

Nitrogen Utilization Mechanism in C3 and C4 Plants
Received date: 2019-06-24
Accepted date: 2019-12-29
Online published: 2019-12-19
Improving nitrogen use efficiency (NUE) of plants is not only an important approach to ensure global food security, but also to achieve sustainable agricultural development. In the past half century, great progress has been made in the study of nitrogen utilization mechanism, but the regulatory mechanism of NUE is not clear and the improvement of NUE is still extremely limited. Photosynthetic carbon assimilation and nitrogen assimilation are integrated in a plant, and only by coordinating carbon and nitrogen metabolism can the carbon/nitrogen balance be maintained and the normal growth and development of plants be regulated. Due to the differences in the photosynthetic nitrogen utilization rate (PNUE) between C3 and C4 plants, there will also be differences in the utilization efficiency of nitrogen. In order to improve crop NUE, it is necessary to understand the functions and regulatory mechanisms of key factors involved in nitrogen absorption, transport, assimilation and signal transduction of C3 and C4 plants. In addition, studies on carbon and nitrogen assimilation and their mechanisms under the conditions of increasing atmospheric CO2 concentration and global warming should not be ignored. This paper reviews the differences of key factors on nitrogen use between C3 and C4 plants and the regulatory mechanisms, and proposes possible ways to improve NUE of C3 crops by using genetic approaches.
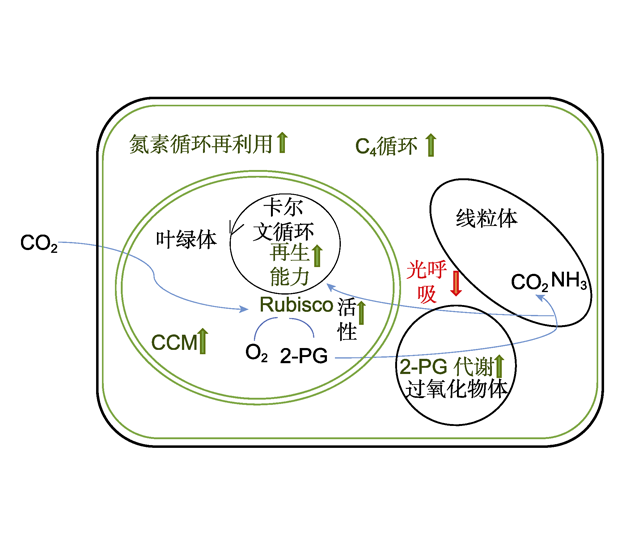
Lu Zhang , Xinhua He . Nitrogen Utilization Mechanism in C3 and C4 Plants[J]. Chinese Bulletin of Botany, 2020 , 55(2) : 228 -239 . DOI: 10.11983/CBB19113
| [1] | 陈冉冉 ( 2012). 不同氮素水平对转玉米PEPC基因水稻碳、氮代谢的影响.博士论文. 北京: 中国农业科学院. pp. 18-37. |
| [2] | 陈太钰 ( 2012). C3/C4光合作用差异表达基因的鉴定与分析. 博士论文. 武汉: 华中农业大学. pp.42-62. |
| [3] | 巩玥, 陈海苗, 姜闯道, 石雷 ( 2014). 植物叶片解剖结构的量化及其在C4植物高粱中的应用. 植物学报 49, 173-182. |
| [4] | 何新华 ( 1995). C3和C4植物的比较营养学及其展望. 见: 张福锁主编. 土壤与植物营养研究新动态(第三卷). 北京: 中国农业出版社. pp.270-279. |
| [5] | 何新华, 安·奥克斯, 李明启 ( 1995). C3和C4禾本科作物的氮素利用效率. 植物学通报 12(3), 20-27. |
| [6] | 何新华, 李明启 ( 1994). C3和C4植物的氮素利用效率. 云南植物研究 16, 93-94. |
| [7] | 李霞, 王超, 陈晏, 孙志伟 ( 2008). PEPC酶活性作为水稻高光效育种筛选指标的研究. 江苏农业学报 24, 559-564. |
| [8] | 罗璇, 郭彤, 胡银岗 ( 2014). 小麦和谷子C4光合途径关键酶活性及其与光合和蒸腾的关系. 麦类作物学报 34, 1083-1091. |
| [9] | 王修兰, 徐师华, 梁红 ( 1998). CO2浓度增加对C3、C4作物生育和产量影响的实验研究. 中国农业科学 31, 55-61. |
| [10] | 魏爱丽, 王志敏, 翟志席, 龚元石 ( 2003). 土壤干旱对小麦旗叶和穗器官C4光合酶活性的影响. 中国农业科学 36, 508-512. |
| [11] | 谢晓金, 李仁英, 张耀鸿, 刘璐, 申双和, 包云轩 ( 2016). CO2浓度升高对水稻和玉米叶片光合生理特性的影响. 江苏农业科学 44(10), 120-123. |
| [12] | 徐晓鹏, 傅向东, 廖红 ( 2016). 植物铵态氮同化及其调控机制的研究进展. 植物学报 51, 152-166. |
| [13] | 曾长立, 王晓明, 张福锁, 王兴仁 ( 2001). 浅析C3植物和C4植物对大气中CO2浓度升高条件下的反应. 江汉大学学报 18(3), 6-14. |
| [14] | 张庆琛, 许为钢, 胡琳, 李艳, 张磊, 齐学礼 ( 2010). 玉米C4型全长pepc基因导入普通小麦的研究. 麦类作物学报 30, 194-197. |
| [15] | 甄晓溪, 刘浩然, 李鑫, 徐凡, 张文忠 ( 2019). 异源过表达OsATG8b基因提高转基因拟南芥的氮/碳胁迫耐受性和产量. 植物学报 54, 23-36. |
| [16] | Appenroth KJ, Me?o R, Jourdan V, Lillo C ( 2000). Phytochrome and post-translational regulation of nitrate reductase in higher plants. Plant Sci 159, 51-56. |
| [17] | Aubry S, Brown NJ, Hibberd JM ( 2011). The role of proteins in C3 plants prior to their recruitment into the C4 pathway. J Exp Bot 62, 3049-3059. |
| [18] | Bandyopadhyay A, Datta K, Zhang J, Yang W, Raychaudhuri S, Miyao M, Datta SK ( 2007). Enhanced photosynthesis rate in genetically engineered indica rice expressing pepc gene cloned from maize. Plant Sci 172, 1204-1209. |
| [19] | Bar-Even A, Noor E, Savir Y, Liebermeister W, Davidi D, Tawfik DS, Milo R ( 2011). The moderately efficient enzyme: evolutionary and physicochemical trends shaping enzyme parameters. Biochemistry 50, 4402-4410. |
| [20] | Basra AS, Dhawan AK, Goyal SS ( 2002). Dcmu inhibits in vivo nitrate reduction in illuminated barley (C3) leaves but not in maize (C4): a new mechanism for the role of light? Planta 215, 855-861. |
| [21] | Betti M, Bauw H, Busch FA, Fernie AR, Keech O, Levey M, Ort Donald R, Parry Martin AJ, Sage R, Timm S, Walker B, Weber APM ( 2016). Manipulating photorespiration to increase plant productivity: recent advances and perspectives for crop improvement. J Exp Bot 67, 2977-2988. |
| [22] | Bloom AJ, Asensio JSR, Randall L, Rachmilevitch S, Cousins AB, Carlisle EA ( 2012). CO2 enrichment inhibits shoot nitrate assimilation in C3 but not C4 plants and slows growth under nitrate in C3 plants. Ecology 93, 355-367. |
| [23] | Bloom AJ, Burger M, Asensio JSR, Cousins AB ( 2010). Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 328, 899-903. |
| [24] | Bloom AJ, Burger M, Kimball BA, Pinter Jr JP ( 2014). Nitrate assimilation is inhibited by elevated CO2 in field- grown wheat. Nat Climate Change 4, 477-480. |
| [25] | Bowes G ( 2010). Single-cell C4 photosynthesis in aquatic plants. In: Raghavendra AS, Sage RF, eds. C4 Photosynthesis and Related CO2 Concentrating Mechanisms. Dordrecht: Springer. pp. 63-80. |
| [26] | Br?utigam A, Gowik U ( 2016). Photorespiration connects C3 and C4 photosynthesis. J Exp Bot 67, 2953-2962. |
| [27] | Br?utigam A, Kajala K, Wullenweber J, Sommer M, Gagneul D, Weber KL, Carr KM, Gowik U, Mass J, Lercher MJ, Westhoff P, Hibberd JM, Weber AP ( 2011). An mRNA blueprint for C4 photosynthesis derived from comparative transcriptomics of closely related C3 and C4 species. Plant Physiol 155, 142-156. |
| [28] | Br?utigam A, Schliesky S, Külahoglu C, Osborne CP, Weber APM ( 2014). Towards an integrative model of C4 photosynthetic subtypes: insights from comparative transcriptome analysis of NAD-ME, NADP-ME, and PEP-CK C4 species. J Exp Bot 65, 3579-3593. |
| [29] | Brown RH ( 1978). A difference in N use efficiency in C3 and C4 plants and its implications in adaptation and evolution. Crop Sci 18, 93-98. |
| [30] | Busch FA, Sage TL, Cousins AB, Sage RF ( 2013). C3 plants enhance rates of photosynthesis by reassimilating photorespired and respired CO2. Plant Cell Environ 36, 200-212. |
| [31] | Campbell WH ( 1999). Nitrate reductase structure, function and regulation: bridging the gap between biochemistry and physiology. Annu Rev Plant Physiol Plant Mol Biol 50, 277-303. |
| [32] | Carmo-Silva E, Scales JC, Madgwick PJ, Parry MAJ ( 2015). Optimizing Rubisco and its regulation for greater resource use efficiency. Plant Cell Environ 38, 1817-1832. |
| [33] | Christin PA, Boxall SF, Gregory R, Edwards EJ, Hartwell J, Osborne CP ( 2013). Parallel recruitment of multiple genes into C4 photosynthesis. Genome Biol Evol 5, 2174-2187. |
| [34] | Christin PA, Osborne CP ( 2014). The evolutionary ecology of C4 plants. New Phytol 204, 765-781. |
| [35] | Edwards EJ, Osborne CP, Stromberg CAE, Smith SA, Bond WJ, Christin PA, Cousins AB, Duvall MR, Fox DL, Freckleton RP, Ghannoum O, Hartwell J, Huang Y, Janis CM, Keeley JE, Kellogg EA, Knapp AK, Leakey ADB, Nelson DM, Saarela JM, Sage RF, Sala OE, Salamin N, Still CJ, Tipple B ( 2010). The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science 328, 587-591. |
| [36] | Erb TJ, Zarzycki J ( 2016). Biochemical and synthetic biology approaches to improve photosynthetic CO2-fixation. Curr Opin Chem Biol 34, 72-79. |
| [37] | Furbank RT ( 2011). Evolution of the C4 photosynthetic mechanism: are there really three C4 acid decarboxylation types? J Exp Bot 62, 3103-3108. |
| [38] | Gowik U, Br?utigam A, Weber KL, Weber AP, Westhoff P ( 2011). Evolution of C4 photosynthesis in the genus flaveria: how many and which genes does it take to make C4? Plant Cell 23, 2087-2105. |
| [39] | Hagemann M, Bauwe H ( 2016). Photorespiration and the potential to improve photosynthesis. Curr Opin Chem Biol 35, 109-116. |
| [40] | He XH, Oaks A, Li M ( 1994). Nitrogen use efficiency in C3 and C4 plants. Agro’s Ann Rev Plant Physiol 1, 147-188. |
| [41] | Hibberd JM, Sheehy JE, Langdale JA ( 2008). Using C4 photosynthesis to increase the yield of rice-rationale and feasibility. Curr Opin Plant Biol 11, 228-231. |
| [42] | Hikosaka K, Shigeno A ( 2009). the role of Rubisco and cell walls in the interspecific variation in photosynthetic capacity. Oecologia 160, 443-451. |
| [43] | IPCC ( 2013). Climate change 2013: the physical science basis. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, eds. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press. pp.119-158. |
| [44] | Jiang ZC, Hull RJ, Sullivan WM ( 2002). Nitrate uptake and reduction in C3 and C4 grasses. J Plant Nutr 25, 1303-1314. |
| [45] | Kajala K, Covshoff S, Karki S, Woodfield H, Tolley BJ, Dionora MJA, Mogul RT, Mabilangan AE, Danila FR, Hibberd JM, Quick WP ( 2011). Strategies for engineering a two-celled C4 photosynthetic pathway into rice. J Exp Bot 62, 3001-3010. |
| [46] | Karki S, Rizal G, Quick WP ( 2013). Improvement of photosynthesis in rice ( Oryza sativa L.) by inserting the C4 pathway. Rice 6, 28. |
| [47] | Ku MSB, Agarie S, Nomura M, Fukayama H, Tsuchida H, Ono K, Hirose S, Toki S, Miyao M, Matsuoka M ( 1999). High-level expression of maize phosphoenolpyruvate carboxylase in transgenic rice plants. Nat Biotechnol 17, 76-80. |
| [48] | Ku MSB, Kano-Murakami Y, Matsuoka M ( 1996). Evolution and expression of C4 photosynthesis genes. Plant Physiol 111, 949-957. |
| [49] | Külahoglu C, Denton AK, Sommer M, Ma? J, Schliesky S, Wrobel TJ, Berckmans B, Gongora-Castillo E, Buell CR, Simon R, De Veylder L, Br?utigam A, Weber APM ( 2014). Comparative transcriptome atlases reveal altered gene expression modules between two cleomaceae C3 and C4 plant species. Plant Cell 26, 3243-3260. |
| [50] | Kurai T, Wakayama M, Abiko T, Yanagisawa S, Aoki N, Ohsugi R ( 2011). Introduction of the ZmDof1 gene into rice enhances carbon and nitrogen assimilation under low- nitrogen conditions. Plant Biotechnol J 9, 826-837. |
| [51] | Langdale JA ( 2011). C4 cycles: past, present, and future research on C4 photosynthesis. Plant Cell 23, 3879-3892. |
| [52] | Li MR, Wedin DA, Tieszen LL ( 1999). C3 and C4 photosynthesis in Cyperus( Cyperaceae) in temperate eastern North America. Can J Bot 77, 209-218. |
| [53] | Long SP, Ainsworth EA, Leakey ADB, N?sberger J, Ort DR ( 2006). Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 312, 1918-1921. |
| [54] | Majeran W, Cai Y, Sun Q, van Wijk KJ ( 2005). Functional differentiation of bundle sheath and mesophyll maize chloroplasts determined by comparative proteomics. Plant Cell 17, 3111-3140. |
| [55] | Majeran W, van Wijk KJ ( 2009). Cell-type-specific differentiation of chloroplasts in C4 plants. Trends Plant Sci 14, 100-109. |
| [56] | Melzer E, O'Leary MH ( 1987). Anapleurotic CO2 fixation by phosphoenolpyruvate carboxylase in C3 plants. Plant Physiol 84, 58-60. |
| [57] | Miyao M, Fukayama H ( 2003). Metabolic consequences of overproduction of phosphoenolpyruvate carboxylase in C3 plants. Arch Biochem Biophys 414, 197-203. |
| [58] | Monson RK, Rawsthorne S ( 2000). CO2 assimilation in C3-C4 intermediate plants. In: Leegood RC, Sharkey TD, von Caemmerer S, eds. Photosynthesis: Physiology and Metabolism. Dordrecht: Springer. pp. 533-550. |
| [59] | NASA ( 2014). Global climate change: vital signs of the planet, available at: http://climate.nasa.gov/400ppmquotes . 2018-12-30. |
| [60] | Nelson T ( 2011). The grass leaf developmental gradient as a platform for a systems understanding of the anatomical specialization of C4 leaves. J Exp Bot 62, 3039-3048. |
| [61] | Nowicka B, Ciura J, Szymańska R, Kruk J ( 2018). Improving photosynthesis, plant productivity and abiotic stress tolerance—current trends and future perspectives. J Plant Physiol 231, 415-433. |
| [62] | Oaks A ( 1994). Efficiency of nitrogen utilization in C3 and C4 cereals. Plant Physiol 106, 407-414. |
| [63] | Ort DR, Merchant SS, Alric J, Barkan A, Blankenship RE, Bock R, Croce R, Hanson MR, Hibberd JM, Long SP, Moore TA, Moroney J, Niyogi KK, Parry MAJ, Peralta-Yahya PP, Prince RC, Redding KE, Spalding MH, van Wijk KJ, Vermaas WFJ, von Caemmerer S, Weber APM, Yeates TO, Yuan JS, Zhu XG ( 2015). Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc Natl Acad Sci USA 112, 8529-8536. |
| [64] | Patterson DT, Flint EP ( 1980). Potential effects of global atmospheric CO2 enrichment on the growth and competitiveness of C3 and C4 weed and crop plants. Weed Sci 28, 71-75. |
| [65] | Peterhansel C, Krause K, Braun HP, Espie GS, Fernie AR, Hanson DT, Keech O, Maurino VG, Mielewczik M, Sage RF ( 2013). Engineering photorespiration: current state and future possibilities. Plant Biol 15, 754-758. |
| [66] | Pinto H, Sharwood RE, Tissue DT, Ghannoum O ( 2014). Photosynthesis of C3, C3-C4, and C4 grasses at glacial CO2. J Exp Bot 65, 3669-3681. |
| [67] | Radchuk R, Radchuk V, G?tz KP, Weichert H, Richter A, Emery RJN, Weschke W, Weber H ( 2007). Ectopic expression of phosphoenolpyruvate carboxylase in Vicia narbonensis seeds: effects of improved nutrient status on seed maturation and transcriptional regulatory networks. Plant J 51, 819-839. |
| [68] | Reich PB, Hobbie SE, Lee TD, Pastore MA ( 2018). Unexpected reversal of C3 versus C4 grass response to elevated CO2 during a 20-year field experiment. Science 360, 317-320. |
| [69] | Ripley BS, Cunniff J, Osborne CP ( 2013). Photosynthetic acclimation and resource use by the C3 and C4 subspecies of Alloteropsis semialata in low CO2 atmospheres. Global Change Biol 19, 900-910. |
| [70] | Ruan CJ, Shao HB, dan Silva JAT ( 2012). A critical review on the improvement of photosynthetic carbon assimilation in C3 plants using genetic engineering. Crit Rev Biotechnol 32, 1-21. |
| [71] | Sage RF ( 2001). C4 plants. In: Levin S, ed. Encyclopedia of Biodiversity. London: Academic Press. pp.575-598. |
| [72] | Sage RF, Christin PA, Edwards EJ ( 2011). The C4 plant lineages of planet earth. J Exp Bot 62, 3155-3169. |
| [73] | Sage RF, Pearcy RW ( 1987). The nitrogen use efficiency of C3 and C4 plants. I. Leaf nitrogen, growth, and biomass partitioning in Chenopodium album(L.) and Amaranthus retroflexus Plant Physiol 84, 954-958. |
| [74] | Sage RF, Sage TL, Kocacinar F ( 2012). Photorespiration and the evolution of C4 photosynthesis. Annu Rev Plant Biol 63, 19-47. |
| [75] | Sage RF, Wedin DA, Li MR ( 1999). The biogeography of C4 photosynthesis: patterns and controlling factors. In: Sage RF, Monson RK, eds. C4 Plant Biology. San Diego: Academic Press. pp.313-375. |
| [76] | Santos PM, Thornton B, Corsi M ( 2002). Nitrogen dynamics in the intact grasses poa trivialis and panicum maximum receiving contrasting supplies of nitrogen. J Exp Bot 53, 2167-2176. |
| [77] | Sehtiya HL, Goyal SS ( 2000). Comparative uptake of nitrate by intact seedlings of C3 (barley) and C4 (corn) plants: effect of light and exogenously supplied sucrose. Plant Soil 227, 185-190. |
| [78] | Spreitzer RJ, Salvucci ME ( 2002). Rubisco: structure, regulatory interactions, and possibilities for a better enzyme. Annu Rev Plant Biol 53, 449-475. |
| [79] | Suzuki S, Murai N, Kasaoka K, Hiyoshi T, Imaseki H, Burnell JN, Arai M ( 2006). Carbon metabolism in transgenic rice plants that express phospho enolpyruvate carboxylase and/or phosphoenolpyruvate carboxykinase. Plant Sci 170, 1010-1019. |
| [80] | Suzuki Y, Miyamoto T, Yoshizawa R, Mae T, Makino A ( 2009). Rubisco content and photosynthesis of leaves at different positions in transgenic rice with an overexpression of RBCS. Plant Cell Environ 32, 417-427. |
| [81] | Suzuki Y, Ohkubo M, Hatakeyama H, Ohashi K, Yoshizawa R, Kojima S, Hayakawa T, Yamaya T, Mae T, Makino A ( 2007). Increased Rubisco content in transgenic rice transformed with the 'Sense' rbcS gene. Plant Cell Physiol 48, 626-637. |
| [82] | Tamoi M, Shigeoka S ( 2005). Improvement of photosynthesis in higher plants. In: Omasa K, Nouchi I, De Kok LJ, eds. Plant Responses to Air Pollution and Global Change. Tokyo: Springer. pp. 141-147. |
| [83] | Taylor SH, Hulme SP, Rees M, Ripley BS, Ian Woodward F, Osborne CP ( 2010). Ecophysiological traits in C3 and C4 grasses: a phylogenetically controlled screening experiment. New Phytol 185, 780-791. |
| [84] | Tissue DT, Dippery JK, Thomas RB, Strain BR ( 1995). Effects of low and elevated CO2 on C3 and C4 annuals. Oecologia 101, 21-28. |
| [85] | Tsuchida H, Tamai T, Fukayama H, Agarie S, Nomura M, Onodera H, Ono K, Nishizawa Y, Lee BH, Hirose S, Toki S, Ku MSB, Matsuoka M, Miyao M ( 2001). High level expression of C4-specific NADP-malic enzyme in leaves and impairment of photoautotrophic growth in a C3 plant, rice. Plant Cell Physiol 42, 138-145. |
| [86] | Vogan PJ, Sage RF ( 2012). Effects of low atmospheric CO2 and elevated temperature during growth on the gas exchange responses of C3, C3-C4 intermediate, and C4 species from three evolutionary lineages of C4 photosynthesis. Oecologia 169, 341-352. |
| [87] | Voznesenskaya EV, Franceschi VR, Kiirats O, Artyusheva EG, Freitag H, Edwards GE ( 2002). Proof of C4 photosynthesis without Kranz anatomy in Bienertia cycloptera(Chenopodiaceae). Plant J 31, 649-662. |
| [88] | Voznesenskaya EV, Franceschi VR, Kiirats O, Freitag H, Edwards GE ( 2001). Kranz anatomy is not essential for terrestrial C4 plant photosynthesis. Nature 414, 543-546. |
| [89] | Walker BJ, VanLoocke A, Bernacchi CJ, Ort DR ( 2016). The costs of photorespiration to food production now and in the future. Annu Rev Plant Biol 67, 107-129. |
| [90] | Wang P, Kelly S, Fouracre JP, Langdale JA ( 2013). Genome-wide transcript analysis of early maize leaf development reveals gene cohorts associated with the differentiation of C4 Kranz anatomy. Plant J 75, 656-670. |
| [91] | Ward JK, Tissue DT, Thomas BR, Strain BDR ( 1999). Comparative responses of model C3 and C4 plants to drought in low and elevated CO2. Global Change Biol 5, 857-867. |
| [92] | Weber APM, von Caemmerer S ( 2010). Plastid transport and metabolism of C3 and C4 plants-comparative analysis and possible biotechnological exploitation. Curr Opin Plant Biol 13, 256-264. |
| [93] | Wessinger ME, Edwards GE, Ku MSB ( 1989). Quantity and kinetic properties of ribulose 1,5-bisphosphate carboxylase in C3, C4, and C3-C4 intermediate species of flaveria (asteraceae). Plant Cell Physiol 30, 665-671. |
| [94] | Whitney SM, Sharwood RE, Orr D, White SJ, Alonso H, Galmés J ( 2011). Isoleucine 309 acts as a C4 catalytic switch that increases ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) carboxylation rate in flaveria. Proc Natl Acad Sci USA 108, 14688-14693. |
| [95] | Yanagisawa S, Akiyama A, Kisaka H, Uchimiya H, Miwa T ( 2004). Metabolic engineering with dof1 transcription factor in plants: improved nitrogen assimilation and growth under low-nitrogen conditions. Proc Natl Acad Sci USA 101, 7833-7838. |
| [96] | Yin ZH, Raven JA ( 1997). A comparison of the impacts of various nitrogen sources on acid-base balance in C3 Triticum aestivum L.and C4 Zea mays L. plants. J Exp Bot 48, 315-324. |
| [97] | Yin ZH, Raven JA ( 1998). Influences of different nitrogen sources on nitrogen- and water-use efficiency, and carbon isotope discrimination, in C3 Triticum aestivum L. and C4 Zea mays L. plants. Planta 205, 574-580. |
| [98] | Zeng Q, Liu B, Gilna B, Zhang YL, Zhu CW, Ma HL, Pang J, Chen GP, Zhu JG ( 2011). Elevated CO2 effects on nutrient competition between a C3 crop (Oryza sativa L.) and a C4 weed(Echinochloa crusgalli L.). Nutr Cycl Agroecosys 89, 93-104. |
| [99] | Zheng ZL ( 2009). Carbon and nitrogen nutrient balance signaling in plants. Plant Signal Behav 4, 584-591. |
/
| 〈 |
|
〉 |