

Identification of the Cysteine Protease Family and Corresponding miRNAs in Jatropha curcas and Their Response to Chill-hardening
Received date: 2021-01-18
Accepted date: 2021-05-07
Online published: 2021-05-07
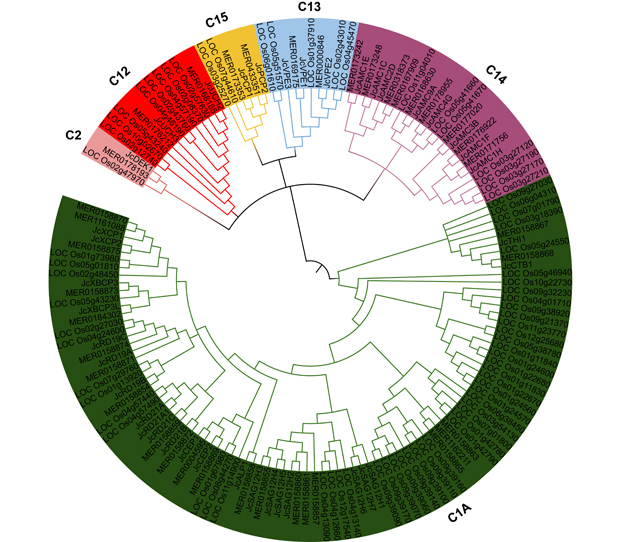
Jatropha curcas is a kind of promising energy plants, but also a chilling-sensitive plant, which can be chill- hardening at 12°C with significant improvement of its chilling tolerance. In this study, the cysteine protease gene family of J. curcas and their corresponding miRNAs were identified at the genome-wide level. The results showed that a total of 39 cysteine protease genes were identified in J. curcas genome, which were located on 11 chromosomes and could be divided into six subfamilies (C1A, C2, C12, C13, C14 and C15); all encoding 181-2 158 amino acids with Cys and His active sites. Based on the sequencing results of miRNAome and degradome, 283 miRNAs were found to be targeted to 14 members of cysteine protease gene family. In addition, the co-expression analysis of those miRNAs targeting to JcDEK1, JcRD21B and JcXBCP3L during chill-hardening demonstrated significantly negative correlation during the chill-hardening at 12°C, suggesting that these miRNAs are involved in the regulation of the cysteine protease genes, and this regulation should be related to the enhancement of chilling tolerance induced by the chill-hardening. This study will be helpful for better understanding the function of cysteine protease gene family in J. curcas and the interaction of the family genes with their corresponding miRNAs, and how this interaction regulates the response of J. curcas to low temperature.
Key words: Jatropha curcas; cysteine protease; gene family; microRNAs; low temperature response
Dandan Wu , Yongkun Chen , Yu Yang , Chunyan Kong , Ming Gong . Identification of the Cysteine Protease Family and Corresponding miRNAs in Jatropha curcas and Their Response to Chill-hardening[J]. Chinese Bulletin of Botany, 2021 , 56(5) : 544 -558 . DOI: 10.11983/CBB21014
| [1] | 郝大海, 龚明 (2020). miRNA作用机制研究进展. 基因组学与应用生物学 39, 3647-3657. |
| [2] | 孔春艳, 陈永坤, 王莎莎, 郝大海, 杨宇, 龚明 (2019). 小桐子低温胁迫下microRNA实时荧光定量PCR内参的筛选与比较. 生物技术通报 35(7), 25-31. |
| [3] | 李忠光, 龚明 (2011). 不同化学消毒剂对小桐子种子萌发和幼苗生长的影响. 种子 30(2), 4-7, 12. |
| [4] | 宋敏, 张瑶, 王丽莹, 彭向永 (2020). 甘蓝型油菜ZF-HD基因家族的鉴定与系统进化分析. 植物学报 54, 699-710. |
| [5] | 王海波, 郭俊云, 唐利洲 (2019). 小桐子MAPKKKK基因家族的全基因组鉴定及表达分析. 植物生理学报 55, 367-377. |
| [6] | 王劲东, 周豫, 余佳雯, 范晓磊, 张昌泉, 李钱峰, 刘巧泉 (2020). miR172-AP2模块调控植物生长发育及逆境响应的研究进展. 植物学报 55, 205-215. |
| [7] | 吴丹丹, 陈永坤, 杨宇, 孔春艳, 龚明 (2021). 小桐子cystatin家族基因和相应miRNAs的鉴定及其在低温响应中可能的作用. 植物生理学报 57, 347-361. |
| [8] | 闫晨阳, 陈赢男 (2020). 4种模式植物LRR VIII-2亚家族基因的鉴定和进化历史分析. 植物学报 55, 442-456. |
| [9] | 张翠桔, 莫蓓莘, 陈雪梅, 崔洁 (2020). 植物miRNA作用方式的分子机制研究进展. 生物技术通报 36(7), 1-14. |
| [10] | Ahmad R, Zuily-Fodil Y, Passaquet C, Bethenod O, Roche R, Repellin A (2014). Identification and characterization of MOR-CP, a cysteine protease induced by ozone and developmental senescence in maize ( Zea mays L.) leaves. Chemosphere 108, 245-250. |
| [11] | Ao PX, Li ZG, Fan DM, Gong M (2013a). Involvement of antioxidant defense system in chill hardening-induced chilling tolerance in Jatropha curcas seedlings. Acta Physiol Plant 35, 153-160. |
| [12] | Ao PX, Li ZG, Gong M (2013b). Involvement of compatible solutes in chill hardening-induced chilling tolerance in Jatropha curcas seedlings. Acta Physiol Plant 35, 3457-3464. |
| [13] | Chen C, Yu Y, Ding XD, Liu BD, Duanmu H, Zhu D, Sun XL, Cao L, Nisa ZU, Li Q, Zhu YM (2018). Genome-wide analysis and expression profiling of PP2C clade D under saline and alkali stresses in wild soybean and Arabidopsis. Protoplasma 255, 643-654. |
| [14] | Chen CJ, Chen H, Zhang Y, Thomas HR, Frank MH, He YH, Xia R (2020). TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant 13, 1194-1202. |
| [15] | Clark K, Franco JY, Schwizer S, Pang Z, Hawara E, Liebrand T (2018). An effector from the huanglongbing-associated pathogen targets citrus proteases. Nat Commun 9, 1718. |
| [16] | Dando PM, Fortunato M, Strand GB, Smith TS, Barrett AJ (2003). Pyroglutamyl-peptidase I: cloning, sequencing, and characterisation of the recombinant human enzyme. Protein Expr Purif 28, 111-119. |
| [17] | Earnshaw WC, Martins LM, Kaufmann SH (1999). Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem 68, 383-424. |
| [18] | Finn RD, Penelope C, Eberhardt RY, Eddy SR, Jaina M, Mitchell AL, Potter SC, Marco P, Matloob Q, Amaia SV (2016). The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res 44, D279-D285. |
| [19] | Grudkowska M, Zagdanska B (2004). Multifunctional role of plant cysteine proteinases. Acta Biochim Pol 51, 609-624. |
| [20] | Hara-Nishimura I (1995). Vacuolar processing enzyme responsible for maturation of vacuolar proteins. Seikagaku 67, 372-377. |
| [21] | Hatsugai N, Kuroyanagi M, Nishimura M, Hara-Nishimura I (2006). A cellular suicide strategy of plants: vacuole- mediated cell death. Apoptosis 11, 905-911. |
| [22] | Kuroyanagi M, Yamada K, Hatsugai N, Kondo M, Nishimura M, Hara-Nishimura I (2005). Vacuolar processing enzyme is essential for mycotoxin-induced cell death in Arabidopsis thaliana. J Biol Chem 280, 32914-32920. |
| [23] | Li ZG, Zeng HZ, Ao PX, Gong M (2014). Lipid response to short-term chilling shock and long-term chill hardening in Jatropha curcas L. seedlings. Acta Physiol Plant 36, 2803-2814. |
| [24] | Liu HJ, Hu MH, Wang Q, Cheng L, Zhang ZB (2018). Role of papain-like cysteine proteases in plant development. Front Plant Sci 9, 1717. |
| [25] | Megha S, Basu U, Kav NNV (2018). Regulation of low temperature stress in plants by microRNAs. Plant Cell Environ 41, 1-15. |
| [26] | Montes JM, Melchinger AE (2016). Domestication and breeding of Jatropha curcas L. Trend Plant Sci 21, 1045-1057. |
| [27] | Niño MC, Kim MS, Kang KK, Cho YG (2020). Genome- wide identification and molecular characterization of cysteine protease genes in rice. Plant Biotechnol Rep 14, 69-87. |
| [28] | Rawlings ND, Barrett AJ, Bateman A (2010). MEROPS: the peptidase database. Nucl Acids Res 38, D227-D233. |
| [29] | Rawlings ND, Salvesen G (2013). Handbook of Proteolytic Enzymes. Salt Lake City: Academic Press. pp. 1253-1257. |
| [30] | Richau KH, Kaschani F, Verdoes M, Pansuriya TC, Niessen S, Stüber K, Colby T, Overkleeft HS, Bogyo M, Van der Hoorn RAL (2012). Subclassification and biochemical analysis of plant papain-like cysteine proteases displays subfamily-specific characteristics. Plant Physiol 158, 1583-1599. |
| [31] | Shimada T, Yamada K, Kataoka M, Nakaune S, Koumoto Y, Kuroyanagi M, Tabata S, Kato T, Shinozaki K, Seki M, Kobayashi M, Kondo M, Nishimura M, Hara-Nishimura I (2003). Vacuolar processing enzymes are essential for proper processing of seed storage proteins in Arabidopsis thaliana. J Biol Chem 278, 32292-32299. |
| [32] | Stroeher VL, Maclagan JL, Good AG (1997). Molecular cloning of a Brassica napus cysteine protease gene inducible by drought and low temperature stress. Physiol Plant 101, 389-397. |
| [33] | Vorster BJ, Cullis CA, Kunert KJ (2019). Plant vacuolar processing enzymes. Front Plant Sci 10, 479. |
| [34] | Wang HB, Gong M, Guo JY, Xin H, Gao Y, Liu C, Dai DQ, Tang LZ (2018a). Genome-wide Identification of Jatropha curcas MAPK, MAPKK, and MAPKKK gene families and their expression profile under cold stress. Sci Rep 8, 16163. |
| [35] | Wang HB, Zou ZR, Wang SS, Gong M (2013a). Global analysis of transcriptome responses and gene expression profiles to cold stress of Jatropha curcas L. PLoS One 8, e82817. |
| [36] | Wang SS, Wang HB, Gong M (2013b). Identification of microRNAs involved in chilling response by deep sequencing of Jatropha curcas L. small RNAs at the global genome level. In: Proceedings of the 21st European Biomass Conference and Exhibition. Copenhagen: European Union. pp. 356-363. |
| [37] | Wang W, Zhou XM, Xiong HX, Mao WY, Zhao P, Sun MX (2018b). Papain-like and legumain-like proteases in rice: genome-wide identification, comprehensive gene feature characterization and expression analysis. BMC Plant Biol 18, 87. |
| [38] | Wani SH, Kumar V, Khare T, Tripathi P, Shah T, Ramakrishna C, Aglawe S, Mangrauthia SK (2020). miRNA applications for engineering abiotic stress tolerance in plants. Biologia 75, 1063-1081. |
| [39] | Wilkinson KD, Laleli-Sahin E, Urbauer J, Larsen CN, Shih GH, Haas AL, Walsh STR, Wand AJ (1999). The binding site for UCH-L3 on ubiquitin: mutagenesis and NMR studies on the complex between ubiquitin and UCH- L3. J Mol Biol 291, 1067-1077. |
| [40] | Wu PZ, Zhou CP, Cheng SF, Wu ZY, Lu WJ, Han JL, Chen YB, Chen Y, Ni PX, Wang Y, Xu X, Huang Y, Song C, Wang ZW, Shi N, Zhang XD, Fang XH, Yang Q, Jiang HW, Chen YP, Li MR, Wang Y, Chen F, Wang J, Wu GJ (2015). Integrated genome sequence and linkage map of physic nut ( Jatropha curcas L), a biodiesel plant. Plant J 81, 810-821. |
| [41] | Zang QW, Wang CX, Li XY, Guo ZA, Jing RL, Zhao J, Chang XP (2010). Isolation and characterization of a gene encoding a polyethylene glycol-induced cysteine protease in common wheat. J Biosci 35, 379-388. |
| [42] | Zhang X, Pan BZ, Chen MS, Chen W, Li J, Xu ZF, Liu CN (2019). JCDB: a comprehensive knowledge base for Jatropha curcas, an emerging model for woody energy plants. BMC Genomics 20, 958. |
| [43] | Zheng L, Chen SS, Xie LH, Lu ZC, Liu MY, Han XJ, Qiao GR, Jiang J, Zhuo RY, Qiu WM, He ZQ (2018). Overexpression of cysteine protease gene from Salix matsudana enhances salt tolerance in transgenic Arabidopsis. Environ Exp Bot 147, 53-62. |
| [44] | Zou Z, Huang QX, Xie GS, Yang LF (2018). Genome-wide comparative analysis of papain-like cysteine protease family genes in castor bean and physic nut. Sci Rep 8, 331. |
/
| 〈 |
|
〉 |