

植物学报 ›› 2021, Vol. 56 ›› Issue (3): 372-387.DOI: 10.11983/CBB20166 cstr: 32102.14.CBB20166
• 专题论坛 • 上一篇
严旭1,2, 左艳春1, 王红林1, 李杨2,3, 李影正2, 寇晶1, 唐祈林1, 周晓康2,*( ), 杜周和1,*(
), 杜周和1,*( )
)
收稿日期:2020-10-09
接受日期:2021-01-21
出版日期:2021-05-01
发布日期:2021-04-30
通讯作者:
周晓康,杜周和
作者简介:duzhouhe@126.com基金资助:
Xu Yan1,2, Yanchun Zuo1, Honglin Wang1, Yang Li2,3, Yingzheng Li2, Jing Kou1, Qilin Tang1, Xiaokang Zhou2,*( ), Zhouhe Du1,*(
), Zhouhe Du1,*( )
)
Received:2020-10-09
Accepted:2021-01-21
Online:2021-05-01
Published:2021-04-30
Contact:
Xiaokang Zhou,Zhouhe Du
摘要: 禾本科三倍体的形成途径包括2n配子融合、倍性间杂交、多精受精和胚乳培养。其中, 2n配子融合和倍性间杂交分别为自然界和人工合成三倍体的主要途径。该文介绍了形态学观测、染色体分析、流式细胞术和分子标记等倍性鉴定方法在禾本科三倍体中的应用及其优缺点。目前, 三倍体在禾谷类作物中无直接应用价值, 但可作为通往多倍体、非整倍体和转移异源基因的遗传桥梁。多年生禾本科三倍体(特别是异源三倍体)在饲草或能源作物中已得到广泛应用, 在该类型禾本科作物中均可直接尝试三倍体育种。多倍体的三倍体育种和无融合生殖三倍体育种可作为未来禾本科三倍体的研究方向。三倍性胚乳培养可以一步合成三倍体, 多精受精可以实现遗传上3个不同基因组的一步融合, 在三倍体研究中应予以重视。鉴于2n配子融合、多精受精的稀有特性和倍性间杂交、胚乳培养频繁的染色体变异, 高通量三倍体鉴定技术的发展将是三倍体研究实现突破的关键。
严旭, 左艳春, 王红林, 李杨, 李影正, 寇晶, 唐祈林, 周晓康, 杜周和. 禾本科三倍体: 形成、鉴定与利用. 植物学报, 2021, 56(3): 372-387.
Xu Yan, Yanchun Zuo, Honglin Wang, Yang Li, Yingzheng Li, Jing Kou, Qilin Tang, Xiaokang Zhou, Zhouhe Du. Triploid in Poaceae: Formation, Detection, and Utilization. Chinese Bulletin of Botany, 2021, 56(3): 372-387.
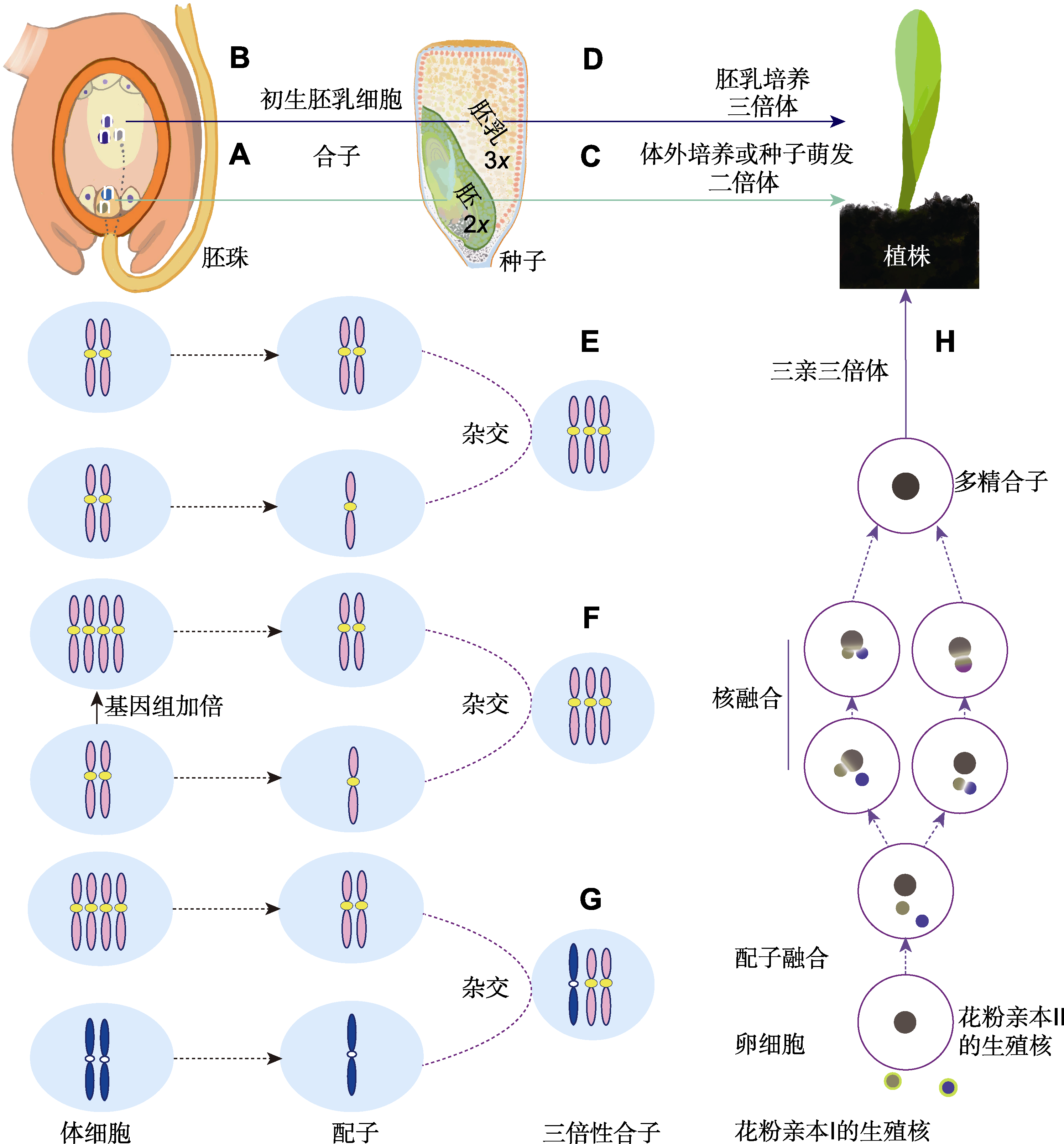
图1 禾本科三倍体的形成途径 (A) 精细胞与卵细胞受精产生二倍性合子后发育成二倍体胚; (B) 精细胞与中央细胞受精产生三倍性初生胚乳细胞后发育成胚乳; (C) 通过离体培养胚或种子萌发发育成二倍体; (D) 通过胚乳组织培养形成三倍体; (E) 通过2n+n生殖方式形成三倍体; (F) 通过二倍体加倍后形成的同源四倍体(2n=4x=AAAA)与二倍体(2n=2x=AA)杂交合成同源三倍体; (G) 通过四倍体(2n=4x=AAAA)与二倍体(2n=2x=BB)杂交合成异源三倍体; (H) 通过多精受精形成三倍体
Figure 1 Pathways to triploidy formation in Poaceae (A) A sperm fertilizes an egg cell to produce diploid zygote which subsequently grows into diploid embryo; (B) A sperm fertilizes a center cell to produce triploid primary endosperm cell which subsequently grows into endosperm; (C) The embryo develops into diploid plant through in vitro culture or seed germination; (D) The endosperm develops into triploid plant via culturing in vitro; (E) Triploid formation by a 2n+n mating; (F) The autotriploid hybrid produced by crossing tetraploid (2n=4x=AAAA) and diploid (2n=2x=AA); (G) The allotriploid hybrid produced by crossing tetraploid (2n=4x=AAAA) and diploid (2n=2x=BB); (H) Formation of triploid by polyspermy
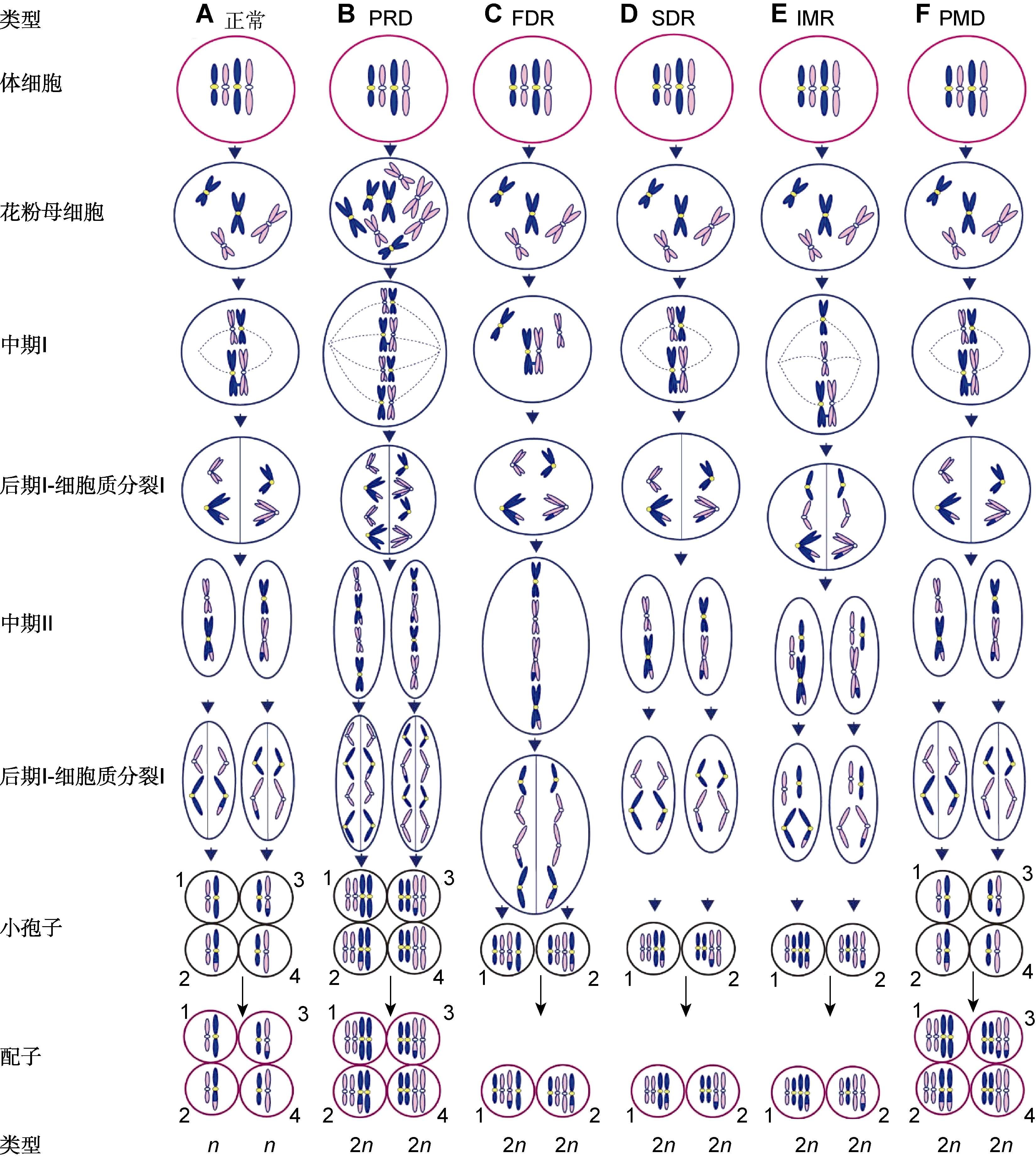
图2 2n配子的形成机制 (A) 与体细胞相比正常配子的染色体数目减半; (B) 通过胞质融合或多核体形成的四倍体花粉母细胞产生PRD型2n配子, 如鸭茅(Falistocco et al., 1995); (C) 在减数分裂I期同源染色体无配对和分裂形成的FDR型2n配子将保留除重组片段外的所有亲本基因, 如雀稗(Filho et al., 2014)和多年生黑麦草(Chen et al., 1997); (D) 在减数分裂II期因分裂缺失形成的SDR型2n配子将保留除重组片段外比正常配子多1套染色体副本的2n配子, 如多年生黑麦草(Chen et al., 1997); (E) 在减数分裂I期部分染色体以姐妹染色单体分离, 其余以二价体分离, 随后在减数分裂II期分裂缺失形成IMR型2n配子, 如百合(Lim et al., 2001); (F) 减数分裂正常而进入配子体发育阶段时基因组加倍形成PMD型2n配子, 如马铃薯(Bastiaanssen et al., 1998)
Figure 2 Mechanisms of 2n gamete formation (A) Chromosome numbers of normal gametes are halved compared with somatic cells; (B) PRD (pre-meiotic doubling) gametes are obtained as a result of the tetraploid pollen mother cells formed by cytomixis or syncytium, e.g., Dactylis glomerata (Falistocco et al., 1995); (C) In the FDR (first division restitution) type, pairing and division of homoeologous chromosomes do not occur during meiosis I, and the FDR 2n gametes maintain all of their parental genes except cross-over fragments, e.g., Paspalum jesuiticum (Filho et al., 2014) and Lolium perenne (Chen et al., 1997); (D) SDR (second division restitution) gametes with two copies of non-recombinant chromosomes are the result of the second division omission or cytokinesis abnormalities after normal anaphase II, e.g., L. perenne (Chen et al., 1997); (E) IMR (indeterminate meiotic restitution) gametes were discovered in Lilium longiflorum × Asiatic hybrid (Lim et al., 2001), in which some chromosomes are separated as univalents during meiosis I whereas the others are separated as bivalents, and subsequently the second division omission occurs during meiosis II; (F) PMD (post-meiotic doubling) gametes occurs due to the genome doubling of haploid spores during microgametogenesis, e.g., Solanum tuberosum (Bastiaanssen et al., 1998)
| 母本 | 父本 | 品种名 | 用途 | 参考文献 |
|---|---|---|---|---|
| 狗牙根(Cynodon dactylon) 2n=4x=36 | 非洲狗牙根(C. transvaalensis) 2n=2x=18 | Tifway、TifwayII、Midiron、TifEagle、Tifgreen、Tifdwarf、MS-Supreme、MS-Express、TifSport和Champion (2n=3x=27) | 草坪草 | |
| 荻(Miscanthus sacchariflorus) 2n=4x=76 | 芒(M. sinensis) 2n=2x=38 | Illinois和Freedom (2n=3x=57) | 能源作物 | |
| 玉米(Zea mays) 2n=2x=20 | 四倍体大刍草(Z. perennis) 2n=4x=40 | 玉草1号(2n=3x=30) | 饲用作物 | 任 |
| 指状摩擦禾(Tripsacum dactyloides) 2n=2x=36 | 指状摩擦禾(T. dactyloides) 2n=4x=72 | Verl (2n=3x=54) | 饲用作物 | |
| 薏苡(Coix lacryma-jobi) 2n=2x=20 | 水生薏苡(C. aquatica) 2n=4x=40 | 丰牧88 (2n=3x=30) | 饲用作物 | |
| 象草(Pennisetum purpureum) 2n=4x=28 | 美洲狼尾草(P. americana) 2n=2x=14 | 热研4号(2n=3x=21) | 饲用作物 | |
| 美洲狼尾草(P. americana) 2n=2x=14 | 象草(P. purpureum) 2n=4x=28 | 闽牧6号和华南1号(2n=3x=21) | 饲用作物 |
表1 禾本科三倍体品种
Table 1 Triploidy cultivars in Poaceae
| 母本 | 父本 | 品种名 | 用途 | 参考文献 |
|---|---|---|---|---|
| 狗牙根(Cynodon dactylon) 2n=4x=36 | 非洲狗牙根(C. transvaalensis) 2n=2x=18 | Tifway、TifwayII、Midiron、TifEagle、Tifgreen、Tifdwarf、MS-Supreme、MS-Express、TifSport和Champion (2n=3x=27) | 草坪草 | |
| 荻(Miscanthus sacchariflorus) 2n=4x=76 | 芒(M. sinensis) 2n=2x=38 | Illinois和Freedom (2n=3x=57) | 能源作物 | |
| 玉米(Zea mays) 2n=2x=20 | 四倍体大刍草(Z. perennis) 2n=4x=40 | 玉草1号(2n=3x=30) | 饲用作物 | 任 |
| 指状摩擦禾(Tripsacum dactyloides) 2n=2x=36 | 指状摩擦禾(T. dactyloides) 2n=4x=72 | Verl (2n=3x=54) | 饲用作物 | |
| 薏苡(Coix lacryma-jobi) 2n=2x=20 | 水生薏苡(C. aquatica) 2n=4x=40 | 丰牧88 (2n=3x=30) | 饲用作物 | |
| 象草(Pennisetum purpureum) 2n=4x=28 | 美洲狼尾草(P. americana) 2n=2x=14 | 热研4号(2n=3x=21) | 饲用作物 | |
| 美洲狼尾草(P. americana) 2n=2x=14 | 象草(P. purpureum) 2n=4x=28 | 闽牧6号和华南1号(2n=3x=21) | 饲用作物 |
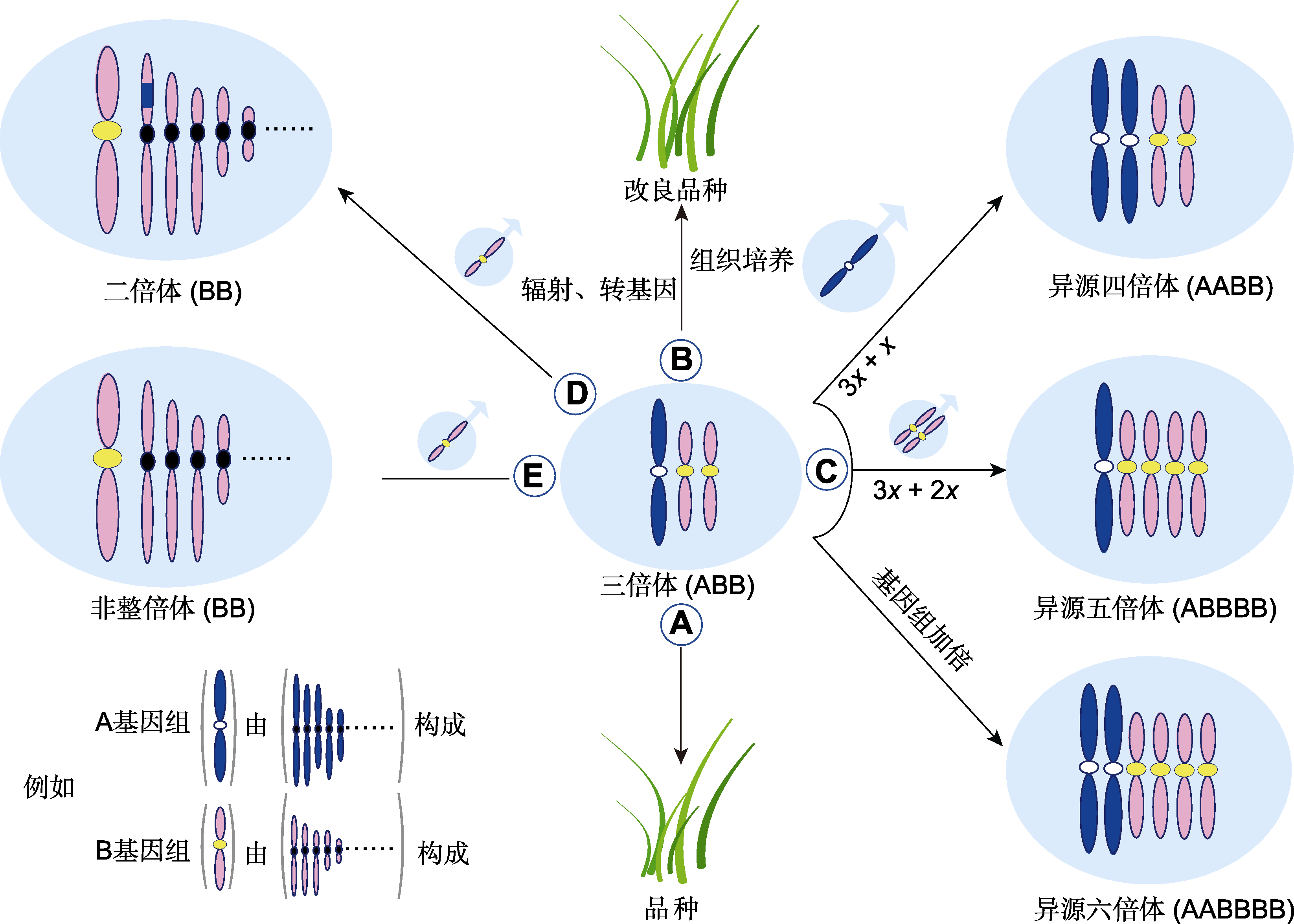
图3 禾本科三倍体的利用 (A) 三倍体作为品种直接利用; (B) 通过辐射、转基因或组织培养等技术改良三倍体品种; (C) 作为通往更高倍性的“三倍体桥”; (D) 转移异源基因; (E) 通过3x/2x合成非整倍体
Figure 3 Application of triploid in Poaceae (A) The direct use of triploid cultivar; (B) The improvement of triploid cultivar through techniques such as radiation, genetic modification, or tissue culture; (C) Use of “triploids bridge” for production of polyploids; (D) Alien genes transfer; (E) Development of aneuploidy by 3x/2x mating
| 1 | 陈平, 吴秀峰, 席嘉宾, 梁红 (2004). 华南1号杂交狼尾草选育初报. 仲恺农业技术学院学报 17, 56-59. |
| 2 | 陈钟佃, 黄勤楼, 黄秀声, 冯德庆, 钟珍梅 (2012). “闽牧6号”狼尾草的选育及田间种植技术. 家畜生态学报 33, 53-55. |
| 3 | 程祝宽, 李欣, 于恒秀, 顾铭洪 (1996). 一套新的籼稻初级三体的选育和细胞学鉴定. 遗传学报 23, 363-371. |
| 4 | 党江波, 宋琴, 李彩, 郭启高, 梁国鲁 (2018). 园艺植物中三倍体的应用现状及育种前景分析. 园艺学报 45, 1813-1830. |
| 5 | 顾洪如, 杨运生, 白淑娟, 陈礼伟 (1992). 牧草新品种“宁牧26-2”狼尾草. 江苏农业科学 ( 4), 61-63. |
| 6 | 黄群策, 孙敬三 (1999). 通过异倍性水稻间杂交获得同源三倍体植株. 植物学报 41, 741-746. |
| 7 | 刘国道, 白昌军, 王东劲, 易克贤, 韦家少, 何华玄, 周家锁 (2002). 热研4号王草选育. 草地学报 10, 92-96. |
| 8 | 刘秀明, 崔腾腾, 葛春霞, 陈翠霞 (2014). 我国野生芒资源的细胞学研究. 草业科学 31, 627-631. |
| 9 | 罗宗志, 林洁荣, 罗虹建, 林志魁, 陈碧成 (2016). 杂交狼尾草和桂牧1号杂交象草的核型分析. 贵州农业科学 44, 8-12. |
| 10 | 吕桂华, 唐祈林, 郭国锦, 陈坚剑, 荣廷昭 (2015). 玉米(Zea mays)×四倍体多年生玉米(Zea perennis)可育三倍体形态学和细胞遗传学研究. 植物遗传资源学报 16, 1152-1156. |
| 11 | 潘志军, 易自力, 杨塞, 肖亮 (2017). 三倍体芒草自然杂交后代数量性状遗传多样性研究. 植物遗传资源学报 18, 984-990. |
| 12 | 任勇, 陈柔屹, 唐祈林, 荣廷昭 (2007). 新型饲草玉米生长动态及收割期的研究. 作物学报 33, 1360-1365. |
| 13 | 孙福艾 (2017). 一份薏苡多倍体材料的创制、鉴定及饲用价值初步评价. 硕士论文. 雅安: 四川农业大学. pp. 1-54. |
| 14 | 孙敬三, 朱至清 (1981). 大麦胚乳植株的诱导及其倍性. 植物学报 23, 262-265. |
| 15 | 田立忠, 徐爱菊 (2000). 高粱(Sorghum bicolor (L.) Moench)未成熟胚乳培养的研究. 辽宁师范大学学报(自然科学版) 23, 395-402. |
| 16 | 王敬驹, 陆文梁, 匡柏健 (1982). 小黑麦杂种胚乳的离体培养研究. 植物学报 24, 420-425. |
| 17 | 王润奇, 高俊华, 王志兴, 王志民 (1994). 谷子三体系列的建立. 植物学报 36, 690-695. |
| 18 | 汪艳, 肖媛, 刘伟, 李婷婷, 胡锐, 乔志仙 (2015). 流式细胞仪检测高等植物细胞核DNA含量的方法. 植物科学学报 33, 126-131. |
| 19 | 许蕾, 陈佩琳, 冯光燕, 钟旻依, 景婷婷, 黄琳凯, 张新全 (2019). 利用流式细胞仪鉴定鸭茅倍性. 草业学报 28, 74-84. |
| 20 | 于卓, 云锦凤, 马有志, 辛志勇 (2004). 加拿大披碱草×野大麦三倍体杂种染色体的分子原位杂交鉴定. 遗传学报 31, 735-739. |
| 21 | 袁金玲, 顾小平, 岳晋军, 吴晓丽 (2015). 一种毛竹胚乳培养的方法. 中国专利, ZL 201510566151.8. 2015-09-08. |
| 22 | 张静, 吴先军, 汪旭东, 周开达, 彭海 (2002). 特异同源三倍体水稻材料SAR-3细胞学研究. 作物学报 28, 704-708. |
| 23 | 张茜, 裘天航, 王安安, 周华健, 袁敏, 李利, 白素兰, 崔素霞 (2020). 北京地区芦苇资源状态及其多样性. 植物学报 55, 693-704. |
| 24 | 张智奇, 钟维瑾, 唐克轩, 周音, 祝明福 (1994). 异源三倍体水稻原生质体培养及植株再生. 作物学报 20, 578-581. |
| 25 | 赵世绪, 刘瑞凝, 敖光明, 陈一心 (1984). 小麦、黑麦未成熟胚乳植株的诱导. 北京农业大学学报 10, 129-132. |
| 26 | 钟声 (2006). 鸭茅不同倍性杂交及后代发育特性的初步研究. 西南农业学报 19, 1034-1038. |
| 27 | 钟小仙, 刘智微, 刘伟国, 崔莉莉, 吴娟子, 张建丽 (2014). 六倍体杂交狼尾草体细胞突变体特异性分析. 草业学报 23, 107-113. |
| 28 | 祝剑峰, 刘幼琪, 王爱云, 宋兆建, 陈冬玲, 蔡得田 (2008). 异源六倍体水稻AACCDD和三倍体水稻ACD生殖特性的细胞胚胎学研究. 植物遗传资源学报 9, 350-357. |
| 29 |
Bajaj YPS, Saini SS, Bidani M (1980). Production of triploid plants from the immature and mature endosperm cultures of rice. Theor Appl Genet 58, 17-18.
DOI PMID |
| 30 |
Bastiaanssen HJM, Van Den Berg PMMM, Lindhout P, Jacobsen E, Ramanna MS (1998). Postmeiotic restitution in 2 n-egg formation of diploid potato. Heredity 81, 20-27.
DOI URL |
| 31 |
Beale KM, Leydon AR, Johnson MA (2012). Gamete fusion is required to block multiple pollen tubes from entering an Arabidopsis ovule. Curr Biol 22, 1090-1094.
DOI URL |
| 32 |
Boller B, Kopeck D (2020). Triploid forage grass hybrids Festuca apennina × F. pratensis display extraordinary heterosis for yield characteristics. Euphytica 216, 143.
DOI URL |
| 33 |
Bretagnolle FO (2001). Pollen production and spontaneous polyploidization in diploid populations of Anthoxanthum alpinum. Biol J Linn Soc 72, 241-247.
DOI URL |
| 34 |
Brownfield L, Köhler C (2011). Unreduced gamete formation in plants: mechanisms and prospects. J Exp Bot 62, 1659-1668.
DOI URL |
| 35 |
Campos JMS, Davide LC, Salgado CC, Santos FC, Costa PN, Silva PS, Alves CCS, Viccini LF, Pereira AV (2009). In vitro induction of hexaploid plants from triploid hybrids of Pennisetum purpureum and Pennisetum glaucum. Plant Breed 128, 101-104.
DOI URL |
| 36 |
Chandra A, Genovesi AD, Wherley BG, Metz SP, Reinert JA, Wu YZ, Skulkaew P, Engelke MC, Hargey D, Nelson LR, Schwartz BM, Raymer PL, Wu YQ, Martin DL, Milla-Lewis SR, Miller G, Kenworthy KE, Munoz P (2015). Registration of ‘DALSA 0605’ St. Augustinegrass. J Plant Regist 9, 27-34.
DOI URL |
| 37 |
Chen C, Sleper DA, Chao S, Johal GS, West CP (1997). RFLP detection of 2 n pollen formation by first and second division restitution in perennial ryegrass. Crop Sci 37, 76-80.
DOI URL |
| 38 | Costich DE, Friebe B, Sheehan MJ, Casler MD, Buckler ES (2010). Genome-size variation in switchgrass ( Panicum virgatum): flow cytometry and cytology reveal rampant aneuploidy. Plant Genome 3, 130-141. |
| 39 |
De Storme N, Zamariola L, Mau M, Sharbel TF, Geelen D (2013). Volume-based pollen size analysis: an advanced method to assess somatic and gametophytic ploidy in flowering plants. Plant Reprod 26, 65-81.
DOI PMID |
| 40 |
Delomas TA (2019). Differentiating diploid and triploid individuals using single nucleotide polymorphisms genotyped by amplicon sequencing. Mol Ecol Resour 19, 1545-1551.
DOI PMID |
| 41 |
Dewald CL, Taliaferro CM, Dunfield PC (1992). Registration of four fertile triploid germplasm lines of eastern gamagrass. Crop Sci 32, 504.
DOI URL |
| 42 |
Duan QH, Liu MCJ, Kita D, Jordan SS, Yeh FLJ, Yvon R, Carpenter H, Federico AN, Garcia-Valencia LE, Eyles SJ, Wang CS, Wu HM, Cheung AY (2020). FERONIA controls pectin- and nitric oxide-mediated male-female interaction. Nature 579, 561-566.
DOI URL |
| 43 |
Dvorak J, Harvey BL, Coulman BE (1973). The use of nitrous oxide for producing eupolyploids and aneuploids in wheat and barley. Can J Genet Cytol 15, 205-214.
DOI URL |
| 44 |
Erichsen AW, Ross JG (1963). A triploid derived from a selfed haploid Sorghum plant. Crop Sci 3, 99-100.
DOI URL |
| 45 |
Falistocco E, Tosti N, Falcinelli M (1995). Cytomixis in pollen mother cells of diploid Dactylis, one of the origins of 2n gametes. J Hered 86, 448-453.
DOI URL |
| 46 |
Filho RAB, Santos ACC, Souza FHD, Valls JFM, Pagliarini MS (2014). Complete asynapsis resulting in 2n pollen formation in Paspalum jesuiticum Parodi (Poaceae). Genet Mol Res 13, 255-261.
DOI URL |
| 47 |
Gao LH, Diarso M, Zhang A, Zhang HK, Dong YZ, Liu LX, Lv ZL, Liu B (2016). Heritable alteration of DNA methylation induced by whole-chromosome aneuploidy in wheat. New Phytol 209, 364-375.
DOI URL |
| 48 |
Ghimire BK, Seong ES, Nguyen TX, Yoo JH, Yu CY, Kim SH, Chung I (2016). Assessment of morphological and phytochemical attributes in triploid and hexaploid plants of the bioenergy crop Miscanthus × giganteus. Ind Crop Prod 89, 231-243.
DOI URL |
| 49 | Hagerup O (1947). The spontaneous formation of haploid, polyploid, and aneuploid embryos in some orchids. Biol Meddel Kongol Danske Vidensk Selsk 20, 1-22. |
| 50 |
Hanna WW, Schertz KF (1971). Trisome identification in Sorghum bicolor( L.) Moench by observing progeny of triploid × translocation stocks. Can J Genet Cytol 13, 105-109.
DOI URL |
| 51 |
Harlan JR, DeWet JMJ (1975). On Ö. Winge and a prayer: the origins of polyploidy. Bot Rev 41, 361-390.
DOI URL |
| 52 |
Henry IM, Dilkes BP, Miller ES, Burkart-Waco D, Comai L (2010). Phenotypic consequences of aneuploidy in Arabidopsis thaliana. Genetics 186, 1231-1245.
DOI |
| 53 |
Hoshino Y, Miyashita T, Thomas TD (2011). In vitro culture of endosperm and its application in plant breeding: approaches to polyploidy breeding. Sci Hortic 130, 1-8.
DOI URL |
| 54 |
Hu FR, Zhang L, Wang XY, Ding J, Wu DX (2005). Agrobacterium-mediated transformed transgenic triploid bermudagrass ( Cynodon dactylon × C. transvaalensis) plants are highly resistant to the glufosinate herbicide liberty. Plant Cell Tissue Organ Cult 83, 13-19.
DOI URL |
| 55 |
Iqbal MZ, Cheng MJ, Zhao YL, Wen XD, Zhang P, Zhang L, Ali A, Rong TZ, Tang QL (2018). Mysterious meiotic behavior of autopolyploid and allopolyploid maize. Comp Cytogenet 12, 247-265.
DOI URL |
| 56 |
Iwata N, Omura T (1975). Studies on the trisomics in rice plants (Oryza sativa L.): III. Relation between trisomics and genetic linkage groups. Jpn J Breed 25, 363-368.
DOI URL |
| 57 |
Jansen RC, Den Nijs APM (1993). A statistical mixture model for estimating the proportion of unreduced pollen grains in perennial ryegrass (Lolium perenne L.) via the size of pollen grains. Euphytica 70, 205-215.
DOI URL |
| 58 |
Johansen B, Von Bothmer R (1994). Pollen size in Hordeum L.: correlation between size, ploidy level, and breeding system. Sex Plant Reprod 7, 259-263.
DOI URL |
| 59 |
Johri BM, Bhojwani SS (1965). Growth responses of mature endosperm in cultures. Nature 208, 1345-1347.
DOI URL |
| 60 |
Kamps TL, Williams NR, Ortega VM, Chamusco KC, Harris-Shultz K, Scully BT, Chase CD (2011). DNA polymorphisms at bermudagrass microsatellite loci and their use in genotype fingerprinting. Crop Sci 51, 1122-1131.
DOI URL |
| 61 |
Kapadia ZJ, Gould FW (1964). Biosystematic studies in the Bouteloua curtipendula complex. III. Pollen size as related to chromosome numbers. Am J Bot 51, 166-172.
DOI URL |
| 62 |
Kato A (1999). Induction of bicellular pollen by trifluralin treatment and occurrence of triploids and aneuploids after fertilization in maize. Genome 42, 154-157.
DOI URL |
| 63 |
Kato A, Birchler JA (2006). Induction of tetraploid derivatives of maize inbred lines by nitrous oxide gas treatment. J Hered 97, 39-44.
DOI URL |
| 64 |
Katsiotis A, Forsberg RA (1995). Pollen grain size in four ploidy levels of genus Avena. Euphytica 83, 103-108.
DOI URL |
| 65 |
Keller B, Feuillet C (2000). Colinearity and gene density in grass genomes. Trends Plant Sci 5, 246-251.
PMID |
| 66 | Kindiger B, Dewald C (1994). Genome accumulation in eastern gamagrass, Tripsacum dactyloides(L.) L. 92, 197-201. |
| 67 |
King IP, Morgan WG, Harper JA, Thomas HM (1999). Introgression mapping in the grasses. II. Meiotic analysis of the Lolium perenne/Festuca pratensis triploid hybrid. Heredity 82, 107-112.
DOI URL |
| 68 |
Kirov I, Divashuk M, Van Laere K, Soloviev A, Khrustaleva L (2014). An easy "SteamDrop" method for high quality plant chromosome preparation. Mol Cytogenet 7, 21.
DOI PMID |
| 69 | Krans JV, Philley HW, Goatley JM Jr, Maddox VL (1999). Registration of ‘MS-Supreme’ bermudagrass. Crop Sci 39, 287. |
| 70 |
Kreiner JM, Kron P, Husband BC (2017). Frequency and maintenance of unreduced gametes in natural plant populations: associations with reproductive mode, life history and genome size. New Phytol 214, 879-889.
DOI URL |
| 71 |
Kron P, Husband BC (2015). Distinguishing 2N gamete nuclei from doublets in pollen using flow cytometry and pulse analysis. Cytometry Part A 87, 943-957.
DOI URL |
| 72 | La Rue CD (1949). Cultures of the endosperm of maize. Am J Bot 36, 798. |
| 73 |
Lamote V, Baert J, Roldán-Ruiz I, De Loose M, Van Bockstaele E (2002). Tracing of 2n egg occurrence in perennial ryegrass (Lolium perenne L.) using interploidy crosses. Euphytica 123, 159-164.
DOI URL |
| 74 |
Lim KB, Ramanna MS, De Jong JH, Jacobsen E, Van Tuyl JM (2001). Indeterminate meiotic restitution (IMR): a novel type of meiotic nuclear restitution mechanism detected in interspecific lily hybrids by GISH. Theor Appl Genet 103, 219-230.
DOI URL |
| 75 |
Loginova DB, Silkova OG (2017). Mechanisms of unreduced gamete formation in flowering plants. Russ J Genet 53, 741-756.
DOI URL |
| 76 |
Lu SY, Peng XX, Guo ZF, Zhang GY, Wang ZC, Wang CY, Pang CS, Fan Z, Wang JH (2007). In vitro selection of salinity tolerant variants from triploid bermudagrass ( Cynodon transvaalensis × C. dactylon) and their physiological responses to salt and drought stress. Plant Cell Rep 26, 1413-1420.
DOI URL |
| 77 |
Maceira NO, De Haan AA, Lumaret R, Billon M, Delay J (1992). Production of 2 n gametes in diploid subspecies of Dactylis glomerata L. 1. Occurrence and frequency of 2n pollen. Ann Bot 69, 335-343.
DOI URL |
| 78 |
Mason AS, Nelson MN, Yan GJ, Cowling WA (2011). Production of viable male unreduced gametes in Brassica interspecific hybrids is genotype specific and stimulated by cold temperatures. BMC Plant Biol 11, 103.
DOI URL |
| 79 | Multani DS, Jena KK, Brar DS, De Los Reyes BG, Angeles ER, Khush GS (1994). Development of monosomic alien addition lines and introgression of genes from Oryza australiensis Domin. to cultivated rice O. sativa L. Theor Appl Genet 88, 102-109. |
| 80 |
Mutlu SS, Mutlu N, Tokgöz S, Çakır M, Selim C (2020). Development of vegetative triploid turf-type bermudagrass [ Cynodon dactylon × C. transvaalensis (C. × mangennisii Hurcombe)]. Genet Resour Crop Evol 67, 177-189.
DOI URL |
| 81 |
Naganowska B, Zwierzykowski Z, Zwierzykowska E (2001). Meiosis and fertility of reciprocal triploid hybrids of Lolium multiflorum with Festuca pratensis. J Appl Genet 42, 247-255.
PMID |
| 82 |
Nakano H, Tashiro T, Maeda E (1975). Plant differentiation in callus tissue induced from immature endosperm of Oryza sauva L. Z Pflanzenphysiol 76, 444-449.
DOI URL |
| 83 |
Nakel T, Tekleyohans DG, Mao YB, Fuchert G, Vo D, Groß-Hardt R (2017). Triparental plants provide direct evidence for polyspermy induced polyploidy. Nat Commun 8, 1033.
DOI URL |
| 84 |
Norstog K, Wall WE, Howland GP (1969). Cytological characteristics of ten-year-old rye-grass endosperm tissue cultures. Bot Gaz 130, 83-86.
DOI URL |
| 85 |
Norstog KJ (1956). Growth of rye-grass endosperm in vitro. Bot Gaz 117, 253-259.
DOI URL |
| 86 |
Nunes JD, Azevedo ALS, Pereira AV, Paula CMP, Campos JMS, Lédo FJS, Santos VB (2013). DNA elimination in embryogenic development of Pennisetum glaucum × Pennisetum purpureum(Poaceae) hybrids. Genet Mol Res 12, 4817-4826.
DOI PMID |
| 87 |
Pagliarini MS, Valle CB, Santos EM, Mendes DV, Bernardo ZH, Mendes-Bonato AB, Silva N, Calisto V (2012). Microsporogenesis in Brachiaria brizantha(Poaceae) as a selection tool for breeding. Genet Mol Res 11, 1309-1318.
DOI PMID |
| 88 |
Pécrix Y, Rallo G, Folzer H, Cigna M, Gudin S, Le Bris M (2011). Polyploidization mechanisms: temperature environment can induce diploid gamete formation in Rosa sp. J Exp Bot 62, 3587-3597.
DOI URL |
| 89 |
Pepin GW, Funk CR (1971). Intraspecific hybridization as a method of breeding Kentucky bluegrass ( Poa pratemis L.) for turf. Crop Sci 11, 445-448.
DOI URL |
| 90 |
Perera D, Barnes DJ, Baldwin BS, Reichert NA (2015). Direct and indirect in vitro regeneration of Miscanthus × giganteus cultivar freedom: effects of explant type and medium on regeneration efficiency. In Vitro Cell Dev Biol Plant 51, 294-302.
DOI URL |
| 91 |
Ramsey J, Schemske DW (1998). Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu Rev Ecol Syst 29, 467-501.
DOI URL |
| 92 |
Rhoades MM (1936). Note on the origin of triploidy in maize. J Genet 33, 355-357.
DOI URL |
| 93 |
Sandfaer J (1975). The occurrence of spontaneous triploids in different barley varieties. Hereditas 80, 149-153.
DOI URL |
| 94 | Schwartz BM, Harris-Shultz KR, Contreras RN, Hans CS, Hanna WW, Milla-Lewis SR (2013). Creation of artificial triploid and tetraploid centipedegrass using colchicine and breeding. Int Turfgrass Soc Res J 12, 327-334. |
| 95 | Sehgal CB (1974). Growth of barley and wheat endosperm in cultures. Curr Sci 43, 38-40. |
| 96 |
Sheidai M, Jafari S, Taleban P, Keshavarzi M (2009). Cytomixis and unreduced pollen grain formation in Alopecurus L. and Catbrosa Beauv.(Poaceae). Cytologia 74, 31-41.
DOI URL |
| 97 |
Sieber VK, Murray BG (1979). The cytology of the genus Alopecurus(Gramineae). Bot J Linn Soc 79, 343-355.
DOI URL |
| 98 |
Springer TL, Dewald CL, Sims PL, Gillen RL, Louthan VH, Cooper WJ, Taliaferro CM, Maura C, Pfaff S, Wynia RL, Douglas JL, Henry J, Bruckerhoff SB, Grinten M, Salon PR, Houck MJ, Esquivel RG (2006). Registration of 'Verl' eastern gamagrass. Crop Sci 46, 477.
DOI URL |
| 99 |
Straus J, LaRue CD (1954). Maize endosperm tissue grown in vitro I. Culture requirements. Am J Bot 41, 687-694.
DOI URL |
| 100 |
Suarez EY, Lopez AG, Naranjo CA (1992). Polyspermy versus unreduced male gametes as the origin of nonaploids (9x) common wheat plants. Caryologia 45, 21-28.
DOI URL |
| 101 |
Sun S, Wu Y, Lin XY, Wang J, Yu JM, Sun Y, Miao YL, Li QP, Sanguinet KA, Liu B (2017). Hybrid weakness in a rice interspecific hybrid is nitrogen-dependent, and accompanied by changes in gene expression at both total transcript level and parental allele partitioning. PLoS One 12, e0172919.
DOI URL |
| 102 |
Tamaoki T, Ullstrup JA (1958). Cultivation in vitro of excised endosperm and meristem tissues of corn. Bull Torrey Bot Club 85, 260-272.
DOI URL |
| 103 |
Tan GY, Dunn GM (1973). Relationship of stomatal length and frequency and pollen-grain diameter to ploidy level in Bromus inermis Leyss. Crop Sci 13, 332-334.
DOI URL |
| 104 |
Thomas H, Morgan WG, Humphreys MW (1988). The use of a triploid hybrid for introgression in Lolium species. Theor Appl Genet 76, 299-304.
DOI PMID |
| 105 |
Thomas TD, Chaturvedi R (2008). Endosperm culture: a novel method for triploid plant production. Plant Cell Tissue Organ Cult 93, 1-14.
DOI URL |
| 106 |
Toda E, Ohnishi Y, Okamoto T (2016). Development of polyspermic rice zygotes. Plant Physiol 171, 206-214.
DOI URL |
| 107 |
Toda E, Okamoto T (2016). Formation of triploid plants via possible polyspermy. Plant Signal Behav 11, e1218107.
DOI URL |
| 108 |
Van Santen E, Hugessen PM, Casler MD (1991). Identification and frequency of tetraploid progeny from 2 x-4x and 4x-2x crosses in Dactylis. Genome 34, 273-278.
DOI URL |
| 109 |
Wang C, Liu Q, Shen Y, Hua YF, Wang JJ, Lin JR, Wu MG, Sun TT, Cheng ZK, Mercier R, Wang KJ (2019). Clonal seeds from hybrid rice by simultaneous genome engineering of meiosis and fertilization genes. Nat Biotechnol 37, 283-286.
DOI URL |
| 110 |
Wang X, Yamada T, Kong FJ, Abe Y, Hoshino Y, Sato H, Takamizo T, Kanazawa A, Yamada T (2011). Establishment of an efficient in vitro culture and particle bombardment-mediated transformation systems in Miscanthus sinensis Anderss, a potential bioenergy crop. GCB Bioenergy 3, 322-332.
DOI URL |
| 111 |
Younis A, Hwang YJ, Lim KB (2014). Exploitation of induced 2 n-gametes for plant breeding. Plant Cell Rep 33, 215-223.
DOI URL |
| 112 |
Yu CY, Kim HS, Rayburn AL, Widholm JM, Juvik JA (2009). Chromosome doubling of the bioenergy crop, Miscanthus × giganteus. GCB Bioenergy 1, 404-412.
DOI URL |
| 113 |
Zeng RZ, Zhu J, Xu SY, Du GH, Guo HR, Chen JJ, Zhang SZ, Xie L (2020). Unreduced male gamete formation in Cymbidium and its use for developing sexual polyploid cultivars. Front Plant Sci 11, 558.
DOI URL |
| [1] | 邸楠,席本野,Jeremiah R.PINTO,王烨,李广德,贾黎明. 宽窄行栽植下三倍体毛白杨根系生物量分布及其对土壤养分因子的响应[J]. 植物生态学报, 2013, 37(10): 961-971. |
| [2] | 李明军, 郭婧, 李翔, 李纪强, 王医鹏, 张晓丽, 刘永康. 盾叶薯蓣胚乳再生体系的建立及其染色体倍性鉴定[J]. 植物学报, 2012, 47(6): 654-660. |
| [3] | 李云. 杨树三倍体选育研究进展[J]. 植物学报, 2001, 18(04): 451-458. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||