

Changes of Protein N-glycosylation in the Growth of Arabidopsis thaliana and Effects of Enzymatic Deglycosylation on Root Development
† These authors contributed equally to this paper
Received date: 2020-09-29
Accepted date: 2020-12-25
Online published: 2020-12-29
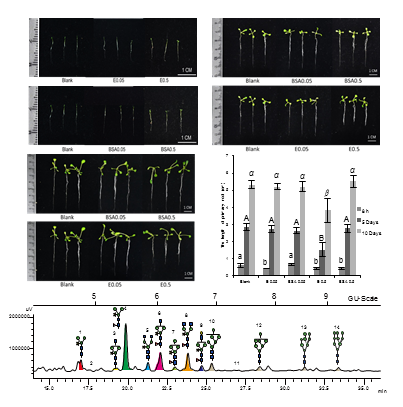
N-glycosylation of proteins plays an important role in plant growth and development. This study explored the changes in protein N-glycosylation in Arabidopsis thaliana at different growth stages and its role in the root development. N-glycans of Arabidopsis Col-0 plants at different growth stages were enzymatically released with N-glycanase and analyzed by HPLC and MALDI-TOF-MS. In addition, Arabidopsis seedlings were treated with N-glycanase, PNGase Rz, for 8 hours before further cultivation in MS medium for five and ten days. The group treated with BSA solution was used as the negative control and the group treated with sterile deionized water was used as blank control. The changes in the primary root length and N-glycosylation composition of the seedlings were measured after treatment. A total number of 12 N-glycan structures were deduced from Arabidopsis, including 4 high-mannose types and 8 complex types. Throughout the entire period, the content of complex type N-glycan was always higher than that of high-mannose; the complex structures modified with xylose and fucose were the dominant component, among which Man3XylFucGlcNAc2 is the highest. The changes of high-mannose N-glycan were as follows: the content steadily increased from 13.87% (seedling stage) to 19.02% (bolting stage), slightly decreased to 17.98% (flowering stage), and dramatically dropped to 2.36% (longhorn ripening stage), then returned 5.23% (aging stage). After treatment with PNGase Rz at high concentration, a significant inhibition of the primary root’s growth was observed, which could not be recovered after cultivation in MS medium for ten days. However, no statistical differences of root length and growth state were found in the treatment group of low concentration (0.05 mg·mL -1), compared with the negative group. N-glycan analysis of seedlings revealed that compared with the control, both treatment groups showed significant changes in the composition of N-glycans. Especially, the total content of high-mannose N-glycans was dramatically lower than that of the blank control group. Meanwhile, these glycoform differences shrank with prolonged time of cultivation and finally disappeared. In conclusion, Arabidopsis thaliana has unique pattern of N-glycosylation during the whole growing period; and glycanase treatment could intermediately alter the N-glycosylation pattern and subsequently inhibit the development of root.
Key words: Arabidopsis thaliana; N-glycan; root development; deglycosylation
Ting Wang , Huanhuan Yang , Hongwei Zhao , Josef Voglmeir , Li Liu . Changes of Protein N-glycosylation in the Growth of Arabidopsis thaliana and Effects of Enzymatic Deglycosylation on Root Development[J]. Chinese Bulletin of Botany, 2021 , 56(3) : 262 -274 . DOI: 10.11983/CBB20163
| 1 | 王梦, 危双, 王婷, Voglmeir J, 刘丽 (2017). Solitalea canadensis源β-N-乙酰氨基己糖苷酶的基因克隆、异源表达和酶学特性. 微生物学报 57, 1270-1282. |
| 2 | Altmann F (2007). The role of protein glycosylation in allergy. Int Arch Allergy Immunol 142, 99-115. |
| 3 | Bao LG, Ma SW, van Huystee RB (2001). The effects of the site-directed removal of N-glycosylation from cationic peanut peroxidase on its function. Arch Biochem Biophys 386, 17-24. |
| 4 | Dam S, Thaysen-Andersen M, Stenkj?r E, Lorentzen A, Roepstorff P, Packer NH, Stougaard J (2013). Combined N-glycome and N-glycoproteome analysis of the Lotus japonicus seed globulin fraction shows conservation of protein structure and glycosylation in legumes. J Proteome Res 12, 3383-3392. |
| 5 | Du YM, Xia T, Gu XQ, Wang T, Ma HY, Voglmeir J, Liu L (2015). Rapid sample preparation methodology for plant N-glycan analysis using acid-stable PNGase H+. J Agric Food Chem 63, 10550-10555. |
| 6 | Ghazarian H, Idoni B, Oppenheimer SB (2011). A glycobiology review: carbohydrates, lectins and implications in cancer therapeutics. Acta Histochem 113, 236-247. |
| 7 | Horiuchi R, Hirotsu N, Miyanishi N (2015). Comparative analysis of N-glycans in the ungerminated and germinated stages of Oryza sativa. Carbohydr Res 418, 1-8. |
| 8 | Ihara Y, Ikezaki M, Takatani M, Ito Y (2020). Calnexin/calreticulin and assays related to N-glycoprotein folding in vitro. In: Hirabayashi J, ed. Lectin Purification and Analysis: Methods and Protocols. Humana: Springer. pp. 295-308. |
| 9 | Kajiura H, Koiwa H, Nakazawa Y, Okazawa A, Kobayashi A, Seki T, Fujiyama K (2010). Two Arabidopsis thaliana Golgi, α-mannosidase I enzymes are responsible for plant N-glycan maturation. Glycobiology 20, 235-247. |
| 10 | Kang JS, Frank J, Kang CH, Kajiura H, Vikram M, Ueda A, Kim S, Bahk JD, Triplett B, Fujiyama K, Lee SY, von Schaewen A, Koiwa H (2008). Salt tolerance of Arabidopsis thaliana requires maturation of N-glycosylated proteins in the Golgi apparatus. Proc Natl Acad Sci USA 105, 5933-5938. |
| 11 | Kato S, Hayashi M, Kitagawa M, Kajiura H, Maeda M, Kimura Y, Igarashi K, Kasahara M, Ishimizu T (2018). Degradation pathway of plant complex-type N-glycans: identification and characterization of a key α1,3-fucosidase from glycoside hydrolase family 29. Biochem J 475, 305-317. |
| 12 | Kimura Y, Matsuo S (2000). Changes in N-linked oligosaccharides during seed development of Ginkgo biloba. Biosci Biotechnol Biochem 64, 562-568. |
| 13 | Kimura Y, Takeoka Y, Inoue M, Maeda M, Fujiyama K (2011). Double-knockout of putative endo-β-N-acetylglucosaminidase (ENGase) genes in Arabidopsis thaliana: loss of ENGase activity induced accumulation of high- mannose type free N-glycans bearing N,N'-acetylchitobiosyl unit. Biosci Biotechnol Biochem 75, 1019-1021. |
| 14 | Koiwa H, Li F, McCully MG, Mendoza I, Koizumi N, Manabe Y, Nakagawa Y, Zhu JH, Rus A, Pardo JM, Bressan RA, Hasegawa PM (2003). The STT3a subunit isoform of the Arabidopsis oligosaccharyltransferase controls adaptive responses to salt/osmotic stress. Plant Cell 15, 2273-2284. |
| 15 | Liebminger E, Hüttner S, Vavra U, Fischl R, Schoberer J, Grass J, Blaukopf C, Seifert GJ, Altmann F, Mach L, Strasser R (2009). Class I α-mannosidases are required for N-glycan processing and root development in Arabidopsis thaliana. Plant Cell 21, 3850-3867. |
| 16 | Liebminger E, Veit C, Pabst M, Batoux M, Zipfel C, Altmann F, Mach L, Strasser R (2011). β-N-acetylhexosaminidases HEXO1 and HEXO3 are responsible for the formation of paucimannosidic N-glycans in Arabidopsis thaliana. J Biol Chem 286, 10793-10802. |
| 17 | Lindner H, Kessler SA, Müller LM, Shimosato-Asano H, Boisson-Dernier A, Grossniklaus U (2015). TURAN and EVAN mediate pollen tube reception in Arabidopsis Synergids through protein glycosylation. PLoS Biol 13, e1002139. |
| 18 | Maeda M, Kimura Y (2014). Structural features of free N-glycans occurring in plants and functional features of de-N-glycosylation enzymes, ENGase, and PNGase: the presence of unusual plant complex type N-glycans. Front Plant Sci 5, 429. |
| 19 | Misaki R, Kimura Y, Fujiyama K, Seki T (2001). Glycoproteins secreted from suspension -cultured tobacco BY2 cells have distinct glycan structures from intracellular glycoproteins. Biosci Biotechnol Biochem 65, 2482-2488. |
| 20 | Nagashima Y, von Schaewen A, Koiwa H (2018). Function of N-glycosylation in plants. Plant Sci 274, 70-79. |
| 21 | Nakamura K, Inoue M, Yoshiie T, Hosoi K, Kimura Y (2008). Changes in structural features of free N-glycan and endoglycosidase activity during tomato fruit ripening. Biosci Biotechnol Biochem 72, 2936-2945. |
| 22 | Rips S, Bentley N, Jeong IS, Welch JL, von Schaewen A, Koiwa H (2014). Multiple N-glycans cooperate in the subcellular targeting and functioning of Arabidopsis KORRIGAN1. Plant Cell 26, 3792-3808. |
| 23 | Saijo Y (2010). ER quality control of immune receptors and regulators in plants. Cell Microbiol 12, 716-724. |
| 24 | Shen JB, Ding Y, Gao CJ, Rojo E, Jiang LW (2014). N-linked glycosylation of AtVSR1 is important for vacuolar protein sorting in Arabidopsis. Plant J 80, 977-992. |
| 25 | Strasser R, Schoberer J, Jin CS, Gl?ssl J, Mach L, Steinkellner H (2006). Molecular cloning and characterization of Arabidopsis thaliana Golgi α-mannosidase II, a key enzyme in the formation of complex N-glycans in plants. Plant J 45, 789-803. |
| 26 | Vi?tor R, Loutelier-Bourhis C, Fitchette AC, Margerie P, Gonneau M, Faye L, Lerouge P (2003). Protein N-glycosylation is similar in the moss Physcomitrella patens and in higher plants. Planta 218, 269-275. |
| 27 | Wang T, Hu XC, Cai ZP, Voglmeir J, Liu L (2017a). Qualitative and quantitative analysis of carbohydrate modification on glycoproteins from seeds of Ginkgo biloba. J Agric Food Chem 65, 7669-7679. |
| 28 | Wang T, Voglmeir J (2014). PNGases as valuable tools in glycoprotein analysis. Protein Pept Lett 21, 976-985. |
| 29 | Wang WL, Wang W, Du YM, Wu H, Yu XB, Ye KP, Li CB, Jung YS, Qian YJ, Voglmeir J, Liu L (2017b). Comparison of anti-pathogenic activities of the human and bovine milk N-glycome: fucosylation is a key factor. Food Chem 235, 167-174. |
| 30 | Wang ZY, Gehring C, Zhu JH, Li FM, Zhu JK, Xiong LM (2015). The Arabidopsis Vacuolar Sorting Receptor 1 is required for osmotic stress-induced abscisic acid biosynthesis. Plant Physiol 167, 137-152. |
| 31 | Wei S, Henquet MGL, Mentink RA, van Dijk AJ, Cordewener JHG, Bosch D, America AHP, van der Krol AR (2011). N-glycoproteomics in plants: perspectives and challenges. J Proteomics 74, 1463-1474. |
| 32 | Wilson IBH, Zeleny R, Kolarich D, Staudacher E, Stroop CJM, Kamerling JP, Altmann F (2001). Analysis of Asn -linked glycans from vegetable foodstuffs: widespread occurrence of Lewis a, core α1,3-linked fucose and xylose substitutions. Glycobiology 11, 261-274. |
| 33 | Xu SL, Medzihradszky KF, Wang ZY, Burlingame AL, Chalkley RJ (2016). N-glycopeptide profiling in Arabidopsis inflorescence. Mol Cell Proteomics 15, 2048-2054. |
| 34 | Zeng W, Ford KL, Bacic A, Heazlewood JL (2018). N- linked glycan micro-heterogeneity in glycoproteins of Arabidopsis. Mol Cell Proteomics 17, 413-421. |
/
| 〈 |
|
〉 |