

Chinese Bulletin of Botany ›› 2017, Vol. 52 ›› Issue (6): 774-782.DOI: 10.11983/CBB16171 cstr: 32102.14.CBB16171
• TECHNIQUES AND METHODS • Previous Articles Next Articles
Hu Tianyuan1, Wang Rui1, Chen Shang1, Ma Baowei1, Gao Wei1,2,*( ), Huang Luqi3
), Huang Luqi3
Received:2016-08-21
Accepted:2017-01-10
Online:2017-11-01
Published:2018-02-22
Contact:
Gao Wei
Hu Tianyuan, Wang Rui, Chen Shang, Ma Baowei, Gao Wei, Huang Luqi. Protoplast Isolation and Establishment of Transient Expression System of Tripterygium wilfordii Suspension Culture Cells[J]. Chinese Bulletin of Botany, 2017, 52(6): 774-782.
| Group | Cellulase (%) | Pectinase (%) | Macerozyme (%) |
|---|---|---|---|
| 1 | 1.5 | 0.3 | 0.5 |
| 2 | 2.0 | 0.3 | 0.5 |
| 3 | 2.0 | 0.5 | 0.5 |
| 4 | 2.0 | 0.7 | 0.5 |
| 5 | 2.5 | 0.3 | 0.5 |
Table 1 Proportion of enzymic digestion to remove Triptery- gium wilfordii suspension cells cytoderm
| Group | Cellulase (%) | Pectinase (%) | Macerozyme (%) |
|---|---|---|---|
| 1 | 1.5 | 0.3 | 0.5 |
| 2 | 2.0 | 0.3 | 0.5 |
| 3 | 2.0 | 0.5 | 0.5 |
| 4 | 2.0 | 0.7 | 0.5 |
| 5 | 2.5 | 0.3 | 0.5 |
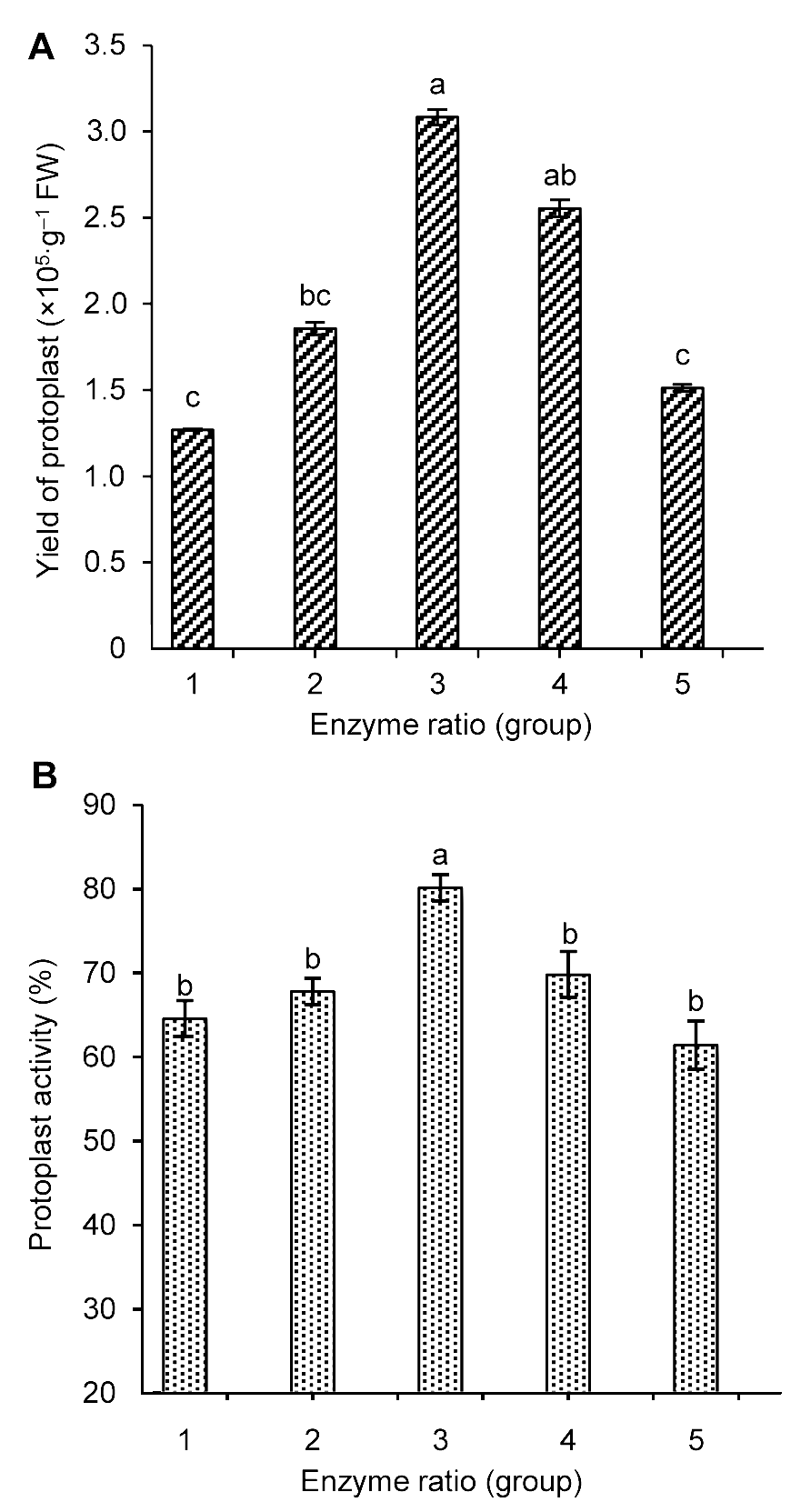
Figure 1 The influence of enzyme concentration on Tripterygium wilfordii suspension cells protoplast yield (A) and activity (B) Different lowercase letters indicate significant differences at P<0.05.
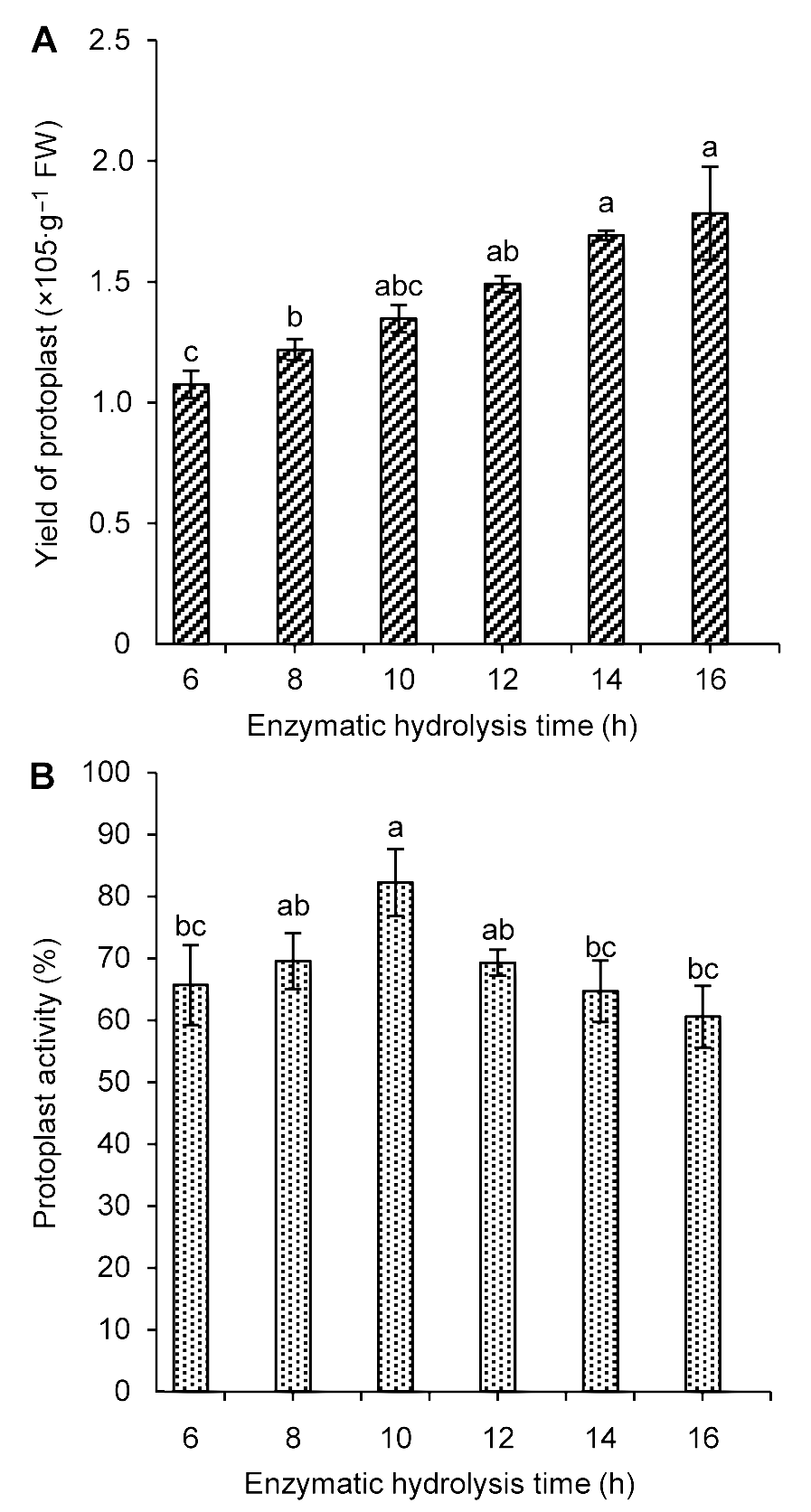
Figure 2 The influence of enzymatic hydrolysis time on Trip- terygium wilfordii suspension cells protoplast yield (A) and activity (B)Different lowercase letters indicate significant differences at P<0.05.
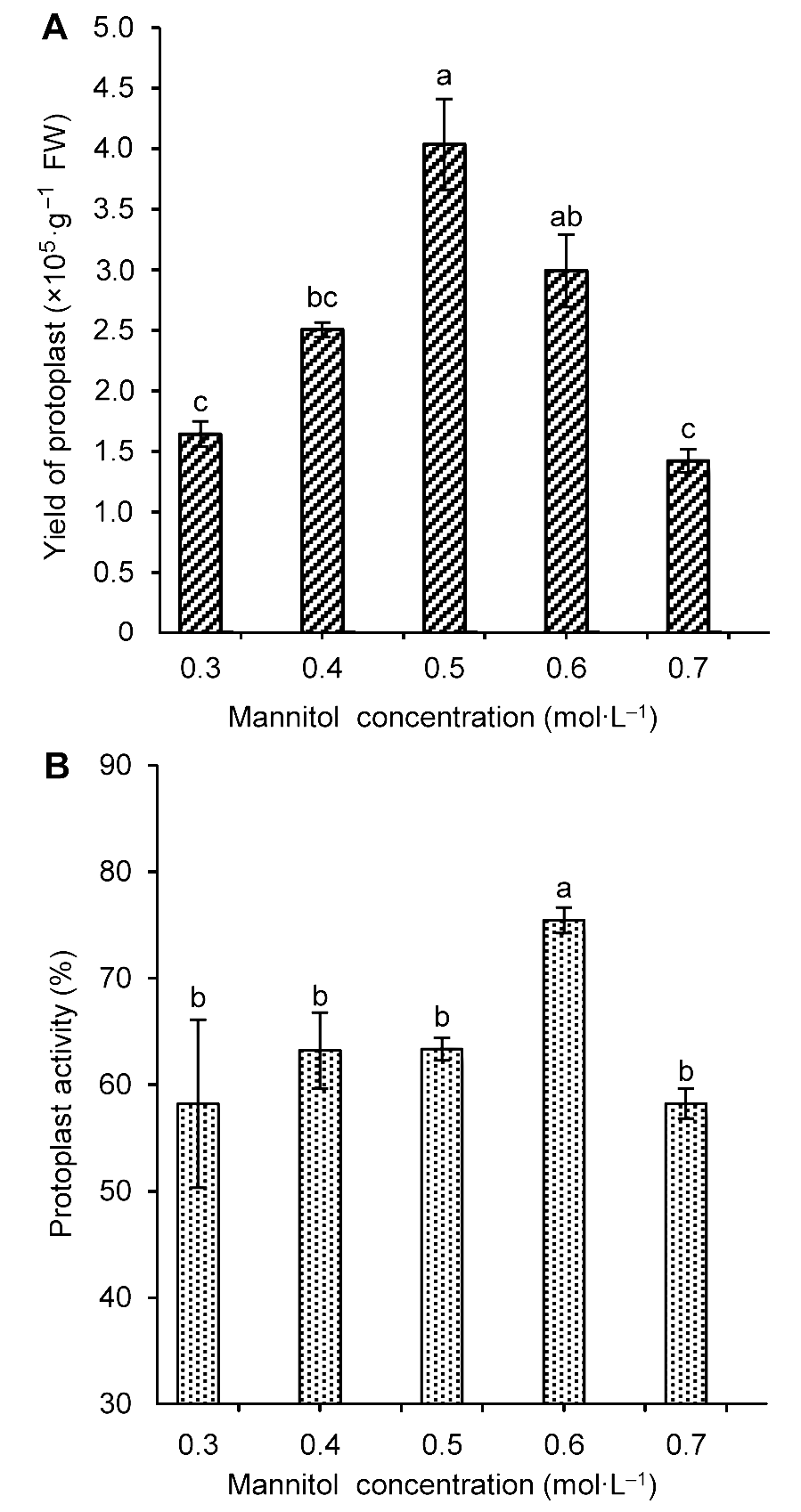
Figure 3 The influence of mannitol concentration on Tripterygium wilfordii suspension cells protoplast yield (A) and activity (B)Different lowercase letters indicate significant differences at P<0.05.
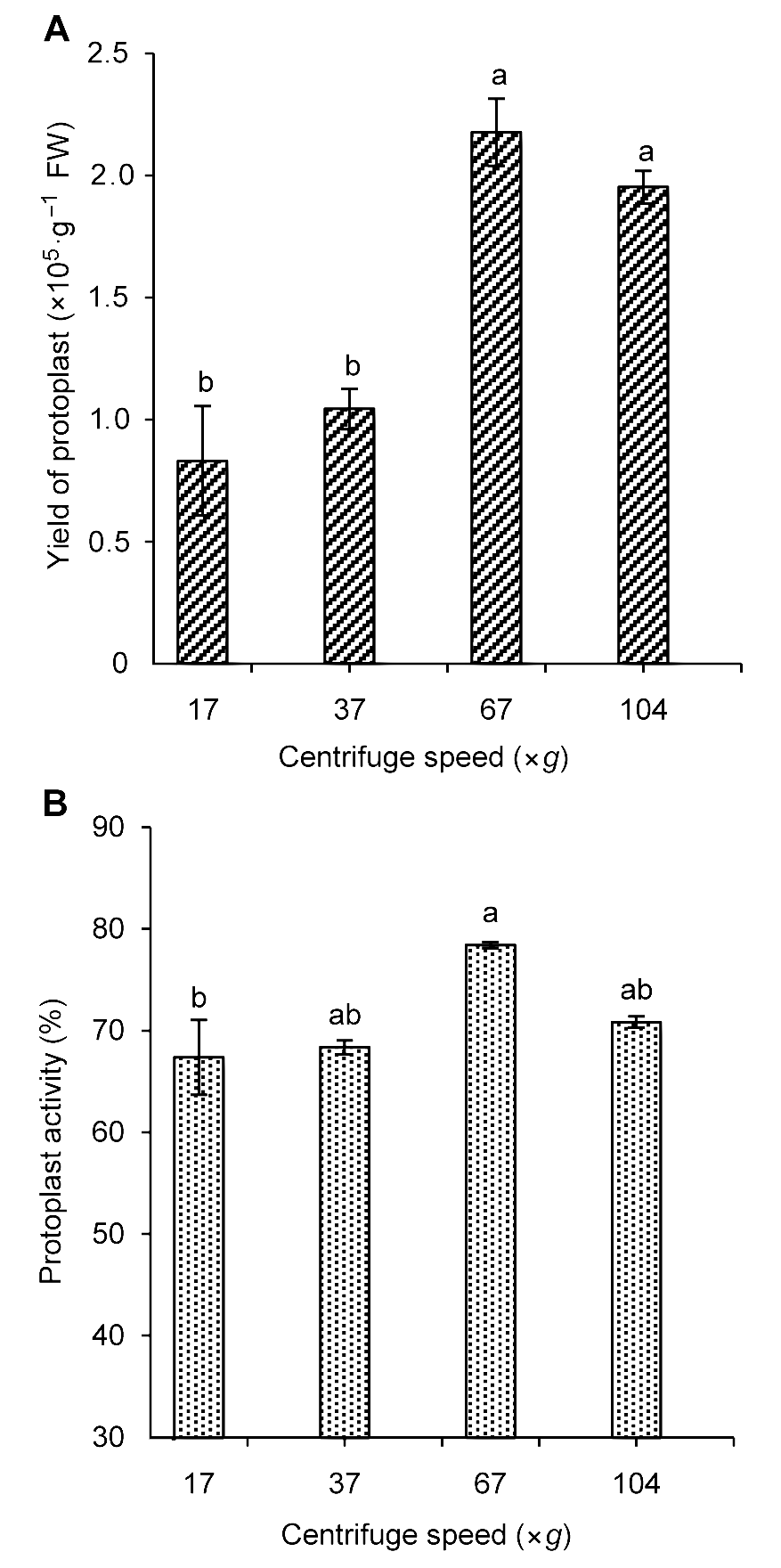
Figure 4 The influence of centrifugal speed on Tripterygium wilfordii suspension cells protoplast yield (A) and activity (B)Different lowercase letters indicate significant differences at P<0.05.
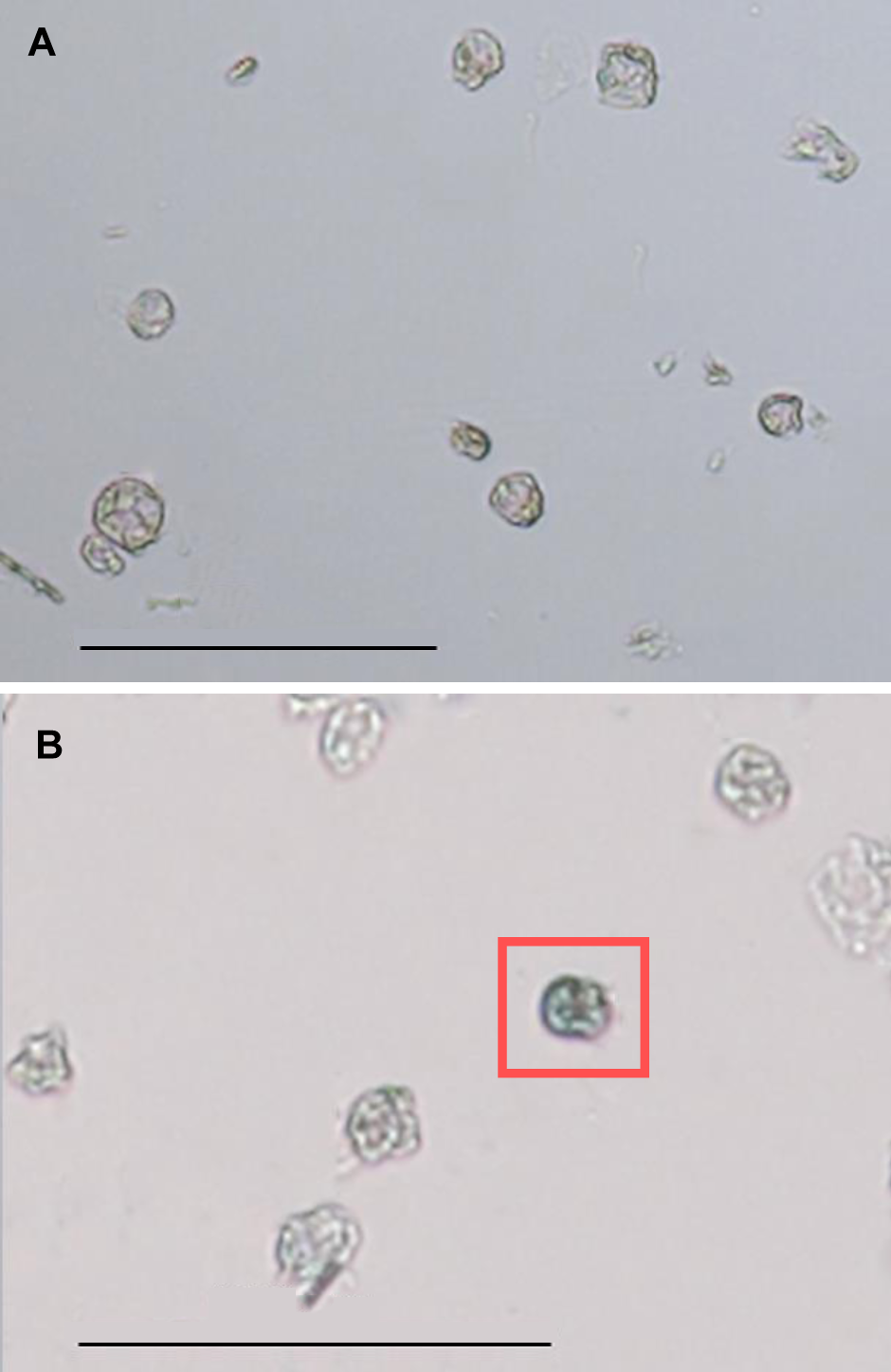
Figure 5 Morphology of Tripterygium wilfordii suspension cells protoplast under optical microscope(A) Morphology of protoplast before trypan blue staining; (B) Morphology of protoplast after trypan blue staining, and the blue cell in red box was dead. Bars=100 μm
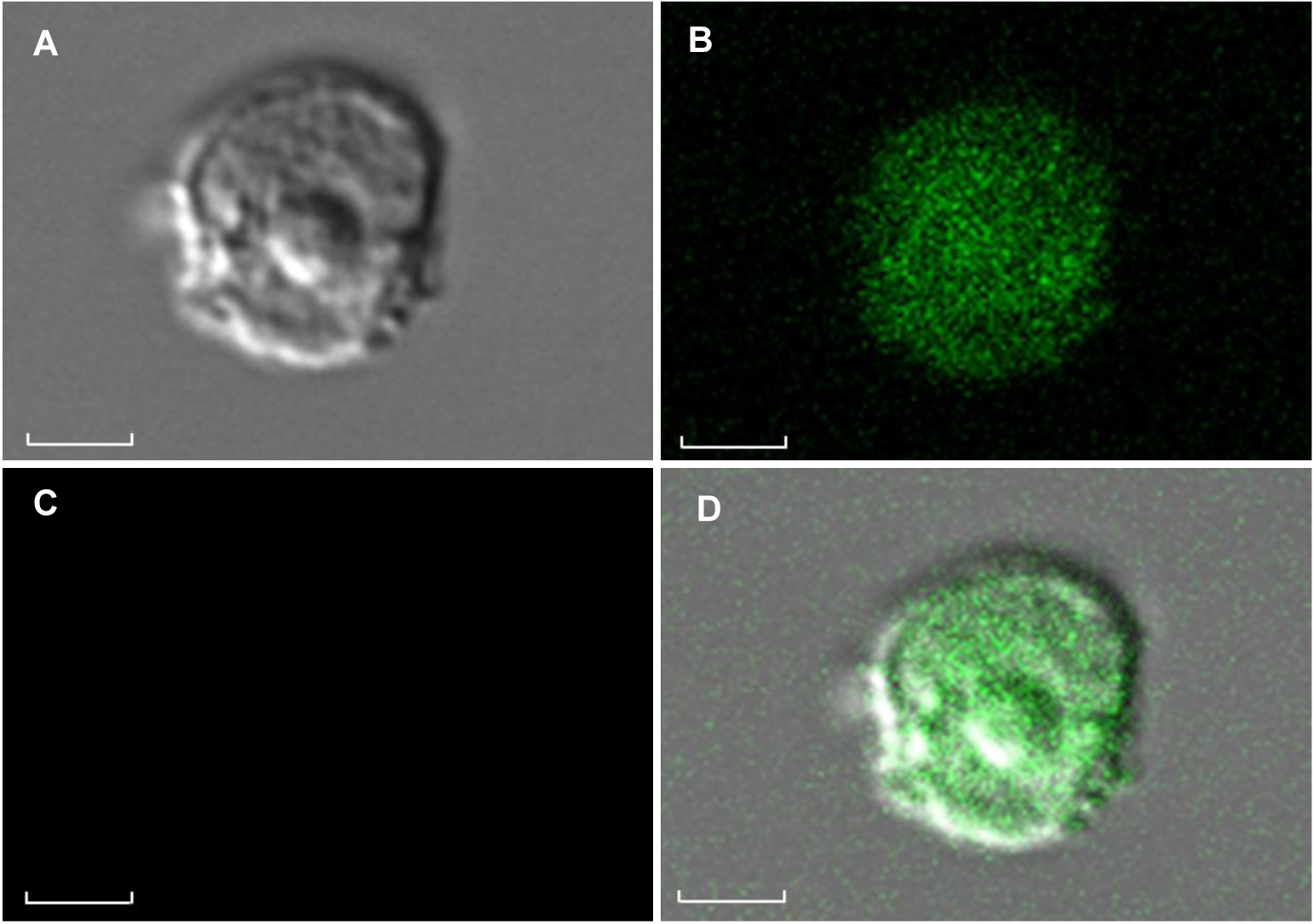
Figure 6 Protoplast of Tripterygium wilfordii suspension cells under laser scanning confocal microscop(A) Protoplasts under the laser confocal microscope in bright field; (B) GFP fluorescence of protoplast under the laser confocal microscope in excitation 488 nm; (C) There is no red chloroplast spontaneous fluorescence of protoplast under the laser confocal microscope in excitation 488 nm; (D) Red chlorophyll fluorescence signals and GFP signals from protoplast. Bars=1 μm
| [1] | 段炼, 钱君, 郭小雨, 朱英 (2014). 一种快速高效的水稻原生质体制备和转化方法的建立. 植物生理学报 50, 351-357. |
| [2] |
李妮娜, 丁林云, 张志远, 郭旺珍 (2014). 棉花叶肉原生质体分离及目标基因瞬时表达体系的建立. 作物学报 40, 231-239.
DOI URL |
| [3] |
刘凡, 赵泓, 秦帆 (2006). 结球白菜下胚轴原生质体培养及其体细胞胚植株再生. 植物学通报 23, 275-280.
DOI URL |
| [4] | 刘继红, 邓秀新 (1999). 植物原生质体非对称融合及其在育种上的应用. 生命科学 11, 88-91. |
| [5] |
张良波, 李培旺, 黄振, 李昌珠 (2011). 木本植物原生质体制备体系的研究进展. 中南林业科技大学学报 31(8), 102-107.
DOI URL |
| [6] |
朱楠, 刘俊, 张馨宇, 董娟娥 (2014). 丹参悬浮培养细胞原生质体的制备和活力检测. 生物工程学报 30, 1612-1621.
DOI URL |
| [7] | Chugh R, Sangwan V, Patil SP, Dudeja V, Dawra RK, Banerjee S, Schumacher RJ, Blazar BR, Georg GI, Vickers SM, Saluja AK (2012). A preclinical evaluation of minnelide as a therapeutic agent against pancreatic cancer. Sci Transl Med 4, 156ra139. |
| [8] |
Cocking EC (1960). A method for the isolation of plant protoplasts and vacuoles.Nature 187, 927-929.
DOI URL PMID |
| [9] |
Duarte P, Ribeiro D, Carqueijeiro I, Bettencourt S, Sottomayor M (2016). Protoplast transformation as a plant- transferable transient expression system.Methods Mol Biol 1405, 137.
DOI URL |
| [10] |
Gilroy S, Jones RL (1992). Gibberellic acid and abscisic acid coordinately regulate cytoplasmic calcium and secre- tory activity in barley aleurone protoplasts.Proc Natl Acad Sci USA 89, 3591-3595.
DOI URL |
| [11] | Guo ZJ, Kallus S, Akiyoshi K, Sunamoto J (2006). Artificial cell wall for plant protoplast. Coating of plasma membrane with hydrophobized polysaccharides.Chem Lett 31, 415-416. |
| [12] |
Knight MR, Campbell AK, Smith SM, Trewavas AJ (1991). Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium.Nature 352, 524-526.
DOI URL |
| [13] |
Liu JL, Lee J, Salazar Hernandez MA, Mazitschek R, Ozcan U (2015). Treatment of obesity with celastrol.Cell 161, 999-1011.
DOI URL |
| [14] |
Lu L, Li FQ, Wang XM (2010). Novel anti-inflammatory and neuroprotective agents for Parkinson’s disease.CNS Neu- rol Disord Drug Targets 9, 232-240.
DOI URL |
| [15] |
Maas C, Werr W (1989). Mechanism and optimized conditions for PEG mediated DNA transfection into plant protoplasts.Plant Cell Rep 8, 148-151.
DOI URL PMID |
| [16] |
Nagata T, Takebe I (1970). Cell wall regeneration and cell division in isolated tobacco mesophyll protoplasts.Planta 92, 301-308.
DOI URL |
| [17] |
Sheen J (2001). Signal transduction in maize and Arabidopsis mesophyll protoplasts.Plant Physiol 127, 1466-1475.
DOI URL PMID |
| [18] | Su P, Tong YR, Cheng QQ, Hu YT, Zhang M, Yang J, Teng QZ, Gao W, Huang LQ (2016). Functional characterization of ent-copalyl diphosphate synthase, kaurene synthase and kaurene oxidase in the Salvia miltiorrhiza gibberellin biosynthetic pathway. Sci Rep 6, 23057. |
| [19] |
Titov DV, Gilman B, He QL, Bhat S, Low WK, Dang YJ, Smeaton M, Demain AL, Miller PS, Kugel JF, Goodrich JA, Liu JO (2011). XPB, a subunit of TFIIH, is a target of the natural product triptolide.Nat Chem Biol 7, 182-188.
DOI URL PMID |
| [20] | Tudses N, Premjet S, Premjet D (2015). Establishment of method for protoplast fusion with peg-mediated between jatropha curcas l. and ricinus communis l.Int J Life Sci Biotech Pharm Res 4, 50-56. |
| [21] |
Wang X, Liang XB, Li FQ, Zhou HF, Liu XY, Wang JJ, Wang XM (2008). Therapeutic strategies for Parkinson’s disease: the ancient meets the future-traditional Chinese herbal medicine, electroacupuncture, gene therapy and stem cells.Neurochem Res 33, 1956-1963.
DOI URL |
| [22] |
Woo JW, Kim J, Kwon SI, Corvalán C, Cho SW, Kim H, Kim SG, Kim ST, Choe S, Kim JS (2015). DNA-free genome editing in plants with preassembled CRISPR- Cas9 ribonucleoproteins. Nat Biotechnol 33, 1162-1164.
DOI URL PMID |
| [23] | Zhang M, Su P, Zhou YJ, Wang XJ, Zhao YJ, Liu YJ, Tong YR, Hu TY, Huang LQ, Gao W (2015). Identification of geranylgeranyl diphosphate synthase genes from Tripterygium wilfordii. Plant Cell Rep 34, 2179-2188. |
| [24] | Zhao YJ, Chen X, Zhang M, Su P, Liu YJ, Tong YR, Wang XJ, Huang LQ, Gao W (2015). Molecular cloning and characterisation of farnesyl pyrophosphate synthase from Tripterygium wilfordii. PLoS One 10, r0125415. |
| [25] |
Zhou ZL, Yang YX, Ding J, Li YC, Miao ZH (2012). Triptolide: structural modifications, structure-activity relations- hips, bioactivities, clinical development and mechanisms.Nat Prod Rep 29, 457-475.
DOI URL PMID |
| [1] | Yuqin Zhang, Jiacheng Wu, Meng He, Renyi Liu, Xiaoyue Zhu. An Efficient Protoplast Transient Expression System in Camellia sinensis var. sinensis cv. ‘Tieguanyin’ [J]. Chinese Bulletin of Botany, 2022, 57(3): 340-349. |
| [2] | Aihua Song, Wenbin Zhang, Shulan Sun, Lingfei Li, Xiaojing Wang. Preparation of Protoplast and Establishment of Transient Expression System in Gerbera hybrida [J]. Chinese Bulletin of Botany, 2017, 52(4): 511-519. |
| [3] | Gaina Zhang;Jingfen Jia*. Interfamilial Somatic Hybridization Between Astragalus melilotoides and Zygophyllum xanthoxylum [J]. Chinese Bulletin of Botany, 2009, 44(04): 442-450. |
| [4] | Zhigang Nie;Yan Wang;Shaoshan Li. Heavy Metal-induced DNA Damage in Arabidopsis thaliana Protoplasts Measured by Single-cell Gel Electrophoresis [J]. Chinese Bulletin of Botany, 2009, 44(01): 117-123. |
| [5] | Bao Zhan;Wenzhong Xu;Mi Ma. Isolation and Analysis for Arsenic Tolerance of Protoplasts from an Arsenic Hyperaccumulator Pteris vittata [J]. Chinese Bulletin of Botany, 2006, 23(4): 363-367. |
| [6] | Fan Liu;Hong Zha;Fan Qin. The Protoplast Culture of Brassica campestris ssp. pekinensis and Plant Regeneration via Somatic Embryogenesis [J]. Chinese Bulletin of Botany, 2006, 23(3): 275-280. |
| [7] | . Research Progress in Plant Cytoplasmic Male Sterility by Protoplast Fusion [J]. Chinese Bulletin of Botany, 2005, 22(增刊): 99-107. |
| [8] | ZHANG Guo-Zeng AN Guo-Yong SONG Chun-Peng. Patch Clamp Recording of Arabidopsis Root Cells Under Different Cultural Conditions [J]. Chinese Bulletin of Botany, 2005, 22(01): 27-31. |
| [9] | LI Xin-Qi YUAN Long-Ping XIAO Jin-Hua DENG Qi-Yun. Breeding of Elite CMS Lines by Reverse Nuclear Substitution [J]. Chinese Bulletin of Botany, 2004, 21(03): 257-262. |
| [10] | WANG Jing-Shan SUN Shi-Meng WANG Wei-Hua BI Ying-Na XU Li-Juan MENG Xiang-Xia. Somatic Hybrids Between Cultivars of the Same Cross-Incompatible Group in Sweet Potato [J]. Chinese Bulletin of Botany, 2004, 21(03): 306-311. |
| [11] | CAO You-Long XU Xing SONG Yu-Xia QU Ling LUO Qing. Establishment of High Efficient Regeneration System from Protoplast of Lycium barbarum L. [J]. Chinese Bulletin of Botany, 2001, 18(05): 605-614. |
| [12] | ZHOU Jun YAN Chang-Hui CHEN Hao-Ming DAI Yao-Ren. Detection of Plant Protoplasts Apoptosis with TUNEL Method [J]. Chinese Bulletin of Botany, 2000, 17(01): 87-89. |
| [13] | CUI Hong and CHEN Mu-Zhuan. Advances in Biotechnological Studies of Sweet Potato [J]. Chinese Bulletin of Botany, 1999, 16(06): 653-657. |
| [14] | LIU Cong-Li;ZHANG Zhi-Fen;XU LI-Juan;MENG Xiang-Xia and WANG Jing-Shan. Efficient Plant Regeneration from Potiole Protoplasts of Sweet Potato [J]. Chinese Bulletin of Botany, 1999, 16(04): 420-424. |
| [15] | LI Yan-Fang;CHENG Xiao-Rui;ZHANG Ya-Lan and PANG Jin-Song. Protoplast Isolation and Culture of Young lnfeorescences of Wild Barley [J]. Chinese Bulletin of Botany, 1999, 16(01): 67-71. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||