

Advances in Plant Flavonoid Transport and Accumulation Mechanism
Received date: 2023-05-22
Accepted date: 2023-10-24
Online published: 2023-12-01
Flavonoids are polyphenols compounds produced during the secondary metabolism of plants, which are widely present in plants and have various functions. Flavonoids biosynthesis takes place at the cytosolic side of the en- doplasmic reticulum (ER), but accumulation of various flavonoids is observed in the vacuole. Efficient transport and ac- cumulation systems are therefore required to transfer flavonoids from the ER into the vacuole. Certain researches for the transport of flavonoids has been done for decades. Current research results showed that: there are three transport mechanisms in plants, including glutathione S-transferase (GST), membrane transporters, and vesicle trafficking. Here, we reviewed the three transport mechanisms and advances of plant flavonoids transport in recent years. The functional cooperation of three distinct but nonexclusive mechanisms were summarized. While the biosynthesis of the flavonoids is well characterized across species, the research on flavonoids transport and accumulation is still relatively insufficient. For better understand the flavonoids transport and accumulation mechanism in plant, the relationship between flavonoids modification and transport, flavonoids transport substrate specificity and preference, and transcriptional regulation of flavonoids transport remain deeply unexplored.
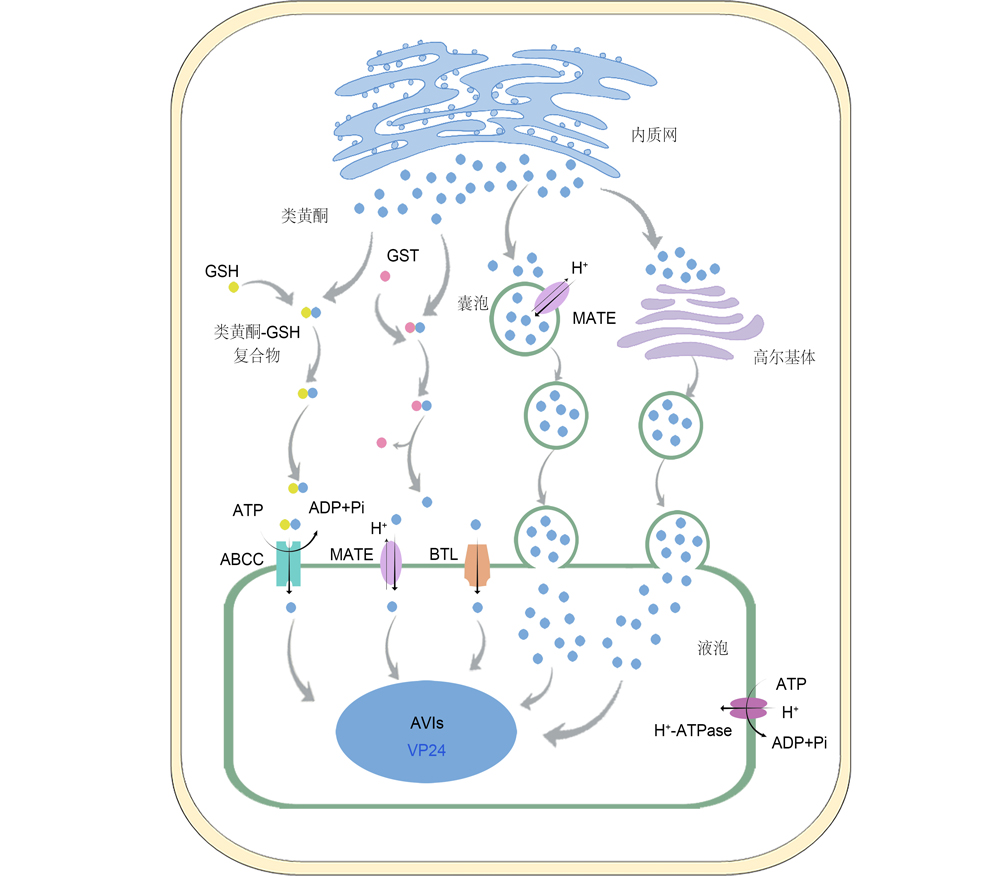
Jingwen Xie , Xiaoyun Cao , Wanqi Pan , Lingjuan Du . Advances in Plant Flavonoid Transport and Accumulation Mechanism[J]. Chinese Bulletin of Botany, 2024 , 59(3) : 463 -480 . DOI: 10.11983/CBB23066
| [1] | Agati G, Matteini P, Goti A, Tattini M (2007). Chloro- plast-located flavonoids can scavenge singlet oxygen. New Phytol 174, 77-89. |
| [2] | Alfenito MR, Souer E, Goodman CD, Buell R, Mol J, Koes R, Walbot V (1998). Functional complementation of an- thocyanin sequestration in the vacuole by widely divergent glutathione S-transferases. Plant Cell 10, 1135-1149. |
| [3] | Appelhagen I, Nordholt N, Seidel T, Spelt K, Koes R, Quattrochio F, Sagasser M, Weisshaar B (2015). TRANS- PARENT TESTA 13 is a tonoplast P3A-ATPase required for vacuolar deposition of proanthocyanidins in Arabidopsis thaliana seeds. Plant J 82, 840-849. |
| [4] | Baxter IR, Young JC, Armstrong G, Foster N, Bogen- schutz N, Cordova T, Peer WA, Hazen SP, Murphy AS, Harper JF (2005). A plasma membrane H+-ATPase is required for the formation of proanthocyanidins in the seed coat endothelium of Arabidopsis thaliana. Proc Natl Acad Sci USA 102, 2649-2654. |
| [5] | Behrens CE, Smith KE, Iancu CV, Choe JY, Dean JV (2019). Transport of anthocyanins and other flavonoids by the Arabidopsis ATP-binding cassette transporter AtABCC2. Sci Rep 9, 437. |
| [6] | Braidot E, Petrussa E, Bertolini A, Peresson C, Ermacora P, Loi N, Terdoslavich M, Passamonti S, Macrì F, Vianello A (2008). Evidence for a putative flavonoid translocator similar to mammalian bilitranslocase in grape berries (Vitis vinifera L.) during ripening. Planta 228, 203-213. |
| [7] | Buer CS, Muday GK, Djordjevic MA (2007). Flavonoids are differentially taken up and transported long distances in Arabidopsis. Plant Physiol 145, 478-490. |
| [8] | Cao YW, Xu LF, Xu H, Yang PP, He GR, Tang YC, Qi XY, Song M, Ming J (2021). LhGST is an anthocyanin-related glutathione S-transferase gene in Asiatic hybrid lilies (Lilium spp.). Plant Cell Rep 40, 85-95. |
| [9] | Chai QC, Wang XL, Gao MW, Zhao XC, Chen Y, Zhang C, Jiang H, Wang JB, Wang YC, Zheng MN, Baltaevich AM, Zhao J, Zhao JS (2023). A glutathione S-transferase GhTT19 determines flower petal pigmentation via regu- lating anthocyanin accumulation in cotton. Plant Biotechnol J 21, 433-448. |
| [10] | Chen KL, Du LJ, Liu HL, Liu YL (2019). A novel R2R3-MYB from grape hyacinth, MaMybA, which is dif- ferent from MaAN2, confers intense and magenta antho- cyanin pigmentation in tobacco. BMC Plant Biol 19, 390. |
| [11] | Chen KL, Liu HL, Lou Q, Liu YL (2017). Corrigendum: ectopic expression of the grape hyacinth (Muscari arme- niacum) R2R3-MYB transcription factor gene, MaAN2, in- duces anthocyanin accumulation in tobacco. Front Plant Sci 8, 1722. |
| [12] | Chen S, Wang XJ, Cheng Y, Gao HS, Chen XH (2023). A review of classification, biosynthesis, biological activities and potential applications of flavonoids. Molecules 28, 4982. |
| [13] | Chen SY, Tang YM, Hu YY, Wang Y, Sun B, Wang XR, Tang HR, Chen Q (2018). FaTT12-1, a multidrug and toxin extrusion (MATE) member involved in proanthocya- nidin transport in strawberry fruits. Sci Hortic 231, 158-165. |
| [14] | Cheng J, Liao L, Zhou H, Gu C, Wang L, Han YP (2015). A small indel mutation in an anthocyanin transporter causes variegated colouration of peach flowers. J Exp Bot 66, 7227-7239. |
| [15] | Conn S, Curtin C, Bézier A, Franco C, Zhang W (2008). Purification, molecular cloning, and characterization of glutathione S-transferases (GSTs) from pigmented Vitis vinifera L. cell suspension cultures as putative anthocya- nin transport proteins. J Exp Bot 59, 3621-3634. |
| [16] | Conn S, Franco C, Zhang W (2010). Characterization of anthocyanic vacuolar inclusions in Vitis vinifera L. cell sus- pension cultures. Planta 231, 1343-1360. |
| [17] | Conn S, Zhang W, Franco C (2003). Anthocyanic vacuolar inclusions (AVIs) selectively bind acylated anthocyanins in Vitis vinifera L. (grapevine) suspension culture. Biotechnol Lett 25, 835-839. |
| [18] | Cui YM, Fan JW, Lu CF, Ren JS, Qi FT, Huang H, Dai SL (2021). ScGST3 and multiple R2R3-MYB transcription factors function in anthocyanin accumulation in Senecio cruentus. Plant Sci 313, 111094. |
| [19] | Dean JV, Willis M, Shaban L (2022). Transport of acylated anthocyanins by the Arabidopsis ATP-binding cassette transporters AtABCC1, AtABCC2, and AtABCC14. Physiol Plant 174, e13780. |
| [20] | Debeaujon I, Peeters AJM, Léon-Kloosterziel KM, Koornneef M (2001). The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter- like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell 13, 853-871. |
| [21] | Feucht W, Treutter D, Polster J (2004). Flavanol binding of nuclei from tree species. Plant Cell Rep 22, 430-436. |
| [22] | Francisco RM, Regalado A, Ageorges A, Burla BJ, Bassin B, Eisenach C, Zarrouk O, Vialet S, Marlin T, Chaves MM, Martinoia E, Nagya R (2013). ABCC1, an ATP binding cassette protein from grape berry, transports anthocyanidin 3-O-glucosides. Plant Cell 25, 1840-1854. |
| [23] | Frank S, Keck M, Sagasser M, Niehaus K, Weisshaar B, Stracke R (2011). Two differentially expressed MATE factor genes from apple complement the Arabidopsis trans- parent testa12mutant. Plant Biol 13, 42-50. |
| [24] | Gani U, Nautiyal AK, Kundan M, Rout B, Pandey A, Misra P (2022). Two homeologous MATE transporter genes, NtMATE21 and NtMATE22, are involved in the modula- tion of plant growth and flavonol transport in Nicotiana tabacum. J Exp Bot 73, 6186-6206. |
| [25] | Gao JS, Wu N, Shen ZL, Lv K, Qian SH, Guo N, Sun X, Cai YP, Lin Y (2016). Molecular cloning, expression analysis and subcellular localization of a Transparent Testa12 ortholog in brown cotton (Gossypium hirsutum L.). Gene 576, 763-769. |
| [26] | Gaxiola RA, Fink GR, Hirschi KD (2002). Genetic ma- nipulation of vacuolar proton pumps and transporters. Plant Physiol 129, 967-973. |
| [27] | Gomez C, Conejero G, Torregrosa L, Cheynier V, Terrier N, Ageorges A (2011). In vivo grapevine anthocyanin transport involves vesicle-mediated trafficking and the contribution of anthoMATE transporters and GST. Plant J 67, 960-970. |
| [28] | Gomez C, Terrier N, Torregrosa L, Vialet S, Fournier- Level A, Verries C, Souquet JM, Mazauric JP, Klein M, Cheynier V, Ageorges A (2009). Grapevine MATE-type proteins act as vacuolar H+-dependent acylated anthocyanin transporters. Plant Physiol 150, 402-415. |
| [29] | Goodman CD, Casati P, Walbot V (2004). A multidrug resistance-associated protein involved in anthocyanin tran- sport in Zea mays. Plant Cell 16, 1812-1826. |
| [30] | Han LL, Zhou L, Zou HZ, Yuan M, Wang Y (2022). PsGSTF3, an anthocyanin-related glutathione S-transferase gene, is essential for petal coloration in tree peony. Int J Mol Sci 23, 1423. |
| [31] | He X, Szewczyk P, Karyakin A, Evin M, Hong WX, Zhang QH, Chang G (2010). Structure of a cation-bound mul- tidrug and toxic compound extrusion transporter. Nature 467, 991-994. |
| [32] | Hsieh K, Huang AHC (2007). Tapetosomes in Brassica tapetum accumulate endoplasmic reticulum-derived fla- vonoids and alkanes for delivery to the pollen surface. Plant Cell 19, 582-596. |
| [33] | Hu B, Zhao JT, Lai B, Qin YH, Wang HC, Hu GB (2016a). LcGST4 is an anthocyanin-related glutathione S-transferase gene in Litchi chinensis Sonn. Plant Cell Rep 35, 831-843. |
| [34] | Hu DG, Sun CH, Ma QJ, You CX, Cheng LL, Hao YJ (2016b). MdMYB1 regulates anthocyanin and malate accumulation by directly facilitating their transport into vacuoles in apples. Plant Physiol 170, 1315-1330. |
| [35] | Hvorup RN, Winnen B, Chang AB, Jiang Y, Zhou XF, Saier MH (2003). The multidrug/oligosaccharidyl-lipid/polysaccharide (MOP) exporter superfamily. Eur J Biochem 270, 799-813. |
| [36] | Ichino T, Fuji K, Ueda H, Takahashi H, Koumoto Y, Takagi J, Tamura K, Sasaki R, Aoki K, Shimada T, Hara-Nishimura I (2014). GFS9/TT9 contributes to in- tracellular membrane trafficking and flavonoid accumula- tion in Arabidopsis thaliana. Plant J 80, 410-423. |
| [37] | Ishikawa T, Li ZS, Lu YP, Rea PA (1997). The GS-X pump in plant, yeast, and animal cells: structure, function, and gene expression. Biosci Rep 17, 189-207. |
| [38] | Jiang SH, Chen M, He NB, Chen XL, Wang N, Sun QG, Zhang TH, Xu HF, Fang HC, Wang YC, Zhang ZY, Wu SJ, Chen XS (2019). MdGSTF6, activated by MdMYB1, plays an essential role in anthocyanin accumulation in apple. Hortic Res 6, 40. |
| [39] | Kaur S, Sharma N, Kapoor P, Chunduri V, Pandey AK, Garg M (2021). Spotlight on the overlapping routes and partners for anthocyanin transport in plants. Physiol Plant 171, 868-881. |
| [40] | Kimble R, Keane KM, Lodge JK, Howatson G (2019). Dietary intake of anthocyanins and risk of cardiovascular disease: a systematic review and meta-analysis of pro- spective cohort studies. Crit Rev Food Sci Nutr 59, 3032-3043. |
| [41] | Kitamura S, Akita Y, Ishizaka H, Narumi I, Tanaka A (2012). Molecular characterization of an anthocyanin-related glutathione S-transferase gene in cyclamen. J Plant Physiol 169, 636-642. |
| [42] | Kitamura S, Matsuda F, Tohge T, Yonekura-Sakakibara K, Yamazaki M, Saito K, Narumi I (2010). Metabolic profiling and cytological analysis of proanthocyanidins in immature seeds of Arabidopsis thaliana flavonoid accu- mulation mutants. Plant J 62, 549-559. |
| [43] | Kitamura S, Oono Y, Narumi I (2016). Arabidopsis pab1, a mutant with reduced anthocyanins in immature seeds from banyuls, harbors a mutation in the MATE transporter FFT. Plant Mol Biol 90, 7-18. |
| [44] | Kitamura S, Shikazono N, Tanaka A (2004). TRANS- PARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis. Plant J 37, 104-114. |
| [45] | Koes R, Verweij W, Quattrocchio F (2005). Flavonoids: a colorful model for the regulation and evolution of bio- chemical pathways. Trends Plant Sci 10, 236-242. |
| [46] | Kou M, Liu YJ, Li ZY, Zhang YG, Tang W, Yan H, Wang X, Chen XG, Su ZX, Arisha MH, Li Q, Ma DF (2019). A novel glutathione S-transferase gene from sweet potato, IbGSTF4, is involved in anthocyanin sequestration. Plant Physiol Biochem 135, 395-403. |
| [47] | Ku YS, Cheng SS, Cheung MY, Niu YC, Liu AL, Chung G, Lam HM (2022). The poly-glutamate motif of GmMATE4 regulates its isoflavone transport activity. Membranes 12, 206. |
| [48] | Kubo H, Nozue M, Kawasaki K, Yasuda H (1995). Intravacuolar spherical bodies in Polygonum cuspidatum. Plant Cell Physiol 36, 1453-1458. |
| [49] | Kulich I, Pe?enková T, Sekere? J, Smetana O, Fendrych M, Foissner I, H?ftberger M, ?ársky V (2013). Arabi- dopsis exocyst subcomplex containing subunit EXO70B1 is involved in autophagy-related transport to the vacuole. Traffic 14, 1155-1165. |
| [50] | Lai B, You Y, Zhang LL, Wang QX, Chen FB, Luo GJ, Du LN, Wang HC (2021). Identification and functional characterization of RsGST1, an anthocyanin-related glutathione S-transferase gene in radish. J Plant Physiol 263, 153468. |
| [51] | Le Roy J, Huss B, Creach A, Hawkins S, Neutelings G (2016). Glycosylation is a major regulator of phenylpro- panoid availability and biological activity in plants. Front Plant Sci 7, 735. |
| [52] | Li CX, Yu WJ, Xu JR, Lu XF, Liu YZ (2022). Anthocyanin biosynthesis induced by MYB transcription factors in plants. Int J Mol Sci 23, 11701. |
| [53] | Lin BW, Gong CC, Song HF, Cui YY (2017). Effects of anthocyanins on the prevention and treatment of cancer. Br J Clin Pharmacol 174, 1226-1243. |
| [54] | Liu HT (2020). Functional Analysis of Anthocyanin Trans- porters GSTF12 and TT12 in ‘Red Zaosu’ Pear. Master’s thesis. Yangling: Northwest A&F University. pp. 35-43. (in Chinese) |
| 刘汉婷 (2020). ‘红早酥’梨花青苷转运蛋白GSTF12和TT12的功能分析. 硕士论文. 杨凌: 西北农林科技大学. pp. 35-43. | |
| [55] | Liu SC, Li YC, Fang HT, Huang BY, Zhao CN, Sun CD, Li SJ, Chen KS (2022). Genome-wide identification and expression analysis of MATE gene family in citrus fruit (Citrus clementina). Genomics 114, 110446. |
| [56] | Liu YF, Qi YW, Zhang AL, Wu HX, Liu ZD, Ren XL (2019). Molecular cloning and functional characterization of AcGST1, an anthocyanin-related glutathione S-transferase gene in kiwifruit (Actinidia chinensis). Plant Mol Biol 100, 451-465. |
| [57] | Lu M, Radchenko M, Symersky J, Nie RX, Guo Y (2013). Structural insights into H+-coupled multidrug extrusion by a MATE transporter. Nat Struct Mol Biol 20, 1310-1317. |
| [58] | Luo HF, Dai C, Li YP, Feng J, Liu ZC, Kang CY (2018). Reduced anthocyanins in petioles codes for a GST an- thocyanin transporter that is essential for the foliage and fruit coloration in strawberry. J Exp Bot 69, 2595-2608. |
| [59] | Marinova K, Pourcel L, Weder B, Schwarz M, Barron D, Routaboul JM, Debeaujon I, Klein M (2007). The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+-antiporter active in proanthocyanidin-accumulating cells of the seed coat. Plant Cell 19, 2023-2038. |
| [60] | Markham KR, Gould KS, Winefield CS, Mitchell KA, Bloor SJ, Boase MR (2000). Anthocyanic vacuolar in- clusions—their nature and significance in flower colouration. Phytochemistry 55, 327-336. |
| [61] | Marrs KA, Alfenito MR, Lloyd AM, Walbot V (1995). A glutathione-S-transferase involved in vacuolar transfer encoded by the maize gene Bronze-2. Nature 375, 397-400. |
| [62] | Mathews H, Clendennen SK, Caldwell CG, Liu XL, Connors K, Matheis N, Schuster DK, Menasco DJ, Wagoner W, Lightner J, Wagner DR (2003). Activation tagging in tomato identifies a transcriptional regulator of anthocyanin biosynthesis, modification, and transport. Plant Cell 15, 1689-1703. |
| [63] | Mentewab A, Stewart CN (2005). Overexpression of an Arabidopsis thaliana ABC transporter confers kanamycin resistance to transgenic plants. Nat Biotechnol 23, 1177-1180. |
| [64] | Mueller LA, Goodman CD, Silady RA, Walbot V (2000). AN9, a petunia glutathione S-transferase required for an- thocyanin sequestration, is a flavonoid-binding protein. Plant Physiol 123, 1561-1570. |
| [65] | Mugford ST, Milkowski C (2012). Serine carboxypepti dase-like acyltransferases from plants. Methods Enzymol 516, 279-297. |
| [66] | Naing AH, Park DY, Park KI, Kim CK (2018). Differential expression of anthocyanin structural genes and transcrip tion factors determines coloration patterns in gerbera flowers. 3 Biotech 8, 393. |
| [67] | Ng MS, Ku YS, Yung WS, Cheng SS, Man CK, Yang L, Song SK, Chung G, Lam HM (2021). MATE-type proteins are responsible for isoflavone transportation and accumulation in soybean seeds. Int J Mol Sci 22, 12017. |
| [68] | Nishizaki Y, Sasaki N, Yasunaga M, Miyahara T, Okamoto E, Okamoto M, Hirose Y, Ozeki Y (2014). Ide- ntification of the glucosyltransferase gene that supplies the p-hydroxybenzoyl-glucose for 7-polyacylation of ant- hocyanin in delphinium. J Exp Bot 65, 2495-2506. |
| [69] | Nishizaki Y, Yasunaga M, Okamoto E, Okamoto M, Hi- rose Y, Yamaguchi M, Ozeki Y, Sasaki N (2013). p-hydroxybenzoyl-glucose is a zwitter donor for the bio- synthesis of 7-polyacylated anthocyanin in Delphinium. Plant Cell 25, 4150-4165. |
| [70] | Niu MY, Bao CJ, Chen JH, Zhou W, Zhang YY, Zhang XY, Su NN, Cui J (2022). RsGSTF12contributes to antho- cyanin sequestration in radish (Raphanus sativus L.). Front Plant Sci 13, 870202. |
| [71] | Nozue M, Baba S, Kitamura Y, Xu WX, Kubo H, Nogawa M, Shioiri H, Kojima M (2003). VP24 found in antho- cyanic vacuolar inclusions (AVIs) of sweet potato cells is a member of a metalloprotease family. Biochem Eng J 14, 199-205. |
| [72] | Nozue M, Kubo H, Nishimura M, Katou A, Hattori C, Usuda N, Nagata T, Yasuda H (1993). Characterization of intravacuolar pigmented structures in anthocyanin- containing cells of sweet potato suspension cultures. Plant Cell Physiol 34, 803-808. |
| [73] | Nozue M, Yamada K, Nakamura T, Kubo H, Kondo M, Nishimura M (1997). Expression of a vacuolar protein (VP24) in anthocyanin-producing cells of sweet potato in suspension culture. Plant Physiol 115, 1065-1072. |
| [74] | Nozzolillo C, Ishikura N (1988). An investigation of the intracellular site of anthocyanoplasts using isolated protoplasts and vacuoles. Plant Cell Rep 7, 389-392. |
| [75] | Oakley A (2011). Glutathione transferases: a structural perspective. Drug Metab Rev 43, 138-151. |
| [76] | Pal L, Dwivedi V, Gupta SK, Saxena S, Pandey A, Chat- topadhyay D (2023). Biochemical analysis of anthocya- nin and proanthocyanidin and their regulation in deter- mining chickpea flower and seed coat colour. J Exp Bot 74, 130-148. |
| [77] | Passamonti S, Cocolo A, Braidot E, Petrussa E, Peresson C, Medic N, Macri F, Vianello A (2005). Cha- racterization of electrogenic bromosulfophthalein transpo- rt in carnation petal microsomes and its inhibition by anti- bodies against bilitranslocase. FEBS J 272, 3282-3296. |
| [78] | Pérez-Díaz R, Madrid-Espinoza J, Salinas-Cornejo J, González-Villanueva E, Ruiz-Lara S (2016). Differential roles for VviGST1, VviGST3, and VviGST4 in proantho- cyanidin and anthocyanin transport in Vitis vinífera. Front Plant Sci 7, 1166. |
| [79] | Pérez-Díaz R, Ryngajllo M, Pérez-Díaz J, Pe?a-Cortés H, Casaretto JA, González-Villa-nueva E, Ruiz-Lara S (2014). VvMATE1 and VvMATE2 encode putative proanthocyanidin transporters expressed during berry development in Vitis vinifera L. Plant Cell Rep 33, 1147-1159. |
| [80] | Petrussa E, Braidot E, Zancani M, Peresson C, Bertolini A, Patui S, Vianello A (2013). Plant flavonoids-biosynthesis, transport and involvement in stress responses. Int J Mol Sci 14, 14950-14973. |
| [81] | Pourcel L, Irani NG, Lu YH, Riedl K, Schwartz S, Grote- wold E (2010). The formation of anthocyanic vacuolar inclusions in Arabidopsis thaliana and implications for the sequestration of anthocyanin pigments. Mol Plant 3, 78-90. |
| [82] | Pucker B, Selmar D (2022). Biochemistry and molecular basis of intracellular flavonoid transport in plants. Plants 11, 963. |
| [83] | Putta S, Yarla NS, Eswar Kumar K, Lakkappa DB, Kamal MA, Scotti L, Scotti MT, Ashraf GM, Rao BSB, Sarala Kumari D, Reddy GV, Tarasov VV, Imandi SB, Aliev G (2018). Preventive and therapeutic potentials of antho- cyanins in diabetes and associated complications. Curr Med Chem 25, 5347-5371. |
| [84] | Qi XL, Liu CL, Song LL, Dong YX, Chen L, Li M (2022). A sweet cherry glutathione S-transferase gene, PavGST1, plays a central role in fruit skin coloration. Cells 11, 1170. |
| [85] | Rea PA (1999). MRP subfamily ABC transporters from plants and yeast. J Exp Bot 50, 895-913. |
| [86] | Sasaki N, Nakayama T (2015). Achievements and perspec- tives in biochemistry concerning anthocyanin modification for blue flower coloration. Plant Cell Physiol 56, 28-40. |
| [87] | Sasaki N, Nishizaki Y, Uchida Y, Wakamatsu E, Umemoto N, Momose M, Okamura M, Yoshida H, Yama- guchi M, Nakayama M, Ozeki Y, Itoh Y (2012). Identifi- cation of the glutathione S-transferase gene responsible for flower color intensity in carnations. Plant Biotechnol 29, 223-227. |
| [88] | Saslowsky DE, Warek U, Winkel BSJ (2005). Nuclear localization of flavonoid enzymes in Arabidopsis. J Biol Chem 280, 23735-23740. |
| [89] | Shao DN, Li YJ, Zhu QH, Zhang XY, Liu F, Xue F, Sun J (2021). GhGSTF12, a glutathione S-transferase gene, is essential for anthocyanin accumulation in cotton (Gos- sypium hirsutum L.). Plant Sci 305, 110827. |
| [90] | Sharma S, Chunduri V, Kumar A, Kumar R, Khare P, Kondepudi KK, Bishnoi M, Garg M (2018). Anthocyanin biofortified colored wheat: nutritional and functional characterization. PLoS One 13, e0194367. |
| [91] | Small CJ, Pecket RC (1982). The ultrastructure of antho- cyanoplasts in red-cabbage. Planta 154, 97-99. |
| [92] | Smith AP, Nourizadeh SD, Peer WA, Xu JH, Bandyopadhyay A, Murphy AS, Goldsbrough PB (2003). Arabidopsis AtGSTF2 is regulated by ethylene and auxin, and encodes a glutathione S-transferase that interacts with flavonoids. Plant J 36, 433-442. |
| [93] | Song XW, Wei JB, Di SK, Pang YZ (2019). Recent advances in the regulation mechanism of transcription fa- ctors and metabolic engineering of anthocyanins. Chin Bull Bot 54, 133-156. (in Chinese) |
| 宋雪薇, 魏解冰, 狄少康, 庞永珍 (2019). 花青素转录因子调控机制及代谢工程研究进展. 植物学报 54, 133-156. | |
| [94] | Sun Y, Li H, Huang JR (2012). Arabidopsis TT19 functions as a carrier to transport anthocyanin from the cytosol to tonoplasts. Mol Plant 5, 387-400. |
| [95] | Sylvia C, Sun JL, Zhang YQ, Ntini C, Ogutu C, Zhao Y, Han YP (2023). Genome-wide analysis of ATP Binding Cassette (ABC) transporters in peach (Prunus persica) and identification of a gene PpABCC1involved in antho- cyanin accumulation. Int J Mol Sci 24, 1931. |
| [96] | Takanashi K, Shitan N, Yazaki K (2014). The multidrug and toxic compound extrusion (MATE) family in plants. Plant Biotechnol 31, 417-430. |
| [97] | Thompson EP, Wilkins C, Demidchik V, Davies JM, Glover BJ (2010). An Arabidopsis flavonoid transporter is required for anther dehiscence and pollen development. J Exp Bot 61, 439-451. |
| [98] | Verweij W, Spelt C, Di Sansebastiano GP, Vermeer J, Reale L, Ferranti F, Koes R, Quattrocchio F (2008). An H+ P-ATPase on the tonoplast determines vacuolar pH and flower colour. Nat Cell Biol 10, 1456-1462. |
| [99] | Vilperte V, Boehm R, Debener T (2021). A highly mutable GST is essential for bract colouration in Euphorbia pul- cherrima Willd. Ex Klotsch. BMC Genomics 22, 208. |
| [100] | Wagner U, Edwards R, Dixon DP, Mauch F (2002). Probing the diversity of the Arabidopsis glutathione S-transferase gene family. Plant Mol Biol 49, 515-532. |
| [101] | Wang JY, Zhang H, Tian ST, Hao WH, Chen KL, Du LJ (2022a). The R2R3MYB transcription factors MaMYBF and MaMYB1 regulate flavonoid biosynthesis in grape hyacinth. Plant Physiol Biochem 194, 85-95. |
| [102] | Wang RR, Lu N, Liu CG, Dixon RA, Wu Q, Mao YW, Yang YT, Zheng XL, He LL, Zhao BL, Zhang F, Yang SC, Chen HT, Jun JH, Li Y, Liu CN, Liu Y, Chen JH (2022b). MtGSTF7, a TT19-like GST gene, is essential for accu- mulation of anthocyanins, but not proanthocyanins in Medicago truncatula. J Exp Bot 73, 4129-4146. |
| [103] | Wang SQ, Pan DZ, Lv XJ, Song XM, Qiu ZM, Huang CM, Huang RH, Chen W (2016). Proteomic approach reveals that starch degradation contributes to anthocyanin accumulation in tuberous root of purple sweet potato. J Proteomics 143, 298-305. |
| [104] | Wang YC, Liu WJ, Jiang HY, Mao ZL, Wang N, Jiang SH, Xu HF, Yang GX, Zhang ZY, Chen XS (2019). The R2R3-MYB transcription factor MdMYB24-like is involved in methyl jasmonate-induced anthocyanin biosynthesis in apple. Plant Physiol Biochem 139, 273-282. |
| [105] | Wei K, Wang LY, Zhang YZ, Ruan L, Li HL, Wu LY, Xu LY, Zhang CC, Zhou XG, Cheng H, Edwards R (2019). A coupled role for CsMYB75 and CsGSTF1 in anthocyanin hyperaccumulation in purple tea. Plant J 97, 825-840. |
| [106] | Winkel-Shirley B (2001). Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and bio- technology. Plant Physiol 126, 485-493. |
| [107] | Wolf AE, Dietz KJ, Schr?der P (1996). Degradation of glutathione S-conjugates by a carboxypeptidase in the plant vacuole. FEBS Lett 384, 31-34. |
| [108] | Wu J, Cheng JH, Yang FC (2006). Transcriptional regula- tion of anthocyanin biosynthesis in plants. Chinese Jour- nal of Cell Biology 28, 453-456. (in Chinese) |
| 吴江, 程建徽, 杨夫臣 (2006). 植物花色素苷生物合成的转录调控. 细胞生物学杂志 28, 453-456. | |
| [109] | Xiong J, Feng JM, Yuan DX, Zhou J, Miao W (2015). Tracing the structural evolution of eukaryotic ATP binding cassette transporter superfamily. Sci Rep 5, 16724. |
| [110] | Xu H, Yang PP, Cao YW, Tang YC, He GR, Xu LF, Ming J (2020). Cloning and functional characterization of a fla- vonoid transport-related MATE gene in Asiatic hybrid lilies (Lilium spp.). Genes 11, 418. |
| [111] | Xu WJ, Dubos C, Lepiniec L (2015). Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci 20, 176-185. |
| [112] | Xue L, Huang XR, Zhang ZH, Lin QH, Zhong QZ, Zhao Y, Gao ZS, Xu CJ (2022). An anthocyanin-related glu- tathione S-transferase, MrGST1, plays an essential role in fruit coloration in Chinese bayberry (Morella rubra). Front Plant Sci 13, 903333. |
| [113] | Yamazaki M, Shibata M, Nishiyama Y, Springob K, Kita- yama M, Shimada N, Aoki T, Ayabe SI, Saito K (2008). Differential gene expression profiles of red and green forms of Perilla frutescens leading to comprehensive iden- tification of anthocyanin biosynthetic genes. FEBS J 275, 3494-3502. |
| [114] | Yang SC, Jiang Y, Xu LQ, Shiratake K, Luo ZR, Zhang QL (2016). Molecular cloning and functional characterization of DkMATE1 involved in proanthocyanidin precursor transport in persimmon (Diospyros kaki Thunb.) fruit. Plant Physiol Biochem 108, 241-250. |
| [115] | Yuan JW, Qiu ZJ, Long Y, Liu YZ, Huang JJ, Liu JX, Yu YX (2023). Functional identification of PhMATE1 in flower color formation in petunia. Physiol Plant 175, e13949. |
| [116] | Zhang HB, Wang L, Deroles S, Bennett R, Davies K (2006a). New insight into the structures and formation of anthocyanic vacuolar inclusions in flower petals. BMC Plant Biol 6, 29. |
| [117] | Zhang TR, Zhang HC, Wu RH (2020). Recent advances on blue flower formation. Chin Bull Bot 55, 216-227. (in Chinese) |
| 张泰然, 张和臣, 武荣花 (2020). 蓝色花形成分子机理研究进展. 植物学报 55, 216-227. | |
| [118] | Zhang XY, Wang XL, Wang XF, Xia GH, Pan QH, Fan RC, Wu FQ, Yu XC, Zhang DP (2006b). A shift of phloem unloading from symplasmic to apoplasmic pathway is in- volved in developmental onset of ripening in grape berry. Plant Physiol 142, 220-232. |
| [119] | Zhang Z, Tian CP, Zhang Y, Li CZY, Li X, Yu Q, Wang S, Wang XY, Chen XS, Feng SQ (2020). Transcriptomic and metabolomic analysis provides insights into antho- cyanin and procyanidin accumulation in pear. BMC Plant Biol 20, 129. |
| [120] | Zhao J (2015). Flavonoid transport mechanisms: how to go, and with whom. Trends Plant Sci 20, 576-585. |
| [121] | Zhao J, Dixon RA (2009). MATE transporters facilitate vacuolar uptake of epicatechin 3'-O-glucoside for proan- thocyanidin biosynthesis in Medicago truncatula and Arabidopsis. Plant Cell 21, 2323-2340. |
| [122] | Zhao J, Dixon RA (2010). The ‘ins’ and ‘outs’ of flavonoid transport. Trends Plant Sci 15, 72-80. |
| [123] | Zhao J, Huhman D, Shadle G, He XZ, Sumner LW, Tang YH, Dixon RA (2011). MATE2 mediates vacuolar se- questration of flavonoid glycosides and glycoside malo- nates in Medicago truncatula. Plant Cell 23, 1536-1555. |
| [124] | Zhao Y, Dong WQ, Zhu YC, Allan AC, Kui LW, Xu CJ (2020). PpGST1, an anthocyanin-related glutathione S-transferase gene, is essential for fruit coloration in peach. Plant Biotechnol J 18, 1284-1295. |
| [125] | Zhu QL, Xie XR, Zhang J, Xiang G, Li Y, Wu HB (2013). In silico analysis of a MRP transporter gene reveals its pos- sible role in anthocyanins or flavonoids transport in Oryza sativa. Am J Plant Sci 4, 555-560. |
| [126] | Zhu ZX, Lu YQ (2016). Plant color mutants and the antho- cyanin pathway. Chin Bull Bot 51, 107-119. (in Chinese) |
| 祝志欣, 鲁迎青 (2016). 花青素代谢途径与植物颜色变异. 植物学报 51, 107-119. |
/
| 〈 |
|
〉 |