

Chinese Bulletin of Botany ›› 2021, Vol. 56 ›› Issue (5): 559-572.DOI: 10.11983/CBB21078 cstr: 32102.14.CBB21078
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Dan Mu1, Zehua Qi1, Qin Li1,2, Kexin Liang1, Shaogui Hua1, Xingyu Zhu1, Mengjie Jiao1, Yuchun Rao2,*( ), Tingzhe Sun1,*(
), Tingzhe Sun1,*( )
)
Received:2021-05-08
Accepted:2021-08-09
Online:2021-09-01
Published:2021-08-31
Contact:
Yuchun Rao,Tingzhe Sun
Dan Mu, Zehua Qi, Qin Li, Kexin Liang, Shaogui Hua, Xingyu Zhu, Mengjie Jiao, Yuchun Rao, Tingzhe Sun. Enhanced Attraction of Mymarids (Stethynium empoascae) by Volatiles from Tea Flowers[J]. Chinese Bulletin of Botany, 2021, 56(5): 559-572.
| Factor | Level A | Level B |
|---|---|---|
| Infestation | Healthy tea shoots (-) | Infested tea shoots (+) |
| Tea flower | Absent (-) | Present (+) |
| Mating status | Virgin mymarid (-) | Mated mymarid (+) |
Table 1 Details for factorial design
| Factor | Level A | Level B |
|---|---|---|
| Infestation | Healthy tea shoots (-) | Infested tea shoots (+) |
| Tea flower | Absent (-) | Present (+) |
| Mating status | Virgin mymarid (-) | Mated mymarid (+) |
| Group | Sum of squares | df | Mean square | F | p value |
|---|---|---|---|---|---|
| Tea flower | 4785.1562 | 1 | 4785.1562 | 42.5105 | 9.6614 × 10-10** |
| Infestation | 3001.5563 | 1 | 3001.5563 | 26.6653 | 7.4251 × 10-7** |
| Mating status | 1531.4062 | 1 | 1531.4062 | 13.6047 | 0.0003** |
| Tea flower × Infestation | 693.0563 | 1 | 693.0563 | 6.1570 | 0.0142* |
| Tea flower × Mating status | 94.5562 | 1 | 94.5562 | 0.8400 | 0.3608 |
| Infestation × Mating status | 45.1562 | 1 | 45.1562 | 0.5274 | |
| Sum of squares for error (SSE) | 17222.3063 | 153 | 112.5641 | ||
| Total | 27373.1938 | 159 |
Table 2 Analysis of variance table for factorial design
| Group | Sum of squares | df | Mean square | F | p value |
|---|---|---|---|---|---|
| Tea flower | 4785.1562 | 1 | 4785.1562 | 42.5105 | 9.6614 × 10-10** |
| Infestation | 3001.5563 | 1 | 3001.5563 | 26.6653 | 7.4251 × 10-7** |
| Mating status | 1531.4062 | 1 | 1531.4062 | 13.6047 | 0.0003** |
| Tea flower × Infestation | 693.0563 | 1 | 693.0563 | 6.1570 | 0.0142* |
| Tea flower × Mating status | 94.5562 | 1 | 94.5562 | 0.8400 | 0.3608 |
| Infestation × Mating status | 45.1562 | 1 | 45.1562 | 0.5274 | |
| Sum of squares for error (SSE) | 17222.3063 | 153 | 112.5641 | ||
| Total | 27373.1938 | 159 |
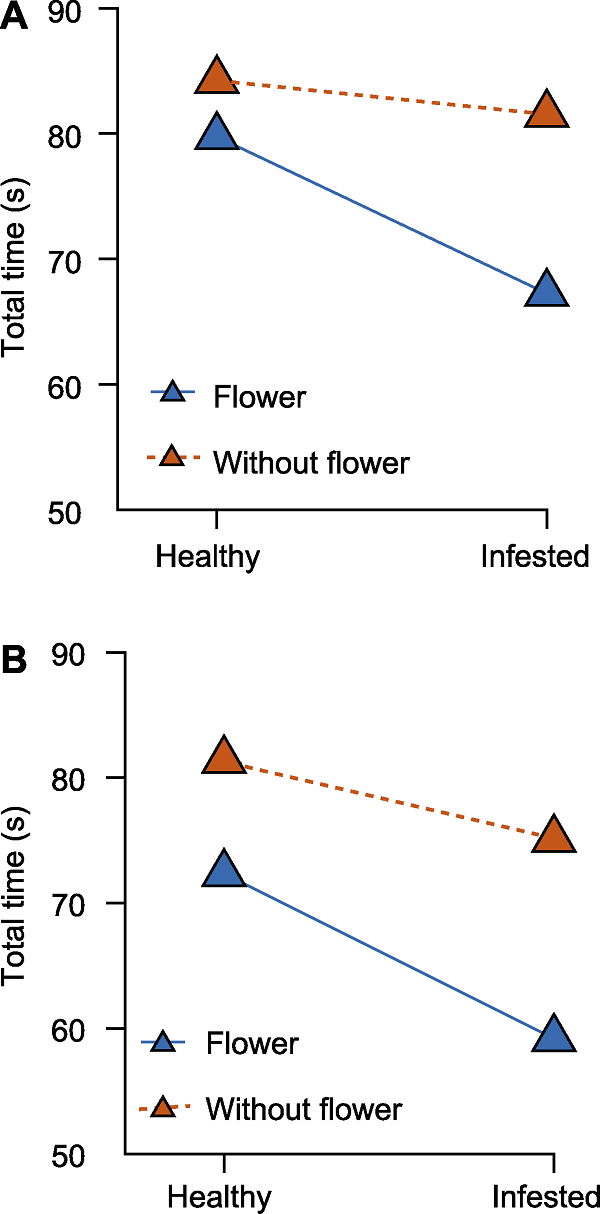
Figure 1 Interactions between tea flowers and leafhopper infestation (A) Virgin mymarids; (B) Mated female mymarids. The effect of interaction between leafhopper infestation and tea flowers on the parasitic behaviour of mymarids. The total time represents the summation of searching, spying and spawning time (unit: second).
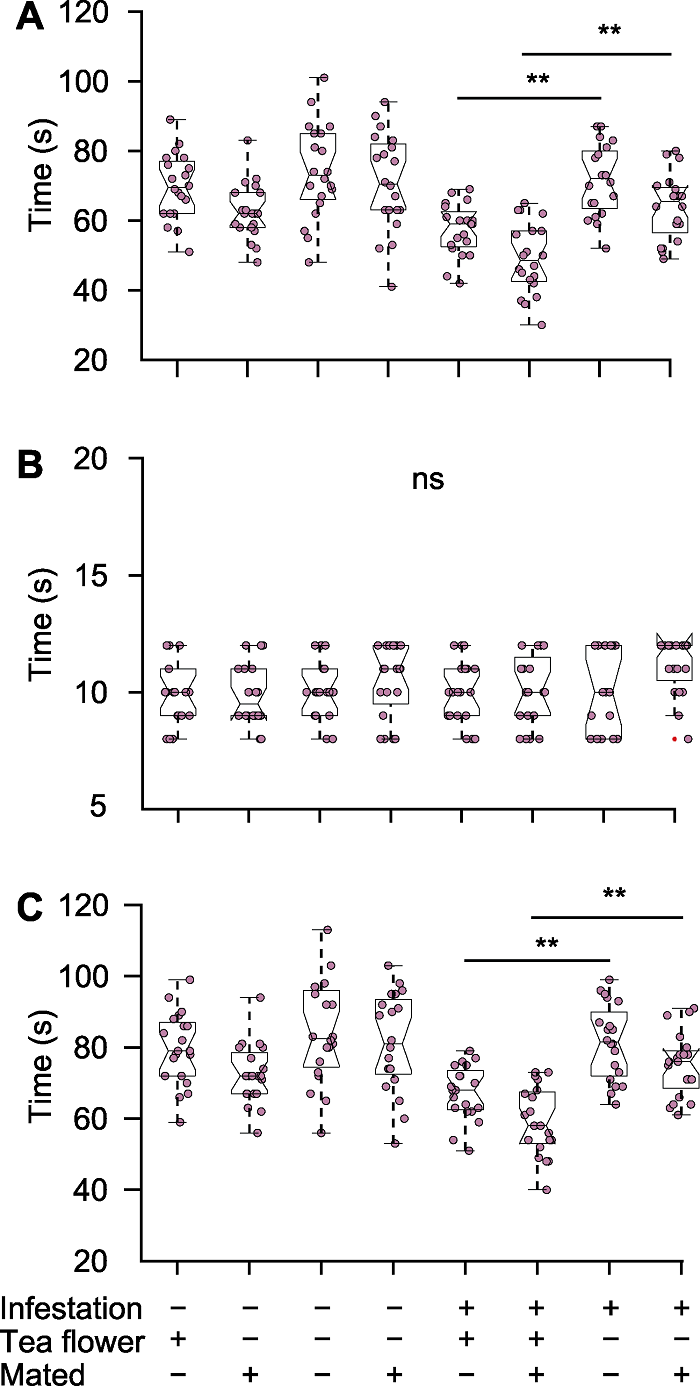
Figure 2 Time distribution for parasitic behavior of female mymarids (A) Searching time for mymarids during parasitism; (B) Distribution for spying and spawning time; (C) Distribution for total time. ns: Not significant. n=20, ** P<0.01
| Group | Mating status | Search time (s) | Spying and spawning time (s) | Total time (s) |
|---|---|---|---|---|
| Intact tea shoot + tea flower | Virgin | 69.75±2.12 | 10.00±0.30 | 79.75±2.26 |
| Mated | 62.50±1.78 | 9.85±0.28 | 72.35±1.88 | |
| Intact tea shoot | Virgin | 74.15±3.02 | 10.10±0.28 | 84.25±3.16 |
| Mated | 70.90±3.12 | 10.50±0.34 | 81.40±3.10 | |
| Tea shoot infested by tea green leafhopper + tea flower | Virgin | 57.35±1.67 | 9.90±0.31 | 67.25±1.70 |
| Mated | 49.10±2.26 | 10.10±0.33 | 59.20±2.15 | |
| Tea shoot infested by tea green leafhopper | Virgin | 71.50±2.26** | 10.05±0.39 | 81.55±2.36** |
| Mated | 63.95±2.10** | 11.15±0.26 | 75.10±1.99** |
Table 3 Parasitic behavior of female mymarids under different conditions (means±SE)
| Group | Mating status | Search time (s) | Spying and spawning time (s) | Total time (s) |
|---|---|---|---|---|
| Intact tea shoot + tea flower | Virgin | 69.75±2.12 | 10.00±0.30 | 79.75±2.26 |
| Mated | 62.50±1.78 | 9.85±0.28 | 72.35±1.88 | |
| Intact tea shoot | Virgin | 74.15±3.02 | 10.10±0.28 | 84.25±3.16 |
| Mated | 70.90±3.12 | 10.50±0.34 | 81.40±3.10 | |
| Tea shoot infested by tea green leafhopper + tea flower | Virgin | 57.35±1.67 | 9.90±0.31 | 67.25±1.70 |
| Mated | 49.10±2.26 | 10.10±0.33 | 59.20±2.15 | |
| Tea shoot infested by tea green leafhopper | Virgin | 71.50±2.26** | 10.05±0.39 | 81.55±2.36** |
| Mated | 63.95±2.10** | 11.15±0.26 | 75.10±1.99** |
| No. | Retention time (min) | Volatile compounds | Relative contents to internal standard (IS) | ||
|---|---|---|---|---|---|
| Healthy tea shoots | Tea shoots infested by tea green leafhopper | Tea flower | |||
| 1 | 3.772 | 4-penten-2-one | - | 4.6679±0.3469 | - |
| 2 | 4.659 | 2-hexanone | - | - | 0.8337±0.1178 |
| 3 | 4.973 | 3-hexenal | 0.6775±0.1432 | 0.6500±0.0644 | - |
| 4 | 6.791 | 3-hexen-1-ol | - | 2.4906±0.2406 | - |
| 5 | 6.973 | Ethylbenzene | - | - | 0.4284±0.0374 |
| 6 | 7.045 | Z-4-hexen-1-ol | - | 3.1843±0.2420 | - |
| 7 | 7.386 | m-xylene | 0.1821±0.0261 | 0.9410±0.1252 | 1.0658±0.1013 |
| 8 | 8.216 | p-xylene | 0.1388±0.0163 | 0.1685±0.0114 | - |
| 9 | 8.571 | 2-heptanone | - | - | 15.7670±0.2817 |
| 10 | 9.415 | Anisole | - | - | 0.4104±0.0497 |
| 11 | 10.061 | α-pinene | - | 0.0849±0.0098 | 0.0467±0.0056 |
| 12 | 11.542 | Benzaldehyde | - | 0.2588±0.0264 | 0.5192±0.0615 |
| 13 | 12.270 | Phenol | - | 0.0915±0.0094 | - |
| 14 | 13.383 | Decane | - | 0.0952±0.0098 | 0.0424±0.0048 |
| 15 | 13.595 | Octanal | - | 0.2073±0.0243 | 0.2724±0.0241 |
| 16 | 13.675 | Z-3-hexenyl acetate | - | 0.3178±0.0256 | - |
| 17 | 13.694 | E-3-hexenyl acetate | - | 0.6841±0.0819 | - |
| 18 | 14.841 | 2-ethyl-1-hexanol | 0.1636±0.0303 | 1.5707±0.1608 | 1.0125±0.1174 |
| 19 | 15.720 | α-phellandrene | - | - | 0.1193±0.0104 |
| 20 | 16.061 | 2-pentylcyclopentanone | - | - | 4.9586±0.3190 |
| 21 | 16.373 | α-methyl benzyl alcohol | - | - | 0.100 0±0.0116 |
| 22 | 16.612 | Acetophenone | 0.0443±0.0077 | 1.0436±0.1229 | 36.3044±3.7347 |
| 23 | 16.900 | cis-linaloloxide | - | - | 0.0395±0.0043 |
| 24 | 17.713 | trans-linaloloxide | - | - | 0.1566±0.0081 |
| 25 | 18.460 | Undecane | 0.0297±0.0048 | 0.2721±0.0370 | 0.2192±0.0237 |
| 26 | 18.693 | Nonanal | 0.0615±0.0105 | 1.0990±0.1109 | 0.7224±0.0835 |
| 27 | 20.668 | Camphor | 0.0989±0.0142 | 1.2977±0.1150 | 0.4835±0.0361 |
| 28 | 21.928 | E-2-nonen-1-ol | 0.0605±0.0074 | 0.2599+0.0135 | 0.1866±0.0158 |
| 29 | 22.381 | Naphthalene | 0.0305±0.0046 | 0.1241±0.0086 | 0.2344±0.0224 |
| 30 | 22.662 | cis-3-hexenyl butyrate | - | 0.0891±0.0081 | - |
| 31 | 23.362 | Dodecane | 0.0176±0.0029 | 0.2182±0.0153 | 0.1281±0.0155 |
| 32 | 23.654 | Decanal | 0.0995±0.0176 | 1.1579±0.1294 | 0.8621±0.0459 |
| 33 | 28.027 | Tridecane | - | 0.1029±0.0097 | 0.0625±0.0079 |
| / | 32.223 | Decanoic acid, ethyl ester (IS) | 1.0000 | 1.0000 | 1.0000 |
| 34 | 32.423 | Tetradecane | 0.0261±0.0055 | 0.3894±0.0406 | 0.1098±0.0113 |
| 35 | 32.625 | Longifolene-(V4) | - | 0.1815±0.0268 | 0.0307±0.0012 |
| 36 | 32.822 | Tetradecanal | - | 0.0467±0.0030 | 0.0275±0.0023 |
| 37 | 34.362 | E-6,10-dimethyl-5,9-undecadien-2-one | 0.0501±0.0065 | 0.5364±0.0388 | 0.2798±0.0269 |
| 38 | 36.594 | Pentadecane | 0.0704±0.0090 | 0.3610±0.0406 | 0.0922±0.0051 |
| 39 | 40.532 | Hexadecane | 0.0552±0.0097 | 0.3384±0.0304 | 0.1625±0.0221 |
| 40 | 44.276 | Heptadecane | - | 0.1006±0.0058 | 0.0437±0.0053 |
| 41 | 49.699 | 1,2-benzenedicarboxylic acid, bis(2- methylpropyl) ester | - | - | 0.0411±0.0023 |
| 42 | 50.477 | Homomenthyl salicylate | - | - | 0.0353±0.0041 |
Table 4 The relative contents of volatiles in healthy tea shoots, leafhopper-infested tea shoots and tea flowers (means±SE)
| No. | Retention time (min) | Volatile compounds | Relative contents to internal standard (IS) | ||
|---|---|---|---|---|---|
| Healthy tea shoots | Tea shoots infested by tea green leafhopper | Tea flower | |||
| 1 | 3.772 | 4-penten-2-one | - | 4.6679±0.3469 | - |
| 2 | 4.659 | 2-hexanone | - | - | 0.8337±0.1178 |
| 3 | 4.973 | 3-hexenal | 0.6775±0.1432 | 0.6500±0.0644 | - |
| 4 | 6.791 | 3-hexen-1-ol | - | 2.4906±0.2406 | - |
| 5 | 6.973 | Ethylbenzene | - | - | 0.4284±0.0374 |
| 6 | 7.045 | Z-4-hexen-1-ol | - | 3.1843±0.2420 | - |
| 7 | 7.386 | m-xylene | 0.1821±0.0261 | 0.9410±0.1252 | 1.0658±0.1013 |
| 8 | 8.216 | p-xylene | 0.1388±0.0163 | 0.1685±0.0114 | - |
| 9 | 8.571 | 2-heptanone | - | - | 15.7670±0.2817 |
| 10 | 9.415 | Anisole | - | - | 0.4104±0.0497 |
| 11 | 10.061 | α-pinene | - | 0.0849±0.0098 | 0.0467±0.0056 |
| 12 | 11.542 | Benzaldehyde | - | 0.2588±0.0264 | 0.5192±0.0615 |
| 13 | 12.270 | Phenol | - | 0.0915±0.0094 | - |
| 14 | 13.383 | Decane | - | 0.0952±0.0098 | 0.0424±0.0048 |
| 15 | 13.595 | Octanal | - | 0.2073±0.0243 | 0.2724±0.0241 |
| 16 | 13.675 | Z-3-hexenyl acetate | - | 0.3178±0.0256 | - |
| 17 | 13.694 | E-3-hexenyl acetate | - | 0.6841±0.0819 | - |
| 18 | 14.841 | 2-ethyl-1-hexanol | 0.1636±0.0303 | 1.5707±0.1608 | 1.0125±0.1174 |
| 19 | 15.720 | α-phellandrene | - | - | 0.1193±0.0104 |
| 20 | 16.061 | 2-pentylcyclopentanone | - | - | 4.9586±0.3190 |
| 21 | 16.373 | α-methyl benzyl alcohol | - | - | 0.100 0±0.0116 |
| 22 | 16.612 | Acetophenone | 0.0443±0.0077 | 1.0436±0.1229 | 36.3044±3.7347 |
| 23 | 16.900 | cis-linaloloxide | - | - | 0.0395±0.0043 |
| 24 | 17.713 | trans-linaloloxide | - | - | 0.1566±0.0081 |
| 25 | 18.460 | Undecane | 0.0297±0.0048 | 0.2721±0.0370 | 0.2192±0.0237 |
| 26 | 18.693 | Nonanal | 0.0615±0.0105 | 1.0990±0.1109 | 0.7224±0.0835 |
| 27 | 20.668 | Camphor | 0.0989±0.0142 | 1.2977±0.1150 | 0.4835±0.0361 |
| 28 | 21.928 | E-2-nonen-1-ol | 0.0605±0.0074 | 0.2599+0.0135 | 0.1866±0.0158 |
| 29 | 22.381 | Naphthalene | 0.0305±0.0046 | 0.1241±0.0086 | 0.2344±0.0224 |
| 30 | 22.662 | cis-3-hexenyl butyrate | - | 0.0891±0.0081 | - |
| 31 | 23.362 | Dodecane | 0.0176±0.0029 | 0.2182±0.0153 | 0.1281±0.0155 |
| 32 | 23.654 | Decanal | 0.0995±0.0176 | 1.1579±0.1294 | 0.8621±0.0459 |
| 33 | 28.027 | Tridecane | - | 0.1029±0.0097 | 0.0625±0.0079 |
| / | 32.223 | Decanoic acid, ethyl ester (IS) | 1.0000 | 1.0000 | 1.0000 |
| 34 | 32.423 | Tetradecane | 0.0261±0.0055 | 0.3894±0.0406 | 0.1098±0.0113 |
| 35 | 32.625 | Longifolene-(V4) | - | 0.1815±0.0268 | 0.0307±0.0012 |
| 36 | 32.822 | Tetradecanal | - | 0.0467±0.0030 | 0.0275±0.0023 |
| 37 | 34.362 | E-6,10-dimethyl-5,9-undecadien-2-one | 0.0501±0.0065 | 0.5364±0.0388 | 0.2798±0.0269 |
| 38 | 36.594 | Pentadecane | 0.0704±0.0090 | 0.3610±0.0406 | 0.0922±0.0051 |
| 39 | 40.532 | Hexadecane | 0.0552±0.0097 | 0.3384±0.0304 | 0.1625±0.0221 |
| 40 | 44.276 | Heptadecane | - | 0.1006±0.0058 | 0.0437±0.0053 |
| 41 | 49.699 | 1,2-benzenedicarboxylic acid, bis(2- methylpropyl) ester | - | - | 0.0411±0.0023 |
| 42 | 50.477 | Homomenthyl salicylate | - | - | 0.0353±0.0041 |
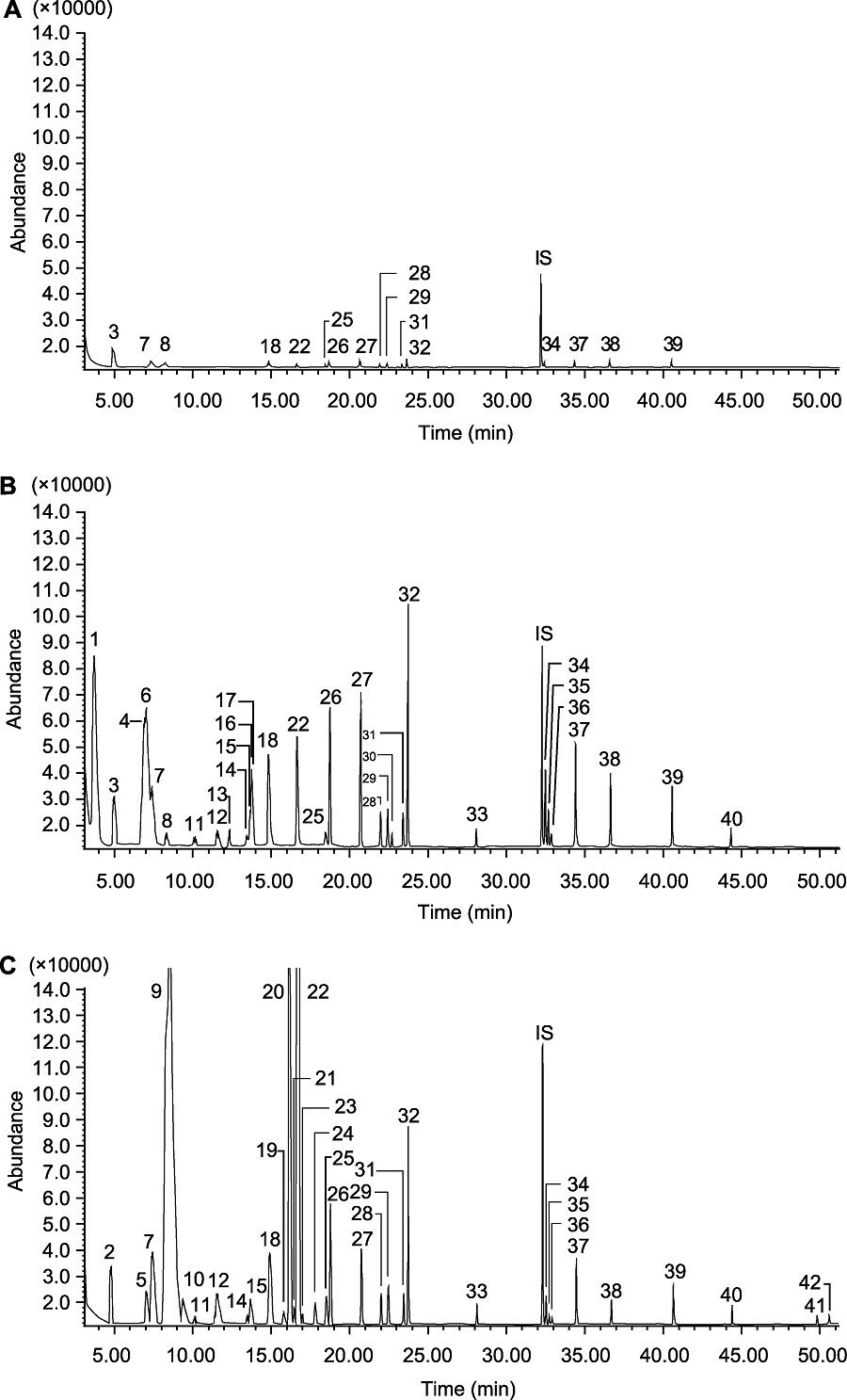
Figure 3 The GC-MS total ion chromatograms of the volatile components from healthy tea shoots (A), leafhopper-infested tea shoots (B) and tea flowers (C) No.1-42 are the same as Table 4. IS: Internal standard
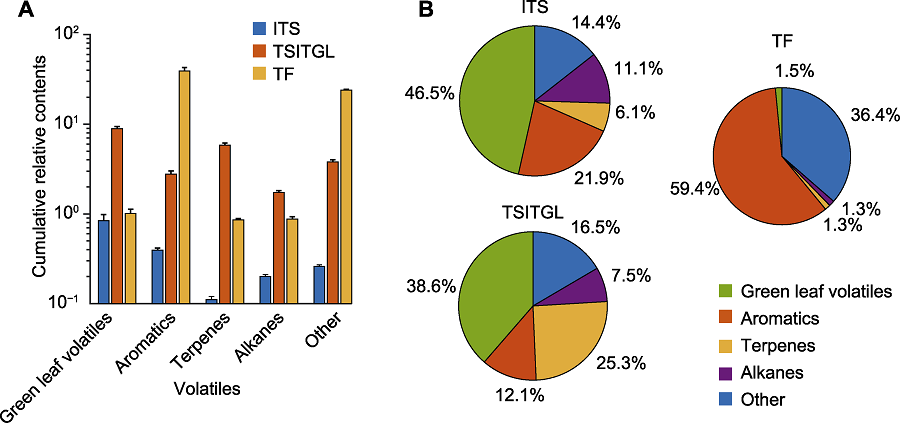
Figure 4 Categories and proportions of volatiles in intact tea shoots (ITS), leafhopper-infested tea shoots (TSITGL) and tea flowers (TF) (A) Variations in volatile categories in intact tea shoots, leafhopper-infested tea shoots and tea flowers; (B) The proportion of five classes of volatile compounds in intact tea shoots, leafhopper-infested tea shoots and tea flowers
| Classification | Volatile compounds |
|---|---|
| Green leaf volatiles | 3-hexenal, 3-hexen-1-ol, Z-4-hexen-1-ol, Z-3-hexenyl acetate, E-3-hexenyl acetate, 2-ethyl-1-hexanol |
| Aromatics | Ethylbenzene, m-xylene, p-xylene, anisole, benzaldehyde, phenol, α-methylbenzyl alcohol, acetophenone, naphthalene, 1,2-benzenedicarboxylic acid, bis (2-methylpropyl) ester |
| Terpenes | 4-penten-2-one, α-pinene, α-phellandrene, cis-linaloloxide, trans-linaloloxide, E-2-nonen-1-ol, cis-3- hexenyl butyrate, longifolene-(V4), E-6,10-dimethyl-5,9-undecadien-2-one |
| Alkanes | Decane, undecane, dodecane, tridecane, tetradecane, pentadecane, hexadecane, heptadecane |
| Other | 2-hexanone, 2-heptanone, octanal, 2-pentylcyclopentanone, nonanal, camphor, decanal, tetradecanal, homomenthyl salicylate |
Table 5 Classification of volatiles from tea plants
| Classification | Volatile compounds |
|---|---|
| Green leaf volatiles | 3-hexenal, 3-hexen-1-ol, Z-4-hexen-1-ol, Z-3-hexenyl acetate, E-3-hexenyl acetate, 2-ethyl-1-hexanol |
| Aromatics | Ethylbenzene, m-xylene, p-xylene, anisole, benzaldehyde, phenol, α-methylbenzyl alcohol, acetophenone, naphthalene, 1,2-benzenedicarboxylic acid, bis (2-methylpropyl) ester |
| Terpenes | 4-penten-2-one, α-pinene, α-phellandrene, cis-linaloloxide, trans-linaloloxide, E-2-nonen-1-ol, cis-3- hexenyl butyrate, longifolene-(V4), E-6,10-dimethyl-5,9-undecadien-2-one |
| Alkanes | Decane, undecane, dodecane, tridecane, tetradecane, pentadecane, hexadecane, heptadecane |
| Other | 2-hexanone, 2-heptanone, octanal, 2-pentylcyclopentanone, nonanal, camphor, decanal, tetradecanal, homomenthyl salicylate |
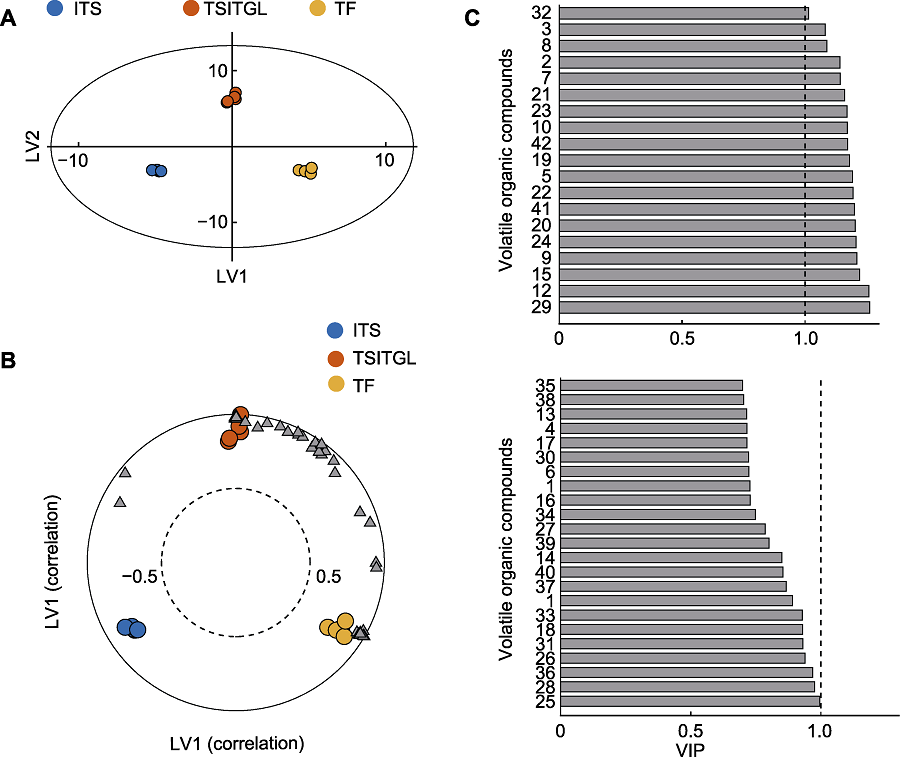
Figure 5 The partial least square discriminant analysis (PLS-DA) for volatiles (A) Scores with respect to the LV1 and LV2 (the eclipse denotes the 95% confidence interval based on Hotelling T2) for volatiles from intact tea shoots (ITS), leafhopper-infested tea shoots (TSITGL) and tea flowers (TF); (B) The biplot in PLS-DA (the dashed line is a guideline for 1.0); (C) The variable importance for the projection (VIP) for volatiles (the number is the same as Table 4). Volatiles with VIP≥1 are shown on top, and volatiles with VIP<1 are displayed at bottom panel.
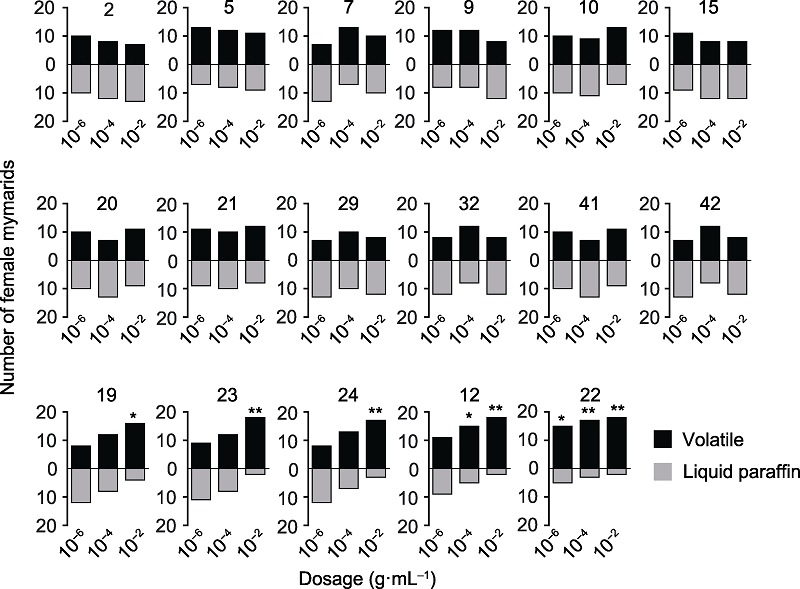
Figure 6 Y-tube olfactometer bioassay to determine the differences between 17 major volatiles from tea flowers and liquid paraffin in attracting mymarids The volatile numbers are shown on top panel (the number is the same as Table 4). * P<0.05; ** P<0.01
| [1] | 边磊 (2014). 基于远程寄主定位机理的假眼小绿叶蝉化学生态和物理调控. 博士论文. 北京: 中国农业科学院. pp. 28-46. |
| [2] | 陈璇, 胡福良 (2009). 蜜蜂花粉采集行为的调控机制. 昆虫知识 46, 490-494. |
| [3] |
董燕梅, 张文颖, 凌正一, 李靖锐, 白红彤, 李慧, 石雷 (2020). 转录因子调控植物萜类化合物生物合成研究进展. 植物学报 55, 340-350.
DOI |
| [4] | 戈林泉, 胡中卫, 吴进才 (2013). 大豆、玉米与水稻配置对稻田寄生蜂的影响. 应用昆虫学报 50, 921-927. |
| [5] | 韩宝瑜, 林金丽, 周孝贵, 章金明 (2009). 假眼小绿叶蝉卵及卵寄生蜂缨小蜂形态观察和寄生率考评. 安徽农业大学学报 36, 13-17. |
| [6] | 韩善捷, 潘铖, 韩宝瑜 (2016). 假眼小绿叶蝉为害致茶梢挥发物变化及其引诱微小裂骨缨小蜂效应. 中国生物防治学报 32, 142-148. |
| [7] | 李飞, 杨丹, 郑姣莉, 姚经武, 朱志刚, 黄大野, 曹春霞 (2020). 中国茶园主要害虫生物防治研究进展. 湖北农业科学 59(10), 5-9, 22. |
| [8] | 李秋玲 (2018). 班氏跳小蜂气味结合蛋白OBPs的结合特性分析. 硕士论文. 武汉: 华中农业大学. pp. 25-28. |
| [9] | 林金丽 (2010). 茶树-假眼小绿叶蝉-缨小蜂间化学和色彩通讯机理研究. 硕士论文. 扬州: 扬州大学. pp. 7-12. |
| [10] | 林郑和, 钟秋生, 陈常颂, 陈志辉, 游小妹 (2015). 不同香型茶树鲜叶挥发性组分与β-葡萄糖苷酶的相关性分析. 植物学报 50, 713-720. |
| [11] | 刘丰静, 冉伟, 李喜旺, 汪素琴, 孙晓玲 (2020). 小贯小绿叶蝉在5个茶树品种(系)上的蜜露排泄量与茶树叶片结构比较. 茶叶科学 40, 625-631. |
| [12] | 孟召娜, 边磊, 罗宗秀, 李兆群, 辛肇军, 蔡晓明 (2018). 全国主产茶区茶树小绿叶蝉种类鉴定及分析. 应用昆虫学报 55, 514-526. |
| [13] | 穆丹 (2011). 茶树挥发性信息素调控假眼小绿叶蝉及叶蝉三棒缨小蜂行为的功效. 博士论文. 北京: 中国农业科学院. pp. 12-62. |
| [14] | 聂晓培 (2017). 班氏跳小蜂寄主定位的嗅觉机制. 硕士论文. 武汉: 华中农业大学. pp. 41-47. |
| [15] | 潘铖, 林金丽, 韩宝瑜 (2016). 茶梢信息物引诱叶蝉三棒缨小蜂效应的检测. 生态学报 36, 3785-3795. |
| [16] | 吴国火, 崔林, 王梦馨, 李红亮, 韩宝瑜 (2020). 茶树花香气及茶叶气味对中华蜜蜂的引诱效应. 生态学报 40, 4024-4031. |
| [17] | 张云宣 (2018). 挥发性物质介导的稻纵卷叶螟姬小蜂搜寻与定位寄主行为机制初步研究. 硕士论文. 南宁: 广西大学. pp. 38-42. |
| [18] | 赵冬香, 陈宗懋, 程家安 (2000). 茶小绿叶蝉优势种的归属. 茶叶科学 20, 101-104. |
| [19] |
左照江, 张汝民, 高岩 (2009). 植物间挥发物信号的研究进展. 植物学报 44, 245-252.
DOI |
| [20] |
Aartsma Y, Leroy B, Van Der Werf W, Dicke M, Poelman EH, Bianchi FJJA (2019). Intraspecific variation in herbivore-induced plant volatiles influences the spatial range of plant-parasitoid interactions. Oikos 128, 77-86.
DOI URL |
| [21] | Boachon B, Junker RR, Miesch L, Bassard JE, Hofer R, Caillieaudeaux R, Seidel DE, Lesot A, Heinrich C, Ginglinger JF, Allouche L, Vincent B, Wahyuni DSC, Paetz C, Beran F, Miesch M, Schneider B, Leiss K, Werck-Reichhart D (2015). CYP76C1 (Cytochrome P450)-mediated linalool metabolism and the formation of volatile and soluble linalool oxides in Arabidopsis flowers. Plant Cell 27, 2972-2990. |
| [22] |
Bouwmeester H, Schuurink RC, Bleeker PM, Schiestl F (2019). The role of volatiles in plant communication. Plant J 100, 892-907.
DOI |
| [23] |
Cao H (2013). Polysaccharides from Chinese tea: recent advance on bioactivity and function. Int J Biol Macromol 62, 76-79.
DOI URL |
| [24] |
Chen D, Chen GJ, Sun Y, Zeng XX, Ye H (2020). Physiological genetics, chemical composition, health benefits and toxicology of tea ( Camellia sinensis L.) flower: a review. Food Res Int 137, 109584.
DOI PMID |
| [25] |
Chen GJ, Yuan QX, Saeeduddin M, Ou SY, Zeng XX, Ye H (2016). Recent advances in tea polysaccharides: extraction, purification, physicochemical characterization and bioactivities. Carbohydr Polym 153, 663-678.
DOI URL |
| [26] |
D'Alessandro M, Brunner V, Von Mérey G, Turlings TCJ (2009). Strong attraction of the parasitoid Cotesia marginiventris towards minor volatile compounds of maize. J Chem Ecol 35, 999-1008.
DOI PMID |
| [27] |
Desurmont GA, Von Arx M, Turlings TCJ, Schiestl FP (2020). Floral odors can interfere with the foraging behavior of parasitoids searching for hosts. Front Ecol Evol 8, 148.
DOI URL |
| [28] |
Fu JY, Han BY, Xiao Q (2014). Mitochondrial COI and 16sRNA evidence for a single species hypothesis of E. vitis, J. formosana and E. onukii in East Asia. PLoS One 9, e115259.
DOI URL |
| [29] |
Gorden NLS, Adler LS (2018). Consequences of multiple flower-insect interactions for subsequent plant-insect interactions and plant reproduction. Am J Bot 105, 1835-1846.
DOI URL |
| [30] |
Han BY, Chen ZM (2002a). Composition of the volatiles from intact and mechanically pierced tea aphid-tea shoot complexes and their attraction to natural enemies of the tea aphid. J Agric Food Chem 50, 2571-2575.
DOI URL |
| [31] |
Han BY, Chen ZM (2002b). Composition of the volatiles from intact and tea aphid-damaged tea shoots and their allurement to several natural enemies of the tea aphid. J Appl Entomol 126, 497-500.
DOI URL |
| [32] |
Huang MS, Sanchez-Moreiras AM, Abel C, Sohrabi R, Lee S, Gershenzon J, Tholl D (2012). The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-β-caryophyllene, is a defense against a bacterial pathogen. New Phytol 193, 997-1008.
DOI URL |
| [33] |
Ishiwari H, Suzuki T, Maeda T (2007). Essential compounds in herbivore-induced plant volatiles that attract the predatory mite Neoseiulus womersleyi. J Chem Ecol 33, 1670-1681.
PMID |
| [34] | Joshi R, Poonam, Saini R, Guleria S, Babu GDK, Kumari M, Gulati A (2011). Characterization of volatile components of tea flowers ( Camellia sinensis) growing in Kangra by GC/MS. Nat Prod Commun 6, 1155-1158. |
| [35] |
Lee LC, Liong CY, Jemain AA (2018). Partial least squares- discriminant analysis (PLS-DA) for classification of high- dimensional (HD) data: a review of contemporary practice strategies and knowledge gaps. Analyst 143, 3526-3539.
DOI URL |
| [36] |
Liu FL, Zhang XW, Chai JP, Yang DR (2006). Pollen phenolics and regulation of pollen foraging in honeybee colony. Behav Ecol Sociobiol 59, 582-588.
DOI URL |
| [37] |
Maffei ME, Gertsch J, Appendino G (2011). Plant volatiles: production, function and pharmacology. Nat Prod Rep 28, 1359-1380.
DOI URL |
| [38] |
McCormick AC, Unsicker SB, Gershenzon J (2012). The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci 17, 303-310.
DOI PMID |
| [39] |
Mu D, Cui L, Ge J, Wang MX, Liu LF, Yu XP, Zhang QH, Han BY (2012). Behavioral responses for evaluating the attractiveness of specific tea shoot volatiles to the tea green leafhopper, Empoaca vitis. Insect Sci 19, 229-238.
DOI URL |
| [40] |
Poveda K, Steffan-Dewenter I, Scheu S, Tscharntke T (2003). Effects of below- and above-ground herbivores on plant growth, flower visitation and seed set. Oecologia 135, 601-605.
PMID |
| [41] |
Qin DZ, Zhang L, Xiao Q, Dietrich C, Matsumura M (2015). Clarification of the identity of the tea green leafhopper based on morphological comparison between Chinese and Japanese Specimens. PLoS One, 10, e0139202.
DOI URL |
| [42] |
Rodriguez-Saona CR, Byers JA, Schiffhauer D (2012). Effect of trap color and height on captures of blunt-nosed and sharp-nosed leafhoppers (Hemiptera: Cicadellidae) and non-target arthropods in cranberry bogs. Crop Prot 40, 132-144.
DOI URL |
| [43] |
Rohrig E, Sivinski J, Teal P, Stuhl C, Aluja M (2008). A floral-derived compound attractive to the tephritid fruit fly parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae). J Chem Ecol 34, 549-557.
DOI PMID |
| [44] | Sanou A, Traoré F, Ba MN, Dabiré-Binso CL, Pittendrigh BR, Sanon A (2019). Effects of volatiles from Clavigralla tomentosicollis Stål. (Hemiptera: Coreidae) adults on the host location behavior of the egg parasitoid Gryon fulviventre (Crawford) (Hymenoptera: Scelionidae). Int J Insect Sci 11, 1-7. |
| [45] |
Schiestl FP (2015). Ecology and evolution of floral volatile-mediated information transfer in plants. New Phytol 206, 571-577.
DOI PMID |
| [46] |
Schnee C, Kollner TG, Held M, Turlings TCJ, Gershenzon J, Degenhardt J (2006). The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc Natl Acad Sci USA 103, 1129-1134.
DOI URL |
| [47] |
Sharma R, Rana A, Kumar S (2020). Phytochemical investigation and bioactivity studies of flowers obtained from different cultivars of Camellia sinensis plant. Nat Prod Res doi: 10.1080/14786419.2020.1844696.
DOI |
| [48] |
Simpson M, Gurr GM, Simmons AT, Wratten SD, James DG, Leeson G, Nicol HI (2011). Insect attraction to synthetic herbivore-induced plant volatile-treated field crops. Agr Forest Entomol 13, 45-57.
DOI URL |
| [49] |
Takemoto H, Takabayashi JJ (2015). Parasitic wasps Aphidius ervi are more attracted to a blend of host-induced plant volatiles than to the independent compounds. J Chem Ecol 41, 801-807.
DOI URL |
| [50] |
Tan XL, Hu NN, Zhang F, Ramirez-Romero R, Desneux N, Wang S, Ge F (2016). Mixed release of two parasitoids and a polyphagous ladybird as a potential strategy to control the tobacco whitefly Bemisia tabaci. Sci Rep 6, 28245.
DOI URL |
| [51] |
Tang GY, Meng X, Gan RY, Zhao CN, Liu Q, Feng YB, Li S, Wei XL, Atanasov AG, Corke H, Li HB (2019). Health functions and related molecular mechanisms of tea components: an update review. Int J Mol Sci 20, 6196.
DOI URL |
| [52] |
Turlings TCJ, Erb M (2018). Tritrophic interactions mediated by herbivore-induced plant volatiles: mechanisms, ecological relevance, and application potential. Annu Rev Entomol 63, 433-452.
DOI PMID |
| [53] | Wang P, Lou YG (2013). Screening and field evaluation of synthetic plant volatiles as attractants for Anagrus nilaparvatae Pang et Wang, an egg parasitoid of rice planthoppers. Chin J Appl Entomol 50, 431-440. |
| [54] |
Wang ZZ, Liu YQ, Shi M, Huang JH, Chen XX (2019). Parasitoid wasps as effective biological control agents. J Integ Agric 18, 705-715.
DOI URL |
| [55] |
Wei JN, Kang L (2011). Roles of (Z)-3-hexenol in plant- insect interactions. Plant Signal Behav 6, 369-371.
DOI URL |
| [56] |
Xiu CL, Zhang W, Xu B, Wyckhuys KAG, Cai XM, Su HS, Lu YH (2019). Volatiles from aphid-infested plants attract adults of the multicolored Asian lady beetle Harmonia axyridis. Biol Control 129, 1-11.
DOI URL |
| [57] |
Ye J, Zhang LL, Zhang X, Wu XJ, Fang RX (2021). Plant defense networks against insect-borne pathogens. Trends Plant Sci 26, 272-287.
DOI URL |
| [58] |
Zhao MY, Wang L, Wang JM, Jin JY, Zhang N, Lei L, Gao T, Jing TT, Zhang SR, Wu Y, Wu B, Hu YQ, Wan XC, Schwab W, Song CK (2020). Induction of priming by cold stress via inducible volatile cues in neighboring tea plants. J Integr Plant Biol 62, 1461-1468.
DOI URL |
| [1] | YIN Xiao-Lei, LIU Xu-Yang, JIN Qiang, LI Xian-De, LIN Shao-Ying, YANG Xiang, WANG Wei-Qi, ZHANG Yong-Xun. Effects of different management methods on carbon, nitrogen, and phosphorus contents and their stoichiometric ratios in tea plants [J]. Chin J Plant Ecol, 2021, 45(7): 749-759. |
| [2] | Tingzhe Sun, Zehua Qi, Kexin Liang, Qin Li, Yuchun Rao, Dan Mu. Clustering Analysis of Volatile Components from the Tea Plants Infested by Tea Aphid (Toxoptera aurantii) [J]. Chinese Bulletin of Botany, 2021, 56(4): 422-432. |
| [3] | LI Chong-Wei, BAI Xin-Fu, CHEN Guo-Zhong, ZHU Ping, ZHANG Shu-Ting, HOU Yu-Ping, ZHANG Xing-Xiao. Differences in soil nutrients and phenolic acid metabolites contents in American ginseng cultivated soils with different restoration years [J]. Chin J Plant Ecol, 2021, 45(11): 1263-1274. |
| [4] | Zhang Xiaoling, Li Yichao, Wang Yunyun, Cai Hongyu, Zeng Hui, Wang Zhiheng. Influence of future climate change in suitable habitats of tea in different countries [J]. Biodiv Sci, 2019, 27(6): 595-606. |
| [5] | Xiaomei Liu,Lili Sun,Xiangdong Fu,Hong Liao. An Effective Method for the Rooting of Tea Cuttings [J]. Chinese Bulletin of Botany, 2019, 54(4): 531-538. |
| [6] | Wenju Zhang, Jun Rong, Chaoling Wei, Lianming Gao, Jiakuan Chen. Domestication origin and spread of cultivated tea plants [J]. Biodiv Sci, 2018, 26(4): 357-372. |
| [7] | Yan Sun, Zhongshi Zhou, Rui Wang, Heinz Müller-Schärer. Biological control opportunities of ragweed are predicted to decrease with climate change in East Asia [J]. Biodiv Sci, 2017, 25(12): 1285-1294. |
| [8] | HUANG Wei, WANG Yi, DING Jian-Qing. A review of adaptive evolution of defense strategies in an invasive plant species, Chinese tallow (Triadica sebifera) [J]. Chin J Plant Ecol, 2013, 37(9): 889-900. |
| [9] | Sheng Qiang, Guoqi Chen, Baoping Li, Ling Meng. Invasive alien species in Chinese agricultural ecosystems and their management [J]. Biodiv Sci, 2010, 18(6): 647-659. |
| [10] | YU Xiang-Qin, FENG Yu-Long, LI Qiao-Ming. Review of research advances and prospects of invasive Chromolaena odorata [J]. Chin J Plant Ecol, 2010, 34(5): 591-600. |
| [11] | Huaqin Xu, Runlin Xiao, Tongqing Song, Wen Luo, Quan Ren, Yao Huang. Effects of mulching and intercropping on the functional diversity of soil microbial communities in tea plantations [J]. Biodiv Sci, 2008, 16(2): 166-174. |
| [12] | LU Ping, SANG Wei-Guo, MA Ke-Ping. PROGRESS AND PROSPECTS IN RESEARCH OF AN EXOTIC INVASIVE SPECIES, EUPATORIUM ADENOPHORUM [J]. Chin J Plant Ecol, 2005, 29(6): 1029-1037. |
| [13] | GAO Lei, LI Bo. THE STUDY OF A SPECIOUS INVASIVE PLANT, WATER HYACINTH (EICHHORNIA CRASSIPES): ACHIEVEMENTS AND CHALLENGES [J]. Chin J Plan Ecolo, 2004, 28(6): 735-752. |
| [14] | LIU Hai-Bo TIAN Shi-Ping. Recent Advances on the Mechanism of Postharvest Biocontrol [J]. Chinese Bulletin of Botany, 2001, 18(06): 657-664. |
| [15] | TIAN Shi-Ping FAN Qing. Biological Technologies for the Control of Postharvest Diseases of Fruits and Vegetables [J]. Chinese Bulletin of Botany, 2000, 17(03): 211-217. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||