

A Regeneration System for Organogenesis and Somatic Embryogenesis Using Leaves of Agapanthus praecox as Explants
Received date: 2020-02-10
Accepted date: 2020-05-08
Online published: 2020-05-12
A regeneration system for organogenesis and somatic embryogenesis in vitro was established by using leaves of Agapanthus praecox as explants, and different cultivation media for transplanting were selected for the best effect. The results showed that picloram (PIC) was effective in callus induction of leaves, and the optimal medium was MS+2.0 mg·L -1 PIC. The callus induction rate was determined by the meristematic activity of leaf segments. The callus induction rate of the basal tissues on the 1 st-2 nd euphyll was 85.71%, and the callus induction rate was 66.48% in meristematic zone of 0-0.5 cm of the same leaf. The results also showed that the callus induction efficiency was higher in the middle of leaf transection compared with that at the edge. The optimal medium for adventitious bud induction was MS+1.5 mg·L -1 PIC+0.3 mg·L -1 6-BA, and the induction rate was 80.27%. The basic MS medium was suitable for somatic embryo induction, but the induction rate would be significantly increased if 0.05 mg·L -1 paclobutrazol and 1.0 mg·L -1abscisic acid were added. Plantlets proliferation was promoted by 1.0 mg·L -1 6-BA, and the proliferation coefficients of organogenesis and somatic embryogenesis pathway were 2.23 and 2.93, respectively. The combination of peat:perlite: vermiculite=1:1:1 (v/v/v) was proved the suitable substrate for transplanting and acclimatization of plantlets, with a survival rate of 100%. This regeneration system provides a rapid and efficient propagation technology for A. praecox, and also provides a reference for the regeneration of monocotyledon explants in vitro.
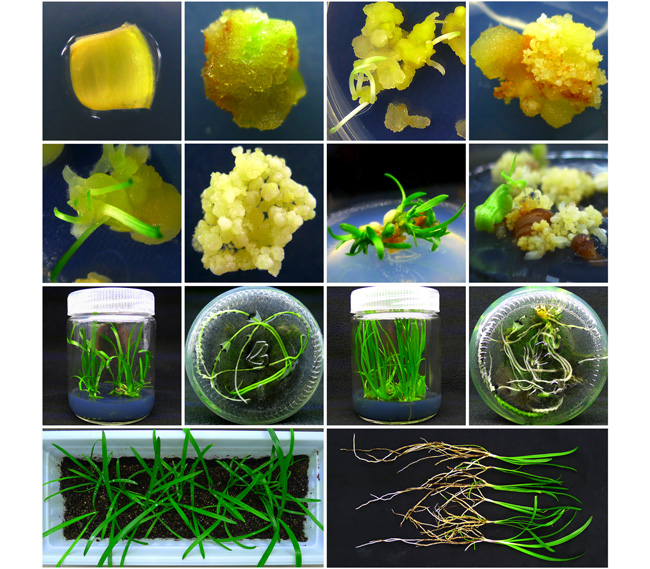
Jianhua Yue , Yan Dong , Xiaohua Wang , Peixia Sun , Siying Wang , Xinnian Zhang , Yan Zhang . A Regeneration System for Organogenesis and Somatic Embryogenesis Using Leaves of Agapanthus praecox as Explants[J]. Chinese Bulletin of Botany, 2020 , 55(5) : 588 -595 . DOI: 10.11983/CBB20019
| [1] | 陈香波, 陆亮, 钱又宇, 范宇婷 ( 2016). 百子莲属种质资源及园林开发应用. 中国园林 32(8), 99-105. |
| [2] | 何叶, 任丽, 孙海龙, 张洁, 邹梦雯, 张荻 ( 2014). 百子莲愈伤组织诱导体系的优化. 西南农业学报 27, 1237-1242. |
| [3] | 胡仲义, 何月秋 ( 2011). 百子莲组织培养及植株再生研究. 北方园艺 ( 10), 118-120. |
| [4] | 康玲 ( 2009). 百子莲的再生体系试验初报. 现代园艺(12), 7-8, 25. |
| [5] | 李黎, 张悦 ( 2014). 外植体类型及年龄对蓝靛果忍冬诱导分化的影响. 林业科技 39(4), 14-15. |
| [6] | 刘芳伊 ( 2013). 单叶刺槐和百子莲组培快繁体系建立. 硕士论文. 保定: 河北农业大学. pp. 17-19. |
| [7] | 彭海峰, 曹友培, 俞新华, 赵晟, 黄晓柯 ( 2007). 仙茅叶片的组织培养及其细胞学观察. 中草药 38, 265-269. |
| [8] | 王文静, 王鹏, 李伟强 ( 2012). 外植体类型和植物生长调节剂浓度对红金银花愈伤组织诱导的影响. 湖北农业科学 51, 4391-4393. |
| [9] | 杨舟, 吕可, 吕珊, 王俊杰, 张荻 ( 2019). 百子莲2个ARF基因与2个Aux/IAA基因的全长克隆与序列分析. 浙江农业学报 31, 86-97. |
| [10] | 张旭红, 王頔, 梁振旭, 孙美玉, 张金政, 石雷 ( 2018). 欧洲百合愈伤组织诱导及植株再生体系的建立. 植物学报 53, 840-847. |
| [11] | 邹梦雯 ( 2015). 毒莠定( PIC)调控百子莲愈伤组织胚性诱导与保持生理生化基础的研究. 硕士论文. 上海: 上海交通大学. pp. 64-66. |
| [12] | Banjac N, Vinterhalter B, Krsti?-Milo?evi? D, Milojevi? J, Tubi? L, Ghalawenji N, Zdravkovi?-Kora? S ( 2019). Somatic embryogenesis and shoot organogenesis from the hypocotyl slices and free radical scavenging activity of regenerants of collard greens (Brassica oleracea L. var. acephala). Plant Cell Tissue Organ Cult 137, 613-626. |
| [13] | Bouamama B, Ben Salem A, Ben Youssef F, Chaieb S, Jaafoura MH, Mliki A, Ghorbel A ( 2011). Somatic embryogenesis and organogenesis from mature caryopses of North African barley accession “Kerkena” ( Hordeum vulgare L.). In Vitro Cell Dev Biol Plant 47, 321-327. |
| [14] | Fehér A, Pasternak TP, Dudits D ( 2003). Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Organ Cult 74, 201-228. |
| [15] | Guo F, Zhang HD, Liu W, Hu XM, Han N, Qian Q, Xu L, Bian HW ( 2018). Callus initiation from root explants employs different strategies in rice and Arabidopsis. Plant Cell Physiol 59, 1782-1789. |
| [16] | Guo HH, Guo HX, Zhang L, Fan YJ, Fan YP, Zeng FC ( 2019). SELTP-assembled battery drives totipotency of somatic plant cell. Plant Biotechnol J 17, 1188-1190. |
| [17] | Hu B, Zhang GF, Liu W, Shi JM, Wang H, Qi MF, Li JQ, Qin P, Ruan Y, Huang H, Zhang YJ, Xu L ( 2017). Divergent regeneration-competent cells adopt a common mechanism for callus initiation in angiosperms. Regeneration 4, 132-139. |
| [18] | Malik MG ( 2008). Comparison of different liquid/solid culture systems in the production of somatic embryos from Narcissus L. ovary explants. Plant Cell Tissue Organ Cult 94, 337-345. |
| [19] | Manchanda P, Gosal SS ( 2012). Effect of activated charcoal, carbon sources and gelling agents on direct somatic embryogenesis and regeneration in sugarcane via leaf roll segments. Sugar Tech 14, 168-173. |
| [20] | Menke-Milczarek I, Zimny J ( 2001). NH4+ and NO3- requirement for wheat somatic embryogenesis. Acta Physiol Plant 23, 37-42. |
| [21] | Pedrali-Noy G, Bernacchia G, do Rosario Alvelos M, Cella R ( 2003). Daucus carota cells contain specific DNA methyltransferase inhibitors that interfere with somatic embryogenesis. Plant Biol 5, 383-392. |
| [22] | Ptak A, Bach A ( 2007). Somatic embryogenesis in tulip (Tulipa gesneriana L.) flower stem cultures. In Vitro Cell Dev Biol Plant 43, 35-39. |
| [23] | Rao K, Chodisetti B, Gandi S, Mangamoori LN, Giri A ( 2011). Direct and indirect organogenesis of Alpinia galanga and the phytochemical analysis. Appl Biochem Biotechnol 165, 1366-1378. |
| [24] | Sugimoto K, Jiao YL, Meyerowitz EM ( 2010). Arabidopsis regeneration from multiple tissues occurs via a root development pathway. Dev Cell 18, 463-471. |
| [25] | Yang L, Wei C, Huang C, Liu HN, Zhang DY, Shen HL, Li YH ( 2019). Role of hydrogen peroxide in stress-induced programmed cell death during somatic embryogenesis in Fraxinus mandshurica. J For Res 30, 767-777. |
| [26] | Zhang N, Fang W, Shi Y, Liu QQ, Yang HY, Gui RY, Lin XC ( 2010). Somatic embryogenesis and organogenesis in Dendrocalamus hamiltonii. Plant Cell Tissue Organ Cult 103, 325-332. |
/
| 〈 |
|
〉 |