

Functional Analysis of Brassica napus BnTTG1-1 Gene
Received date: 2016-12-05
Accepted date: 2017-04-03
Online published: 2018-08-09
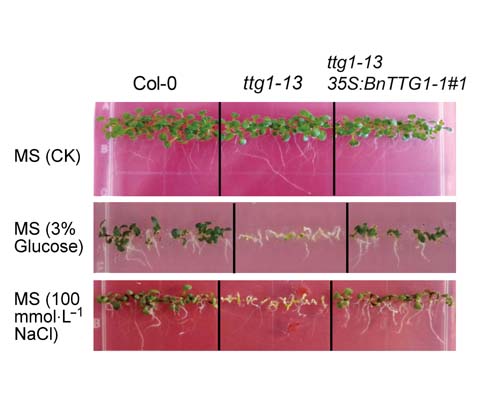
AtTTG1 existing in the nucleus as a WD40 repeat transcription factor plays important roles in regulating trichome initiation, anthocyanin biosynthesis, and storage reserve accumulation in Arabidopsis thaliana. In the present study, we cloned the full-length coding domain sequence (CDS) of the BnTTG1-1 gene from the Brassica napus cv. ‘QINYOU Seven’, analyzed its subcellular localization, detected its temporal and spatial expression patterns in different tissues, and investigated its functions in several biological processes. BnTTG1-1 was localized in the nucleus of tobacco leaf cells, so it may function as a transcription factor. BnTTG1-1 was widely expressed in various vegetative tissues and developing seeds in QINYOU Seven. Moreover, introducing 35S:BnTTG1-1 into the mutant ttg1-13 fully rescued many phenotypes of the mutant, such as no trichomes and anthocyanins, yellow seed coat, higher contents of seed fatty acids and storage proteins, and sensitivity to higher sucrose or salinity stresses during seed germination and seedling establishment. Thus, BnTTG1-1 and AtTTG1 exhibited conserved functions on many biological processes during plant growth and development.
Key words: TTG1; Brassica napus; Arabidopsis thaliana; functional complementation
Liu Kaige , Qi Shuanghui , Duan Shaowei , Li Dong , Jin Changyu , Gao Chenhao , Liu Mingxun Chen Xuanxia . Functional Analysis of Brassica napus BnTTG1-1 Gene[J]. Chinese Bulletin of Botany, 2017 , 52(6) : 713 -722 . DOI: 10.11983/CBB16239
| [1] | 刘后利, 傅廷栋, 陈怀庆, 易淑梅, 熊双娥 (1979). 甘蓝型黄籽油菜的发现及其遗传行为的初步研究. 遗传学报 6, 54. |
| [2] | 张子龙, 李加纳 (2001). 甘蓝型黄籽油菜粒色遗传及其育种研究进展. 作物杂志 (6), 37-40. |
| [3] | Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the |
| [4] | principle of protein-dye binding.Anal Biochem 72, 248-254. |
| [5] | Cavell AC, Lydiate DJ, Parkin IAP, Dean C, Trick M (1998). Collinearity between a 30-centimorgan segment of Arabidopsis thaliana chromosome 4 and duplicated regions within the Brassica napus genome. Genome 41, 62-69. |
| [6] | Cernac A, Andre C, Hoffmann-Benning S, Benning C (2006). WRI1 is required for seed germination and seedling establishment.Plant Physiol 141, 745-757. |
| [7] | Chen MX, Du X, Zhu Y, Wang Z, Hua SJ, Li ZL, Guo WL, Zhang GP, Peng JR, Jiang LX (2012a).Seed Fatty Acid Reducer acts downstream of gibberellin signaling pathway to lower seed fatty acid storage in Arabidopsis. Plant Cell Environ 35, 2155-2169. |
| [8] | Chen MX, Wang Z, Zhu YN, Li ZL, Hussain N, Xuan LJ, Guo WL, Zhang GP, Jiang LX (2012b). The effect of TRANSPARENT TESTA2 on seed fatty acid biosynthesis and tolerance to environmental stresses during young seedling establishment in Arabidopsis. Plant Physiol 160, 1023-1036. |
| [9] | Chen MX, Xuan LJ, Wang Z, Zhou LH, Li ZL, Du X, Ali E, Zhang GP, Jiang LX (2014). TRANSPARENT TESTA8 inhibits seed fatty acid accumulation by targeting several seed development regulators in Arabidopsis.Plant Phy- siol 165, 905-916. |
| [10] | Chen MX, Zhang B, Li CX, Kulaveerasingam H, Chew FT, Yu H (2015). TRANSPARENT TESTA GLABRA1 regulates the accumulation of seed storage reserves in Ara- bidopsis. Plant Physiol 169, 391-402. |
| [11] | Clough SJ, Bent AF (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16, 735-743. |
| [12] | Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010). Abscisic acid: emergence of a core signaling network.Annu Rev Plant Biol 61, 651-679. |
| [13] | Debeaujon I, Léon-Kloosterziel KM, Koornneef M (2000). Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis.Plant Physiol 122, 403-414. |
| [14] | Debeaujon I, Nesi N, Perez P, Devic M, Grandjean O, Caboche M, Lepiniec L (2003). Proanthocyanidin- accumulating cells in Arabidopsis testa: regulation of differentiation and role in seed development.Plant Cell 15, 2514-2531. |
| [15] | Finkelstein RR, Gampala SSL, Rock CD (2002). Abscisic acid signaling in seeds and seedlings.Plant Cell 14, S15-S45. |
| [16] | Gibson SI (2001). Plant sugar-response pathways. Part of a complex regulatory web.Plant Physiol 125, 2203-2203. |
| [17] | Hong JK, Choi HW, Hwang IS, Kim DS, Kim NH, Choi DS, Kim YJ, Hwang BK (2008). Function of a novel GDSL- type pepper lipase gene, CaGLIP1, in disease susceptibility and abiotic stress tolerance. Planta 227, 539-558. |
| [18] | Koes RE, Quattrocchio F, Mol JNM (1994). The flavonoid biosynthetic pathway in plants: function and evolution.BioEssays 16, 123-132. |
| [19] | Koornneef M (1981). The complex syndrome of ttg mutants. Arabidopsis Inf Serv 18, 45-51. |
| [20] | Lepiniec L, Debeaujon I, Routaboul JM, Baudry A, Pourcel L, Nesi N, Caboche M (2006). Genetics and biochemistry of seed flavonoids.Annu Rev Plant Biol 57, 405-430. |
| [21] | Liu KG, Qi SH, Li D, Jin CY, Gao CH, Duan SW, Feng BL, Chen MX (2017). TRANSPARENT TESTA GLABRA 1 ubiquitously regulates plant growth and development from Arabidopsis to foxtail millet (Setaria italica). Plant Sci 254, 60-69. |
| [22] | Livak KJ, Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method.Methods 25, 402-408. |
| [23] | Lu J, Li JN, Lei B, Wang SG, Chai YR (2009). Molecular cloning and characterization of two Brassica napus TTG1 genes reveal genus-specific nucleotide preference, extreme protein-level conservation and fast divergence of organ-specificity. Genes Genom 31, 129-142. |
| [24] | Mol J, Grotewold E, Koes R (1998). How genes paint flowers and seeds.Trends Plant Sci 3, 212-217. |
| [25] | Mu JY, Tan HL, Zheng Q, Fu FY, Liang Y, Zhang J, Yang XH, Wang T, Chong K, Wang XJ, Zuo JR (2008). LEAFY COTYLEDON1 is a key regulator of fatty acid biosynthesis in Arabidopsis. Plant Physiol 148, 1042-1054. |
| [26] | Nakabayashi R, Yonekura-Sakakibara K, Urano K, Suzuki M, Yamada Y, Nishizawa T, Matsuda F, Kojima M, Sakakibara H, Shinozaki K, Michael AJ, Tohge T, Yamazaki M, Saito K (2014). Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids.Plant J 77, 367-379. |
| [27] | Nesi N, Jond C, Debeaujon I, Caboche M, Lepiniec L (2001). The Arabidopsis TT2 gene encodes an R2R3 MYB domain protein that acts as a key determinant for proanthocyanidin accumulation in developing seed. Plant Cell 13, 2099-2114. |
| [28] | Nguyen HN, Kim JH, Hyun WY, Nguyen NT, Hong SW, Lee H (2013). TTG1-mediated flavonols biosynthesis alleviates root growth inhibition in response to ABA.Plant Cell Rep 32, 503-514. |
| [29] | Osborn TC, Kole C, Parkin IAP, Sharpe AG, Kuiper M, Lydiate DJ, Trick M (1997). Comparison of flowering time genes inBrassica rapa, B. napus and Arabidopsis tha- liana. Genetics 146, 1123-1129. |
| [30] | Peer WA, Murphy AS (2007). Flavonoids and auxin transport: modulators or regulators?Trends Plant Sci 12, 556-563. |
| [31] | Petrussa E, Braidot E, Zancani M, Peresson C, Bertolini A, Patui S, Vianello A (2013). Plant flavonoids-biosyn- thesis, transport and involvement in stress responses.Int J Mol Sci 14, 14950-14973. |
| [32] | Shi L, Katavic V, Yu YY, Kunst L, Haughn G (2012). Arabidopsis glabra2 mutant seeds deficient in mucilage biosynthesis produce more oil. Plant J 69, 37-46. |
| [33] | Shirley BW (1996). Flavonoid biosynthesis: ‘new’ functions for an ‘old’ pathway.Trends Plant Sci 1, 377-382. |
| [34] | Shirley BW, Kubasek WL, Storz G, Bruggemann E, Koornneef M, Ausubel FM, Goodman HM (1995). Analysis of Arabidopsis mutants deficient in flavonoid bio- synthesis.Plant J 8, 659-671. |
| [35] | Szymanski DB, Lloyd AM, Marks MD (2000). Progress in the molecular genetic analysis of trichome initiation and morphogenesis in Arabidopsis.Trends Plant Sci 5, 214-219. |
| [36] | Tsuchiya Y, Nambara E, Naito S, McCourt P (2004). The FUS3 transcription factor functions through the epidermal regulator TTG1 during embryogenesis in Arabidopsis. Plant J 37, 73-81. |
| [37] | Walker AR, Davison PA, Bolognesi-Winfield AC, James CM, Srinivasan N, Blundell TL, Esch JJ, Marks MD, Gray JC (1999). The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. Plant Cell 11, 1337-1350. |
| [38] | Wang Z, Chen MX, Chen TL, Xuan LJ, Li ZL, Du X, Zhou LH, Zhang GP, Jiang LX (2014). TRANSPARENT TESTA2 regulates embryonic fatty acid biosynthesis by targeting FUSCA3 during the early developmental stage of Arabidopsis seeds. Plant J 77, 757-769. |
| [39] | Western TL, Burn J, Tan WL, Skinner DJ, Martin- McCaffrey L, Moffatt BA, Haughn GW (2001). Isolation and characterization of mutants defective in seed coat mucilage secretory cell development in Arabidopsis.Plant Physiol 127, 998-1011. |
| [40] | Winkel-Shirley B (2002). Biosynthesis of flavonoids and ef- fects of stress.Curr Opin Plant Biol 5, 218-223. |
| [41] | Xu WJ, Grain D, Bobet S, Le Gourrierec J, Thévenin J, Kelemen Z, Lepiniec L, Dubos C (2014). Complexity and robustness of the flavonoid transcriptional regulatory network revealed by comprehensive analyses of MYB- bHLH-WDR complexes and their targets in Arabidopsis seed.New Phytol 202, 132-144. |
| [42] | Zhang YM, Rock CO (2004). Evaluation of epigallocatechin gallate and related plant polyphenols as inhibitors of the FabG and FabI reductases of bacterial type II fatty-acid synthase.J Biol Chem 279, 30994-31001. |
/
| 〈 |
|
〉 |