

Chinese Bulletin of Botany ›› 2019, Vol. 54 ›› Issue (3): 328-334.DOI: 10.11983/CBB18117 cstr: 32102.14.CBB18117
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Guangqian Cheng1,†,Keli Jia1,2,†,Na Li1,Chuanliang Deng1,Shufen Li1,Wujun Gao1,*
Received:2018-05-11
Accepted:2018-08-06
Online:2019-05-01
Published:2019-11-24
Contact:
Wujun Gao
Guangqian Cheng,Keli Jia,Na Li,Chuanliang Deng,Shufen Li,Wujun Gao. Bioinformatics Analysis and Chromosome Location of Nuclear Integrants of Plastid DNA in Asparagus officinalis[J]. Chinese Bulletin of Botany, 2019, 54(3): 328-334.
| IR | LSC | SSC | |
|---|---|---|---|
| Total sequence length (bp) | 26471 | 83821 | 17904 |
| Number of NUPTs | 726 | 1227 | 286 |
| Average density (No./kb) | 27.9 | 14.6 | 15.9 |
| Average length of NUPTs (bp) | 241 | 258 | 246 |
| The length of NUPTs (bp) | 175215 | 316626 | 70394 |
Table 1 Origin analysis of nuclear integrants of plastid DNAs (NUPTs) sequences on the chloroplast genome of Asparagus officinalis
| IR | LSC | SSC | |
|---|---|---|---|
| Total sequence length (bp) | 26471 | 83821 | 17904 |
| Number of NUPTs | 726 | 1227 | 286 |
| Average density (No./kb) | 27.9 | 14.6 | 15.9 |
| Average length of NUPTs (bp) | 241 | 258 | 246 |
| The length of NUPTs (bp) | 175215 | 316626 | 70394 |
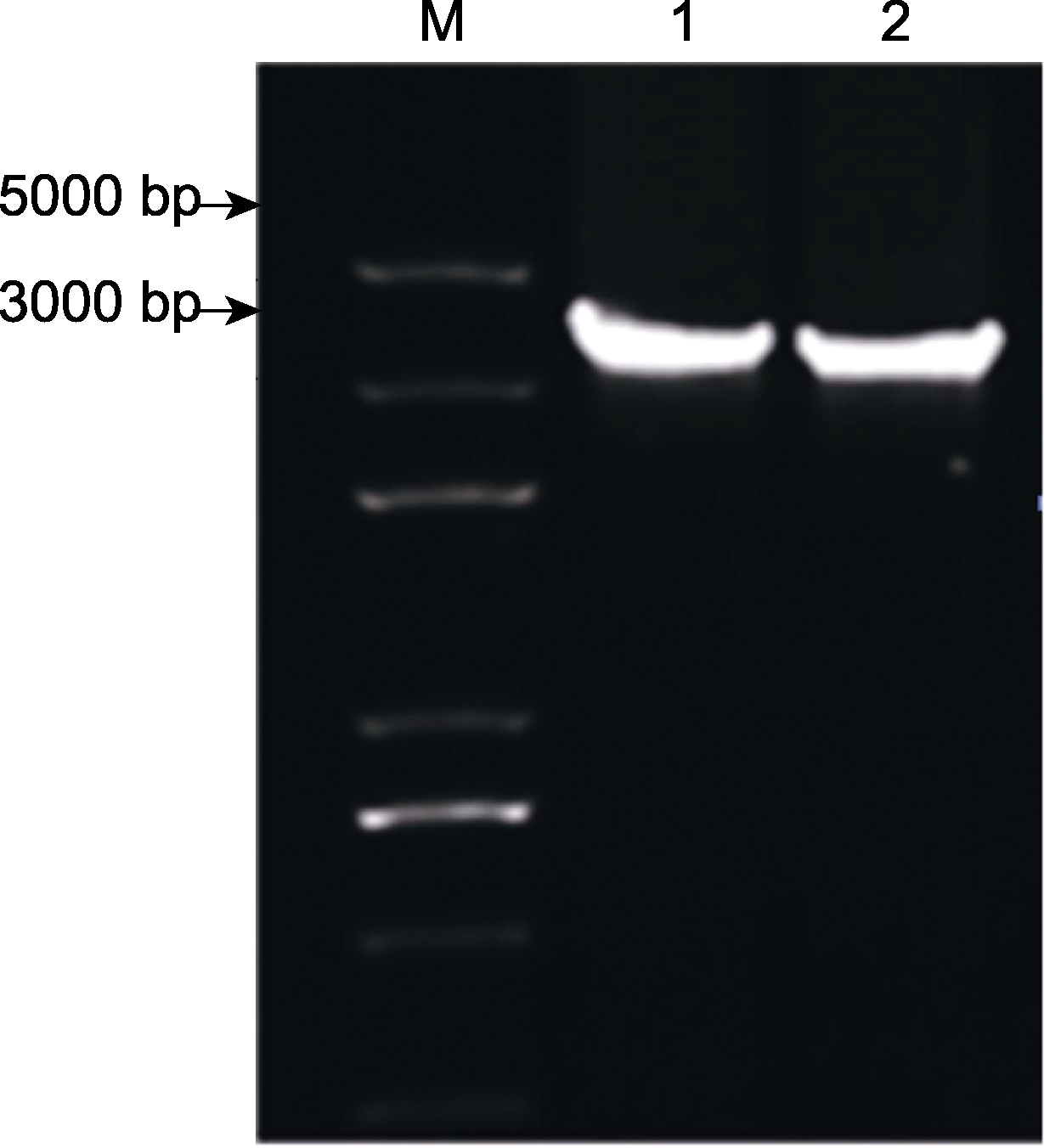
Figure 3 PCR amplification of inverted repeat region (IR) two sequences of region in Asparagus officinalis 1, 2 respresents the amplification results of AocplR1 and AocplR2, respectively.
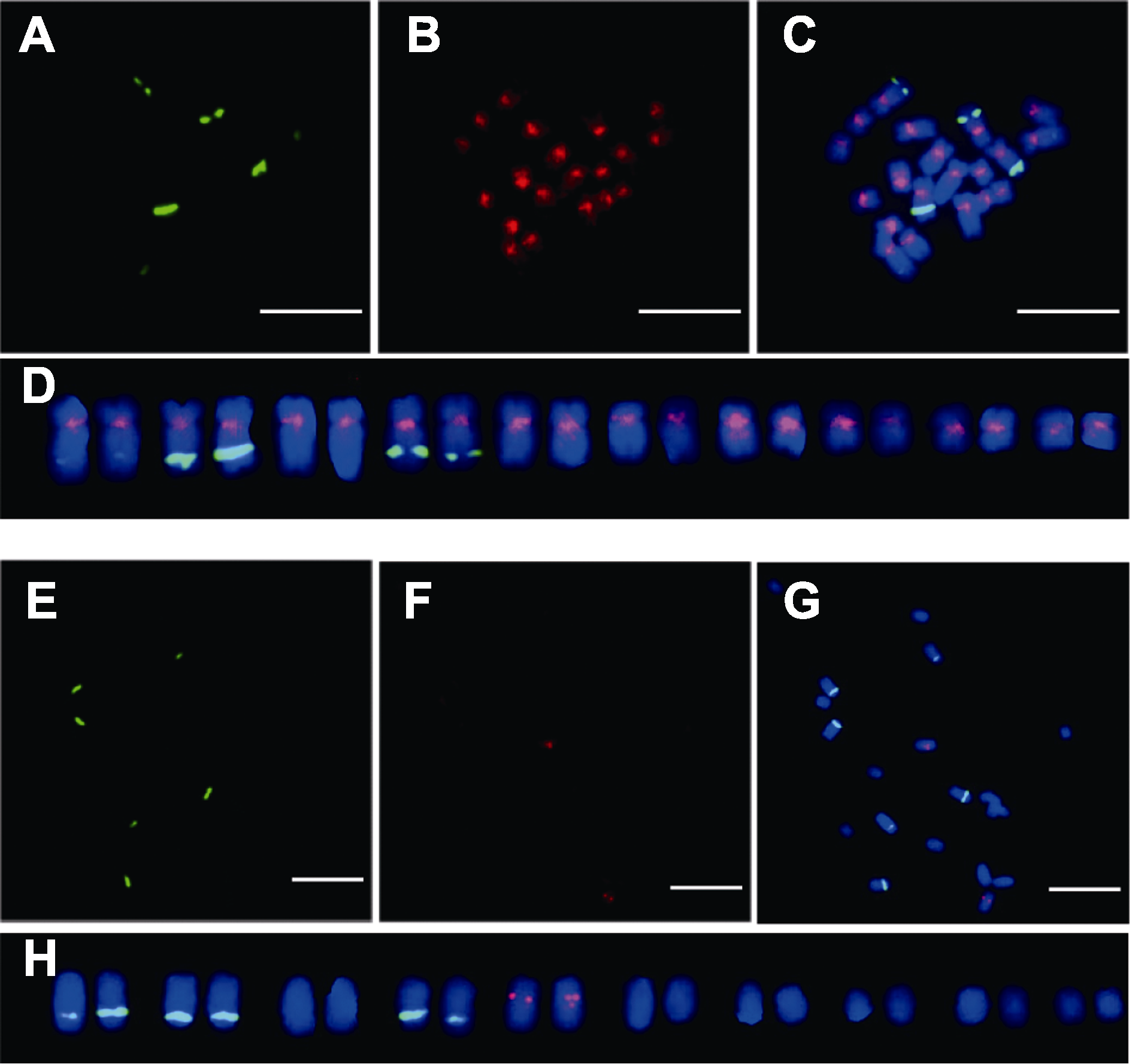
Figure 4 Mitosis metaphase chromosome locations of AocpIR1 and AocpIR2 of Asparagus officinalis (A), (E) FISH result of 45S rDNA; (B), (F) FISH result of AocpIR1 and AocpIR2; (C), (G) Merged picture; (D), (H) Karyotype analysis. Bars=10 μm
| [1] |
高东迎, 何冰, 孙立华 ( 2007). 水稻转座子研究进展. 植物学通报 24, 667-676.
DOI URL |
| [2] |
李巧丽, 延娜, 宋琼, 郭军战 ( 2018). 鲁桑叶绿体基因组序列及特征分析. 植物学报 53, 94-103.
DOI URL |
| [3] |
李书粉, 李旭, 王冰肖, 袁金红, 邓传良, 高武军 ( 2016). 石刁柏雄性偏向核质体DNA的克隆与分析. 西北植物学报 36, 2385-2390.
DOI URL |
| [4] |
Abbott JK, Nordén AK, Hansson B ( 2017). Sex chromosome evolution: historical insights and future perspectives. Proc Roy Soc B 284, 20162806.
DOI URL |
| [5] |
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ ( 1990). Basic local alignment search tool. J Mol Biol 215, 403-410.
DOI URL |
| [6] | Doyle JJ, Doyle JL ( 1987). A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19, 11-15. |
| [7] |
Feschotte C, Pritham EJ ( 2007). DNA transposons and the evolution of eukaryotic genomes. Annu Rev Genet 41, 331-368.
DOI URL PMID |
| [8] |
Guo XY, Ruan SL, Hu WM, Cai DG, Fan LJ ( 2008). Chloroplast DNA insertions into the nuclear genome of rice: the genes, sites and ages of insertion involved. Funct Integr Genomics 8, 101-108.
DOI URL |
| [9] |
Harkess A, Zhou JS, Xu CY, Bowers JE, van der Hulst R, Ayyampalayam S, Mercati F, Riccardi P, McKain MR, Kakrana A, Tang HB, Ray J, Groenendijk J, Arikit S, Mathioni SM, Nakano M, Shan HY, Telgmann-Rauber A, Kanno A, Yue Z, Chen HX, Li WQ, Chen YL, Xu XY, Zhang YP, Luo SC, Chen HL, Gao JM, Mao ZC, Pires JC, Luo MZ, Kudrna D, Wing RA, Meyers BC, Yi KX, Kong HZ, Lavrijsen P, Sunseri F, Falavigna A, Ye Y, Leebens-Mack JH, Chen GY ( 2017). The asparagus genome sheds light on the origin and evolution of a young Y chromosome. Nat Commun 8, 1279.
DOI URL PMID |
| [10] |
Kejnovsky E, Hobza R, Cermak T, Kubat Z, Vyskot B ( 2009). The role of repetitive DNA in structure and evolution of sex chromosomes in plants. Heredity 102, 533-541.
DOI |
| [11] |
Kejnovsky E, Kubat Z, Hobza R, Lengerova M, Sato S, Tabata S, Fukui K, Matsunaga S, Vyskot B ( 2006). Accumulation of chloroplast DNA sequences on the Y chromosome of Silene latifolia. Genetica 128, 167-175.
DOI URL PMID |
| [12] |
Kleine T, Maier UG, Leister D ( 2009). DNA transfer from organelles to the nucleus: the idiosyncratic genetics of endosymbiosis. Annu Rev Plant Biol 60, 115-138.
DOI URL PMID |
| [13] |
Li SF, Zhang GJ, Yuan JH, Deng CL, Gao WJ ( 2016). Repetitive sequences and epigenetic modification: inseparable partners play important roles in the evolution of plant sex chromosomes. Planta 243, 1083-1095.
DOI URL |
| [14] | Löptien H ( 1979). Identification of the sex chromosome pair in asparagus ( Asparagus officinalis L.). Z Pflanzenzüecht 82, 162-173. |
| [15] |
Martis MM, Klemme S, Banaei-Moghaddam AM, Blattner FR, Macas J, Schmutzer T, Scholz U, Gundlach H, Wicker T, Šimková H, Novák P, Neumann P, Kubaláková M, Bauer E, Haseneyer G, Fuchs J, Doležel J, Stein N, Mayer KFX, Houben A ( 2012). Selfish supernumerary chromosome reveals its origin as a mosaic of host genome and organellar sequences. Proc Natl Acad Sci USA 109, 13343-13346.
DOI URL |
| [16] |
Matsuo M, Ito Y, Yamauchi R, Obokata J ( 2005). The rice nuclear genome continuously integrates, shuffles, and eli- minates the chloroplast genome to cause chloroplast- nuclear DNA flux. Plant Cell 17, 665-675.
DOI URL |
| [17] |
Michalovova M, Vyskot B, Kejnovsky E ( 2013). Analysis of plastid and mitochondrial DNA insertions in the nucleus (NUPTs and NUMTs) of six plant species: size, relative age and chromosomal localization. Heredity 111, 314-320.
DOI URL PMID |
| [18] |
Richly E, Leister D ( 2004). NUMTs in sequenced eukaryotic genomes. Mol Biol Evol 21, 1081-1084.
DOI URL PMID |
| [19] | Sheng WT, Chai XW, Rao YS, Tu XT, Du SG ( 2017). Complete chloroplast genome sequence of asparagus ( Asparagus officinalis L.) and its phylogenetic position within asparagales. J Plant Breed Genet 5, 121-128. |
| [20] | Steflova P, Hobza R, Vyskot B, Kejnovsky E ( 2014). Strong accumulation of chloroplast DNA in the Y chromosomes of Rumex acetosa and Silene latifolia. Cytogenet Genome Res 142, 59-65. |
| [21] | Timmis JN, Ayliffe MA, Huang CY, Martin W ( 2004). Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes. Nat Rev Genet 5, 123-135. |
| [22] |
VanBuren R, Ming R ( 2013). Organelle DNA accumulation in the recently evolved papaya sex chromosomes. Mol Genet Genomics 288, 277-284.
DOI URL |
| [23] |
Wang RJ, Cheng CL, Chang CC, Wu CL, Su TM, Chaw SM ( 2008). Dynamics and evolution of the inverted repeat-large single copy junctions in the chloroplast genomes of monocots. BMC Evol Biol 8, 36.
DOI URL PMID |
| [24] |
Yoshida T, Furihata HY, Kawabe A ( 2014). Patterns of genomic integration of nuclear chloroplast DNA fragments in plant species. DNA Res 21, 127-140.
DOI URL PMID |
| [1] | Lansha Luo, Wenpei Song, Qingzhu Hua, Dawei Li, Hong Liang, Xianzhi Zhang. Research Progress on Plant Sex-determination Genes and Their Epigenetic Regulation [J]. Chinese Bulletin of Botany, 2024, 59(2): 278-290. |
| [2] | Dan Peng, Zhiqiang Wu. Progress on sex determination of dioecious plants [J]. Biodiv Sci, 2022, 30(3): 21416-. |
| [3] | Ge Li,Xiaoqing Meng,Zongyun Li,Mingku Zhu. Expression Patterns and Bioinformatic Analyses of Salt Stress Responsive Gene IbMYB3 in Ipomoea batatas [J]. Chinese Bulletin of Botany, 2020, 55(1): 38-48. |
| [4] | Li Qin, Jingli Chen, Changtian Pan, Lei Ye, Gang Lu. Research Progress in Plant Sex Chromosome Evolution and Sex Determination Genes [J]. Chinese Bulletin of Botany, 2016, 51(6): 841-848. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||