pyrophosphatases cooperatively regulate pyrophosphate le- vels in Arabidopsis thaliana. Plant Cell 30, 1040-1061.
DOI URL |
| [36] |
Shiratake K, Kanayama Y, Maeshima M, Yamaki S (1997). Changes in H+-pumps and a tonoplast intrinsic protein of vacuolar membranes during the development of pear fruit. Plant Cell Physiol 38, 1039-1045.
PMID |
| [37] |
Shiroguchi K, Jia TZ, Sims PA, Xie XS (2012). Digital RNA sequencing minimizes sequence-dependent bias and amplification noise with optimized single-molecule barcodes. Proc Natl Acad Sci USA 109, 1347-1352.
DOI URL |
| [38] |
Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P (2006). Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol 140, 637-646.
PMID |
| [39] |
Sonnewald U (1992). Expression of E. coli inorganic pyrophosphatase in transgenic plants alters photoassimilate partitioning. Plant J 2, 571-581.
PMID |
| [40] |
Stitt M (1987). Fructose 2,6-bisphosphate and plant carbohydrate metabolism. Plant Physiol 84, 201-204.
DOI PMID |
| [41] |
Stitt M (1990). Fructose-2,6-bisphosphate as a regulatory molecule in plants. Annu Rev Plant Physiol Plant Mol Biol 41, 153-185.
DOI URL |
| [42] |
Strand Å, Foyer CH, Gustafsson P, Gardeström P, Hurry V (2003). Altering flux through the sucrose biosynthesis pathway in transgenic Arabidopsis thaliana modifies photosynthetic acclimation at low temperatures and the development of freezing tolerance. Plant Cell Environ 26, 523-535.
DOI URL |
| [43] |
Strand Å, Zrenner R, Trevanion S, Stitt M, Gustafsson P, Gardeström P (2000). Decreased expression of two key enzymes in the sucrose biosynthesis pathway, cytosolic fructose-1,6-bisphosphatase and sucrose phosphate synthase, has remarkably different consequences for photosynthetic carbon metabolism in transgenic Arabidopsis thaliana. Plant J 23, 759-770.
PMID |
| [44] |
Teng S, Keurentjes J, Bentsink L, Koornneef M, Smeekens S (2005). Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/ PAP1 gene. Plant Physiol 139, 1840-1852.
DOI URL |
| [45] |
Wang JW, Wang HQ, Xiang WW, Chai TY (2014). A Medicago truncatula H+-pyrophosphatase gene, MtVP1, improves sucrose accumulation and anthocyanin biosynthesis in potato (Solanum tuberosum L.). Genet Mol Res 13, 3615-3626.
DOI PMID |
| [46] |
Wang XL, Wang HW, Liu SX, Ferjani A, Li JS, Yan JB, Yang XH, Qin F (2016). Genetic variation in ZmVPP1 contributes to drought tolerance in maize seedlings. Nat Genet 48, 1233-1241.
DOI URL |
| [47] |
Yang HB, Knapp J, Koirala P, Rajagopal D, Peer WA, Silbart LK, Murphy A, Gaxiola RA (2007). Enhanced phosphorus nutrition in monocots and dicots over-expressing a phosphorus-responsive type I H+-pyrophosphatase. Plant Biotechnol J 5, 735-745.
DOI URL |
| [48] |
Zhen RG, Kim EJ, Rea PA (1997a). Acidic residues necessary for pyrophosphate-energized pumping and inhibition of the vacuolar H+-pyrophosphatase by N,N'-dicyclohexylcarbodiimide. J Biol Chem 272, 22340-22348.
DOI URL |
| [49] | Zhen RG, Kim EJ, Rea PA (1997b). The molecular and biochemical basis of pyrophosphate-energized proton translocation at the vacuolar membrane. Adv Bot Res 25, 297-337. |
| [1] | 肖银燕, 袁伟娜, 刘静, 孟建, 盛奇明, 谭烨欢, 徐春香. 木葡聚糖及其在植物抗逆过程中的功能研究进展[J]. 植物学报, 2020, 55(6): 777-787. |
| [2] | 刘慧英 朱祝军. 转化酶在高等植物蔗糖代谢中的作用研究进展[J]. 植物学报, 2002, 19(06): 666-674. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||