

The Arabidopsis HSP1 Mediates Chitin-induced Defense Response by Regulating CERK1 Protein Level
Received date: 2022-06-07
Accepted date: 2022-11-15
Online published: 2022-11-29
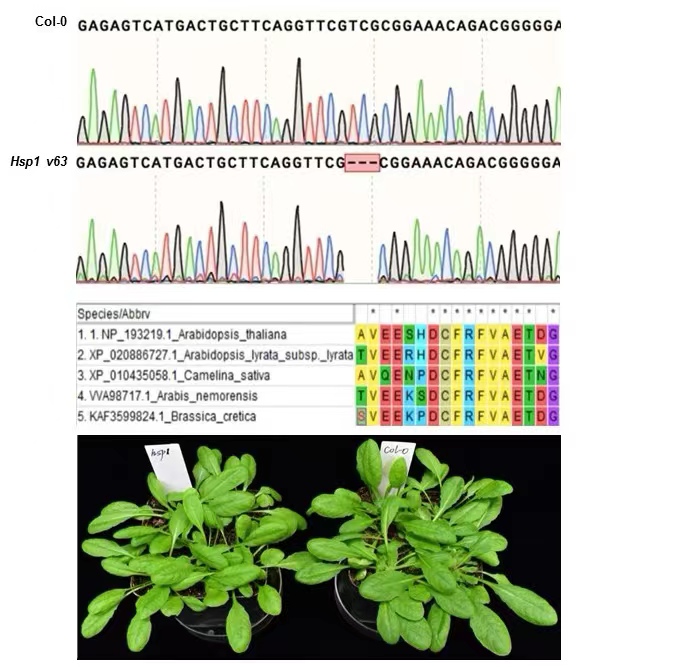
Key words: chitin; receptor defense response; protein level; molecular chaperones
Shi Junxing, Yan Yijia, Dong Ru, Tao Xuan, Sun Xiaolong, Huang Congcong . The Arabidopsis HSP1 Mediates Chitin-induced Defense Response by Regulating CERK1 Protein Level[J]. Chinese Bulletin of Botany, 2023 , 58(5) : 712 -719 . DOI: 10.11983/CBB22117
| [1] | 欧阳石文, 赵开军, 冯兰香 (2001). 植物几丁质酶的结构与功能、分类及进化. 植物学通报 18, 418-426. |
| [2] | 张静, 杨洪强, 魏钦平 (2003). 几丁质及其衍生物的生物活性与在农业中的应用. 植物学通报 20, 178-183. |
| [3] | Antolín-Llovera M, Ried MK, Binder A, Parniske M (2012). Receptor kinase signaling path-ways in plant-microbe in-teractions. Annu Rev Phytopathol 50, 451-473. |
| [4] | Bi GZ, Zhou ZY, Wang WB, Li L, Rao SF, Wu Y, Zhang XJ, Menke FLH, Chen S, Zhou JM (2018). Receptor-like cytoplasmic kinases directly link diverse pattern recogni-tion receptors to the activation of mitogen-activated pro-tein kinase cascades in Arabidopsis. Plant Cell 30, 1543-1561. |
| [5] | Bigeard J, Colcombet J, Hirt H (2015). Signaling mecha-nisms in pattern-triggered immunity (PTI). Mol Plant 8, 521-539. |
| [6] | Chen LT, Hamada S, Fujiwara M, Zhu TH, Thao NP, Wong HL, Krishna P, Ueda T, Kaku H, Shibuya N, Kawasaki T, Shimamoto K (2010). The Hop/Sti1-Hsp90 chaperone complex facilitates the maturation and transport of a PAMP receptor in rice innate immunity. Cell Host Microbe 7, 185-196. |
| [7] | Gong BQ, Guo JH, Zhang NN, Yao XR, Wang HB, Li JF (2019). Cross-microbial protection via priming a conserved immune co-receptor through juxtamembrane phos-phorylation in plants. Cell Host Microbe 26, 810-822. |
| [8] | Hong Z, Jin H, Tzfira T, Li JM (2008). Multiple mechanism-mediated retention of adefective brassinosteroid receptor in the endoplasmic reticulum of Arabidopsis. Plant Cell 20, 3418-3429. |
| [9] | Huang CC, Yan YJ, Zhao HL, Ye Y, Cao YR (2020). Ara-bidopsis CPK5 phosphorylates the chitinreceptor LYK5 to regulate plant innate immunity. Front Plant Sci 11, 702. |
| [10] | Jones JDG, Dangl JL (2006). The plant immune system. Nature 444, 323-329. |
| [11] | Li J, Zhao-Hui C, Batoux M, Nekrasov V, Roux M, Chin-chilla D, Zipfel C, Jones JDG (2009). Specific ER quality control components required for biogenesis of the plant innate immune receptor EFR. Proc Natl Acad Sci USA 106, 15973-15978. |
| [12] | Li W, Deng YW, Ning YS, He ZH, Wang GL (2020). Ex-ploiting broad-spectrum disease resistance in crops: from molecular dissection to breeding. Annu Rev Plant Biol 71, 575-603. |
| [13] | Liu J, Liu B, Chen SF, Gong BQ, Chen LJ, Zhou Q, Xiong F, Wang ML, Feng DR, Li JF, Wang HB, Wang JF (2018). A tyrosine phosphorylation cycle regulates fungal activation of a plant receptor Ser/Thr kinase. Cell Host Microbe 23, 241-253. |
| [14] | Lu D, Lin W, Gao X, Wu S, Cheng C, Avila J, Heese A, Devarenne TP, He P, Shan L (2011). Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant in-nate immunity. Science 332, 1439-1442. |
| [15] | Nagashima Y, von Schaewen A, Koiwa H (2018). Function of N-glycosylation in plants. Plant Sci 274, 70-79. |
| [16] | Savary S, Willocquet L, Pethybridge SJ, Esker P, McRoberts N, Nelson A (2019). The global burden of pathogens and pests on major food crops. Nat Ecol Evol 3, 430-439. |
| [17] | Strasser R (2018). Protein quality control in the endoplasmic reticulum of plants. Annu Rev Plant Biol 69, 147-172. |
| [18] | Tang DZ, Wang GX, Zhou JM (2017). Receptor kinases in plant-pathogen interactions: more than pattern recognition. Plant Cell 29, 618-637. |
| [19] | Zhou JG, Liu DR, Wang P, Ma XY, Lin WW, Chen SX, Mishev K, Lu DP, Kumar R, Vanhoutte I, Meng XZ, He P, Russinova E, Shan LB (2018). Regulation of Arabidopsis brassinosteroid receptor BRI1 endocytosis and degradation by plant U-box PUB12/PUB-13-mediated ubiquitination. Proc Natl Acad Sci USA 115, E1906-E1915. |
| [20] | Zou JJ, Wei FJ, Wang C, Wu JJ, Ratnasekera D, Liu WX, Wu WH (2010). Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid- and Ca2+-mediated stomatal regulation in response to drought stress. Plant Physiol 154, 1232-1243. |
/
| 〈 |
|
〉 |