

Chinese Bulletin of Botany ›› 2022, Vol. 57 ›› Issue (1): 56-68.DOI: 10.11983/CBB21220 cstr: 32102.14.CBB21220
• SPECIAL TOPICS • Previous Articles Next Articles
Yi Qin1, Yanshuang Liu1,2, Liuliu Qiu1, Min Zhou1, Xiaoshan Du1, Shaojun Dai1,*( ), Meihong Sun1,*(
), Meihong Sun1,*( )
)
Received:2021-12-16
Accepted:2022-01-18
Online:2022-01-01
Published:2022-01-19
Contact:
Shaojun Dai,Meihong Sun
Yi Qin, Yanshuang Liu, Liuliu Qiu, Min Zhou, Xiaoshan Du, Shaojun Dai, Meihong Sun. Advance in Molecular Mechanism of MBF1 Regulating Plant Heat Response and Development[J]. Chinese Bulletin of Botany, 2022, 57(1): 56-68.
| 物种 | 拉丁名 | 蛋白名 | 蛋白编号(JGI数据库) | 参考文献 |
|---|---|---|---|---|
| 拟南芥 | Arabidopsis thaliana | AtMBF1a | AT2G42680.1 | Tsuda et al., |
| AtMBF1b | AT3G58680.1 | |||
| AtMBF1c | AT3G24500.1 | |||
| 番茄 | Solanum lycopersicum | SlMBF1a | Solyc10g007350.3.1 | Zhang et al., |
| SlMBF1b | Solyc12g014290.2.1 | |||
| SlMBF1c | Solyc07g062400.3.1 | |||
| SlMBF1d | Solyc09g055470.1.1 | |||
| SlER24 | Solyc01g104740.3.1 | |||
| 水稻 | Oryza sativa | OsMBF1a | LOC_Os08g27850.1 | Zhang et al., |
| OsMBF1c | LOC_Os06g39240.1 | |||
| 菠菜 | Spinacia oleracea | SoMBF1b | Spov3_C0009.00073 | Xu et al., |
| SoMBF1c | Spov3_C0062.00022 | |||
| 马铃薯 | S. tuberosum | StMBF1a | PGSC0003DMP400012592 | Yu et al., |
| StMBF1b | PGSC0003DMP400026892 | |||
| StMBF1c | PGSC0003DMP400051869 | |||
| StMBF1d | PGSC0003DMP400051868 | |||
| 小麦 | Triticum aestivum | TaMBF1a-1 | Traes_2AL_A9D390619.1 | Qin et al., |
| TaMBF1a-2 | Traes_2BL_4F31B5695.1 | |||
| TaMBF1a-3 | Traes_2DL_D4AB94C53.1 | |||
| TaMBF1a-4 | Traes_3AS_719D37CCA.1 | |||
| TaMBF1a-5 | Traes_3B_EC2B74116.1 | |||
| TaMBF1b | Traes_4DS_F1C77E7B0.1 | |||
| TaMBF1c-7A | Traes_7AL_FA77CC1F41.1 | |||
| TaMBF1c-7B | Traes_7BL_A002364C5.1 | |||
| TaMBF1c-7C | Traes_7DL_D5AD8EB4B.1 | |||
| 葡萄 | Vitis vinifera | VvMBF1a | VIT_212s0028g02020.1 | Yan et al., |
| VvMBF1a-like | VIT_219s0014g01260.1 | |||
| VvMBF1c | VIT_211s0016g04080.1 | |||
| 大豆 | Glycine max | GmMBF1a-1 | Glyma.06G276200.1.p | Tsuda et al., |
| GmMBF1a-2 | Glyma.06G276300.1.p | |||
| GmMBF1a-3 | Glyma.12G129100.1.p | |||
| 玉米 | Zea mays | ZmMBF1a | ZmPHB47.01G345100.1.p | Tsuda et al., |
| ZmMBF1b | ZmPHB47.04G054300.1.p | |||
| ZmMBF1c | ZmPHB47.09G119100.1.p | |||
| 蓖麻 | Ricinus communis | RcMBF1b | 27894.m000799 | Tsuda et al., |
| RcMBF1c | 29912.m005549 | |||
| 蒺藜苜蓿 | Medicago truncatula | MtMBF1b-1 | Medtr2g084220.1 | Tsuda et al., |
| MtMBF1b-2 | Medtr4g080090.1 | |||
| MtMBF1b-3 | Medtr6g018330.1 | |||
| MtMBF1c | Medtr6g086280.1 |
Table 1 Members of the MBF1 protein family in different plant species
| 物种 | 拉丁名 | 蛋白名 | 蛋白编号(JGI数据库) | 参考文献 |
|---|---|---|---|---|
| 拟南芥 | Arabidopsis thaliana | AtMBF1a | AT2G42680.1 | Tsuda et al., |
| AtMBF1b | AT3G58680.1 | |||
| AtMBF1c | AT3G24500.1 | |||
| 番茄 | Solanum lycopersicum | SlMBF1a | Solyc10g007350.3.1 | Zhang et al., |
| SlMBF1b | Solyc12g014290.2.1 | |||
| SlMBF1c | Solyc07g062400.3.1 | |||
| SlMBF1d | Solyc09g055470.1.1 | |||
| SlER24 | Solyc01g104740.3.1 | |||
| 水稻 | Oryza sativa | OsMBF1a | LOC_Os08g27850.1 | Zhang et al., |
| OsMBF1c | LOC_Os06g39240.1 | |||
| 菠菜 | Spinacia oleracea | SoMBF1b | Spov3_C0009.00073 | Xu et al., |
| SoMBF1c | Spov3_C0062.00022 | |||
| 马铃薯 | S. tuberosum | StMBF1a | PGSC0003DMP400012592 | Yu et al., |
| StMBF1b | PGSC0003DMP400026892 | |||
| StMBF1c | PGSC0003DMP400051869 | |||
| StMBF1d | PGSC0003DMP400051868 | |||
| 小麦 | Triticum aestivum | TaMBF1a-1 | Traes_2AL_A9D390619.1 | Qin et al., |
| TaMBF1a-2 | Traes_2BL_4F31B5695.1 | |||
| TaMBF1a-3 | Traes_2DL_D4AB94C53.1 | |||
| TaMBF1a-4 | Traes_3AS_719D37CCA.1 | |||
| TaMBF1a-5 | Traes_3B_EC2B74116.1 | |||
| TaMBF1b | Traes_4DS_F1C77E7B0.1 | |||
| TaMBF1c-7A | Traes_7AL_FA77CC1F41.1 | |||
| TaMBF1c-7B | Traes_7BL_A002364C5.1 | |||
| TaMBF1c-7C | Traes_7DL_D5AD8EB4B.1 | |||
| 葡萄 | Vitis vinifera | VvMBF1a | VIT_212s0028g02020.1 | Yan et al., |
| VvMBF1a-like | VIT_219s0014g01260.1 | |||
| VvMBF1c | VIT_211s0016g04080.1 | |||
| 大豆 | Glycine max | GmMBF1a-1 | Glyma.06G276200.1.p | Tsuda et al., |
| GmMBF1a-2 | Glyma.06G276300.1.p | |||
| GmMBF1a-3 | Glyma.12G129100.1.p | |||
| 玉米 | Zea mays | ZmMBF1a | ZmPHB47.01G345100.1.p | Tsuda et al., |
| ZmMBF1b | ZmPHB47.04G054300.1.p | |||
| ZmMBF1c | ZmPHB47.09G119100.1.p | |||
| 蓖麻 | Ricinus communis | RcMBF1b | 27894.m000799 | Tsuda et al., |
| RcMBF1c | 29912.m005549 | |||
| 蒺藜苜蓿 | Medicago truncatula | MtMBF1b-1 | Medtr2g084220.1 | Tsuda et al., |
| MtMBF1b-2 | Medtr4g080090.1 | |||
| MtMBF1b-3 | Medtr6g018330.1 | |||
| MtMBF1c | Medtr6g086280.1 |
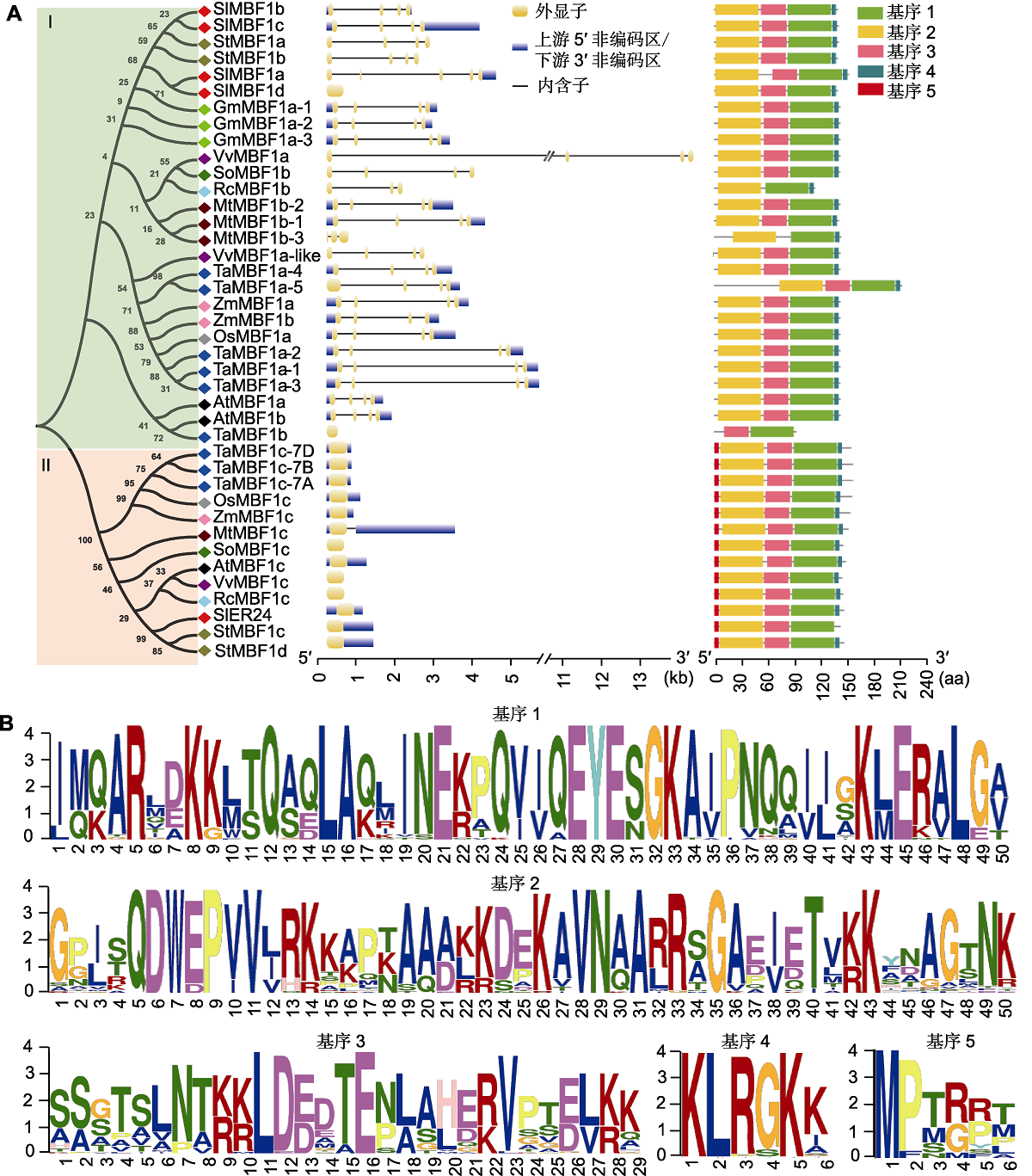
Figure 1 The analysis of the phylogenetic relationships, gene structures and conserved motifs of plant MBF1 (A) Phylogenetic relationship, gene structure and conserved motif distribution map of plant MBF1. The full-length protein sequences of MBF1 proteins from 11 species are downloaded from the JGI database (https://phytozome-next.jgi.doe.gov/) (table 1). MEGA7.0 software is used to construct a Neighbor-Joining homologous phylogenetic tree. The green background indicates the type I subfamily, and the red background indicates the type II subfamily. GSDS2.0 software (http://gsds.cbi.pku.edu.cn/) is used to draw a gene structure diagram online. 5'UTR/3'UTR are represented by blue boxes, exons are represented by yellow boxes, and introns are represented by black straight lines. The MEME website (https://meme-suite.org/meme/tools/meme) is used to predict conserved motifs. Conserved domains and functions are identified through the NCBI website CDD database (https://www.ncbi.nlm.nih.gov/cdd). Tbtools software is used to draw a map of the conserved motifs, and the 5 conserved motifs are indicated by boxes with different colors. (B) The predicted conserved motif sequences in MBF1 proteins of 11 species.
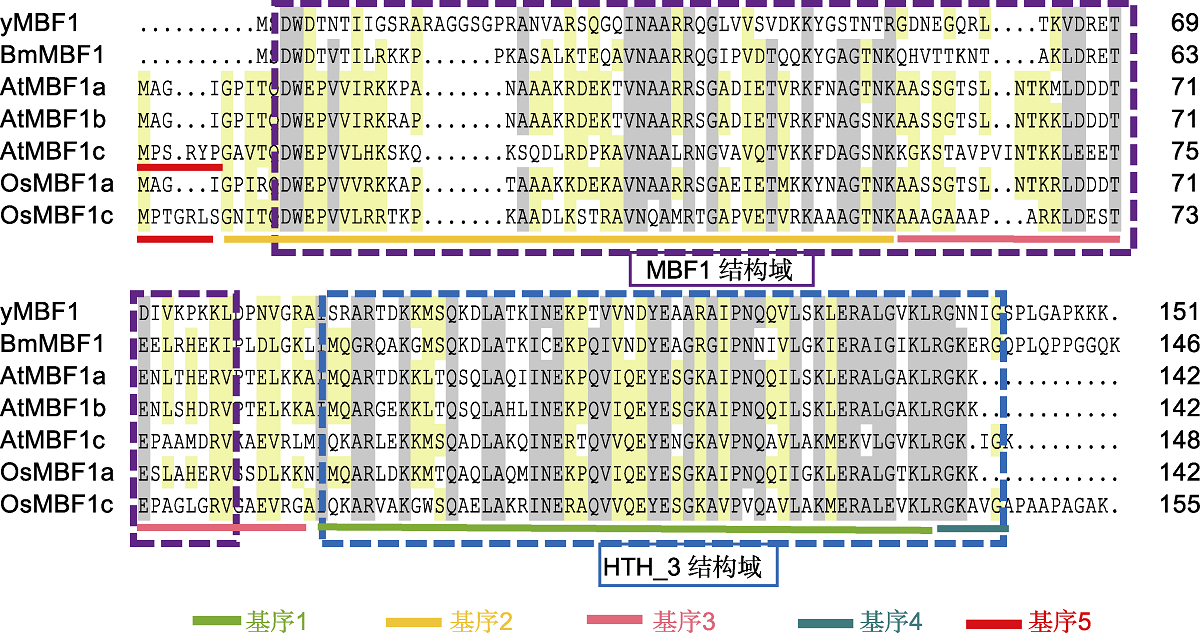
Figure 2 Homologous sequence alignment analysis of MBF1s of different species The protein sequences of Bombyx mori BmMBF1 and Saccharomyces cerevisiae yMBF1 are downloaded from Ensembl database (https://ensemblgenomes.org/), the corresponding protein accession number is BGIBMGA007702 and YOR298C-A. DNAMAN software is used for sequence alignment. The purple box indicates the N-terminal MBF1 domain, and the blue box indicates the C-terminal HTH_3 domain. The protein sequences and protein accession number of Arabidopsis and rice MBF1 are listed in Table 1.
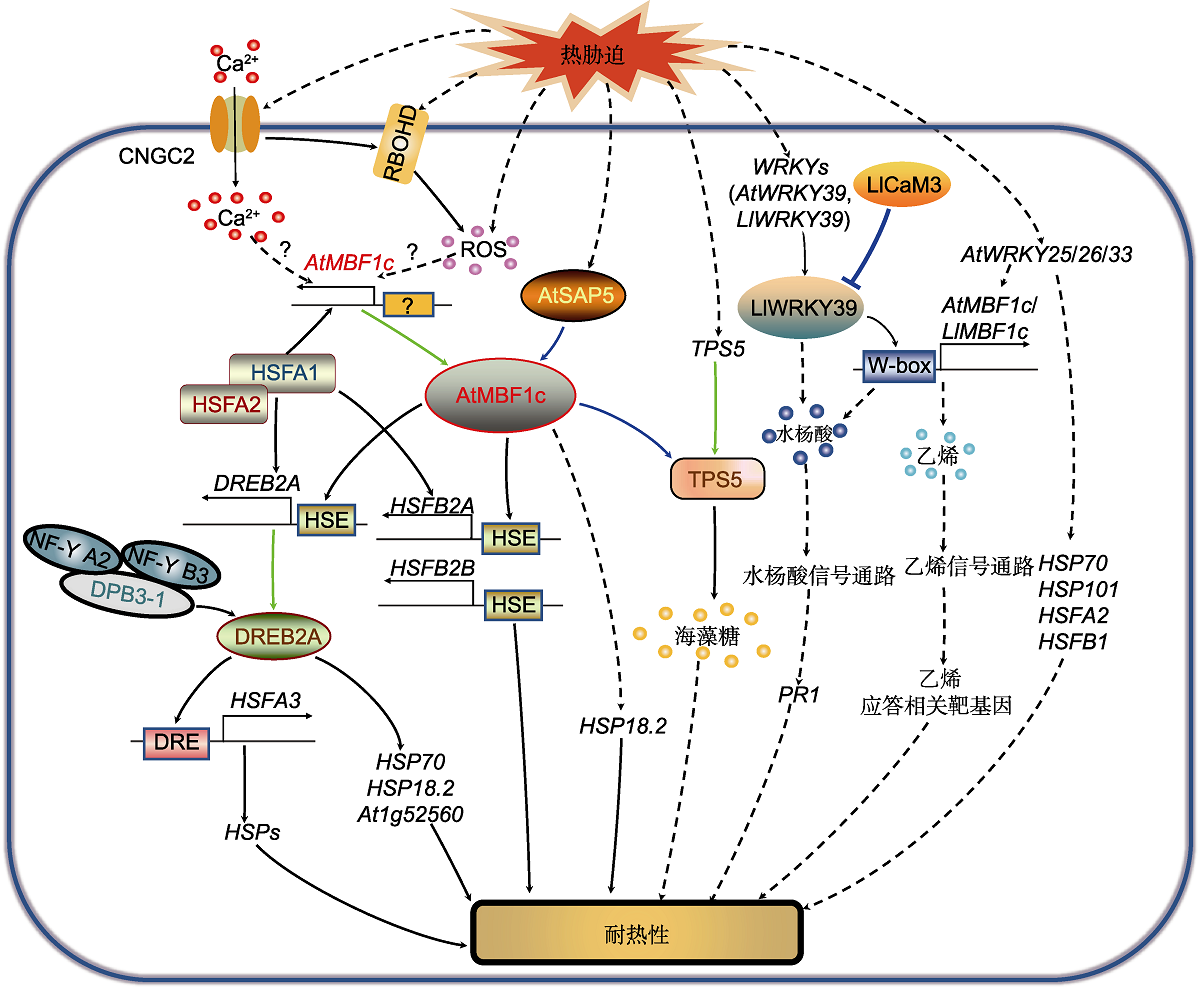
Figure 3 Signaling pathway of MBF1 regulating heat stress response Heat stress causes Ca2+ influx by activating the plasma membrane-localized protein CNGC2, and leads to the accumulation of ROS by activating the plasma membrane-bound RBOHD. The Ca2+ signal and ROS signal activate the heat stress response by regulating AtMBF1c and its downstream target genes through unknown pathways. The heat stress transcription factor HSFA1 interacts with HSFA2 and directly regulates the expression of AtMBF1c, HSFA2, HSFBs and DREB2A. At the same time, AtMBF1c binds to the HSE elements of DREB2A, HSFB2A and HSFB2B promoters to regulate their gene expression and improve heat stress tolerance. DREB2A interacts with the trimeric co-activation complex formed by DPB3-1, NF-Y A2 and NF-Y B3 to enhance the transcriptional activation of the downstream target gene HSFA3 and improve plant heat tolerance. DREB2A also promotes the expression of HSP70, HSP18.2 and At1g52560 to enhance plant heat tolerance. As the upstream regulator, AtSAP5 interacts with and activates AtMBF1c in the nucleus, regulating the expression of HSP18.2 and improving plant heat tolerance. Heat stress induces the expression of TPS5. AtMBF1c interacts with TPS5 to improve heat tolerance by promoting the synthesis and accumulation of trehalose. Heat stress induces the expression of AtWRKY39, AtWRKY25, AtWRKY26 and AtWRKY33. AtWRKY39 regulates the expression of the downstream gene of salicylic acid (SA) signaling pathway PR1 through AtMBF1c to improve heat tolerance. AtWRKY25, AtWRKY26 and AtWRKY33 regulate the expression of downstream genes in the ethylene (ET) signaling pathway through AtMBF1c to improve heat resistance, and at the same time promote the expression of HSP70, HSP101, HSFA2, and HSFB1. CNGC2: Cyclic nucleotide-gated channel 2; DPB3-1 (NF-YC10): DNA polymerase II subunit B3-1; DRE: Dehydration-responsive element; DREB2A: Dehydration responsive element-binding protein 2A; HSE: Heat shock elements; HSFA1/2/3: Heat stress transcription factor A1/2/3; HSFB2A/B: Heat stress transcription factor B2A/B; HSP18.2/ 70/101: Heat shock protein 18.2/70/101; MBF1c: Multiprotein bridging factor 1c; NF-Y A2/B3: Nuclear factor Y A2/B3; PR1: Pathogenesis-related factor 1; RBOHD: Respiratory burst oxidase homologue D; ROS: Reactive oxygen species; SAP5: Stress-associated protein 5; TPS5: Trehalose phosphate synthetase 5. The blue solid arrow indicates protein interaction; the green solid arrow indicates gene encoding protein; the black solid arrow indicates direct transcription activation; the black dashed arrow indicates indirect transcription activation.
| [1] | 李思佳, 张咏雪, 贾明生, 李莹, 戴绍军 (2020). 植物类LORELEI糖基磷脂酰肌醇锚定蛋白研究进展. 植物学报 55, 541-550. |
| [2] |
邱丽丽, 赵琪, 张玉红, 戴绍军 (2017). 植物质膜蛋白质组的逆境应答研究进展. 植物学报 52, 128-147.
DOI |
| [3] |
张洵, 喻娟娟, 王思竹, 李莹, 戴绍军 (2019). 植物DREPP基因家族研究进展. 植物学报 54, 582-595.
DOI |
| [4] | Arc E, Sechet J, Corbineau F, Rajjou L, Marion-Poll A (2013). ABA crosstalk with ethylene and nitric oxide in seed dormancy and germination. Front Plant Sci 4, 63. |
| [5] |
Arce DP, Godoy AV, Tsuda K, Yamazaki KI, Valle EM, Iglesias MJ, Di Mauro MF, Casalongué CA (2010). The analysis of an Arabidopsis triple knock-down mutant reveals functions for MBF1 genes under oxidative stress conditions. J Plant Physiol 167, 194-200.
DOI URL |
| [6] |
Avonce N, Leyman B, Mascorro-Gallardo JO, Van Dijck P, Thevelein JM, Iturriaga G (2004). The Arabidopsis trehalose-6-P synthase AtTPS1 gene is a regulator of glucose, abscisic acid, and stress signaling. Plant Physiol 136, 3649-3659.
DOI URL |
| [7] | Bita CE, Gerats T (2013). Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci 4, 273. |
| [8] |
Blombach F, Launay H, Snijders APL, Zorraquino V, Wu H, de Koning B, Brouns SJJ, Ettema TJG, Camilloni C, Cavalli A, Vendruscolo M, Dickman MJ, Cabrita LD, La Teana A, Benelli D, Londei P, Christodoulou J, van der Oost J (2014). Archaeal MBF1 binds to 30S and 70S ribosomes via its helix-turn-helix domain. Biochem J 462, 373-384.
DOI PMID |
| [9] |
Busk PK, Wulf-Andersen L, Strøm CC, Enevoldsen M, Thirstrup K, Haunsø S, Sheikh SØP (2003). Multiprotein bridging factor 1 cooperates with c-jun and is necessary for cardiac hypertrophy in vitro. Exp Cell Res 286, 102-114.
DOI URL |
| [10] |
Clarke SM, Mur LAJ, Wood JE, Scott IM (2004). Salicylic acid dependent signaling promotes basal thermotolerance but is not essential for acquired thermotolerance in Arabidopsis thaliana. Plant J 38, 432-447.
DOI URL |
| [11] | Corbineau F, Xia Q, Bailly C, El-Maarouf-Bouteau H (2014). Ethylene, a key factor in the regulation of seed dormancy. Front Plant Sci 5, 539. |
| [12] |
De Boeck HJ, Bassin S, Verlinden M, Zeiter M, Hiltbrunner E (2016). Simulated heat waves affected alpine grassland only in combination with drought. New Phytol 209, 531-541.
DOI PMID |
| [13] |
Di Mauro MF, Iglesias MJ, Arce DP, Valle EM, Arnold RB, Tsuda K, Yamazaki KI, Casalongué CA, Godoy AV (2012). MBF1s regulate ABA-dependent germination of Arabidopsis seeds. Plant Signal Behav 7, 188-192.
DOI URL |
| [14] |
Ding LP, Wu Z, Teng RD, Xu SJ, Cao X, Yuan GZ, Zhang DH, Teng NJ (2021). LlWRKY39 is involved in thermotolerance by activating LlMBF1c and interacting with LlCaM3 in lily (Lilium longiflorum). Hortic Res 8, 36.
DOI URL |
| [15] |
Eulgem T, Somssich IE (2007). Networks of WRKY transcription factors in defense signaling. Curr Opin Plant Biol 10, 366-371.
DOI URL |
| [16] |
Finka A, Cuendet AFH, Maathuis FJM, Saidi Y, Goloubinoff P (2012). Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell 24, 3333-3348.
DOI URL |
| [17] |
Gao F, Han XW, Wu JH, Zheng SZ, Shang ZL, Sun DY, Zhou RG, Li B (2012). A heat-activated calcium-permeable channel-Arabidopsis cyclic nucleotide-gated ion channel 6-is involved in heat shock responses. Plant J 70, 1056-1069.
DOI URL |
| [18] | Godoy AV, Zanetti ME, San Segundo B, Casalongué CA (2001). Identification of a putative Solanum tuberosum transcriptional coactivator up-regulated in potato tubers by Fusarium solani f. sp. eumartii infection and wounding. Physiol Plant 112, 217-222. |
| [19] |
Gong ZZ, Xiong LM, Shi HZ, Yang SH, Herrera-Estrella LR, Xu GH, Chao DY, Li JR, Wang PY, Qin F, Li JJ, Ding YL, Shi YT, Wang Y, Yang YQ, Guo Y, Zhu JK (2020). Plant abiotic stress response and nutrient use efficiency. Sci China Life Sci 63, 635-674.
DOI URL |
| [20] |
Grennan AK (2007). The role of trehalose biosynthesis in plants. Plant Physiol 144, 3-5.
PMID |
| [21] |
Higashi Y, Ohama N, Ishikawa T, Katori T, Shimura A, Kusakabe K, Yamaguchi-Shinozaki K, Ishida J, Tanaka M, Seki M, Shinozaki K, Sakata Y, Hayashi T, Taji T (2013). HsfA1d, a protein identified via FOX hunting using Thellungiella salsuginea cDNAs improves heat tolerance by regulating heat-stress-responsive gene expression. Mol Plant 6, 411-422.
DOI PMID |
| [22] |
Hommel M, Khalil-Ahmad Q, Jaimes-Miranda F, Mila I, Pouzet C, Latché A, Pech JC, Bouzayen M, Regad F (2008). Over-expression of a chimeric gene of the transcriptional co-activator MBF1 fused to the EAR repressor motif causes developmental alteration in Arabidopsis and tomato. Plant Sci 175, 168-177.
DOI URL |
| [23] |
Hozain M, Abdelmageed H, Lee J, Kang M, Fokar M, Allen RD, Holaday AS (2012). Expression of AtSAP5 in cotton up-regulates putative stress-responsive genes and improves the tolerance to rapidly developing water deficit and moderate heat stress. J Plant Physiol 169, 1261-1270.
DOI URL |
| [24] |
Jaimes-Miranda F, Montes RAC (2020). The plant MBF1 protein family: a bridge between stress and transcription. J Exp Bot 71, 1782-1791.
DOI PMID |
| [25] |
Jegadeesan S, Beery A, Altahan L, Meir S, Pressman E, Firon N (2018). Ethylene production and signaling in tomato (Solanum lycopersicum) pollen grains is responsive to heat stress conditions. Plant Reprod 31, 367-383.
DOI PMID |
| [26] |
Katano K, Honda K, Suzuki N (2018a). Integration between ROS regulatory systems and other signals in the regulation of various types of heat responses in plants. Int J Mol Sci 19, 3370.
DOI URL |
| [27] |
Katano K, Kataoka R, Fujii M, Suzuki N (2018b). Differences between seedlings and flowers in anti-ROS based heat responses of Arabidopsis plants deficient in cyclic nucleotide gated channel 2. Plant Physiol Biochem 123, 288-296.
DOI URL |
| [28] |
Kim GD, Cho YH, Yoo SD (2015). Regulatory functions of evolutionarily conserved AN1/A20-like Zinc finger family proteins in Arabidopsis stress responses under high temperature. Biochem Biophys Res Commun 457, 213-220.
DOI URL |
| [29] |
Kotak S, Larkindale J, Lee U, von Koskull-Döring P, Vierling E, Scharf KD (2007). Complexity of the heat stress response in plants. Curr Opin Plant Biol 10, 310-316.
DOI URL |
| [30] |
Larkindale J, Hall JD, Knight MR, Vierling E (2005). Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol 138, 882-897.
PMID |
| [31] |
Li BJ, Gao K, Ren HM, Tang WQ (2018). Molecular mechanisms governing plant responses to high temperatures. J Integr Plant Biol 60, 757-779.
DOI URL |
| [32] |
Li SJ, Fu QT, Chen LG, Huang WD, Yu DQ (2011). Arabidopsis thaliana WRKY25, WRKY26, and WRKY33 coordinate induction of plant thermotolerance. Planta 233, 1237-1252.
DOI URL |
| [33] |
Li SJ, Zhou X, Chen LG, Huang WD, Yu DQ (2010). Functional characterization of Arabidopsis thaliana WRKY39 in heat stress. Mol Cells 29, 475-483.
DOI URL |
| [34] | Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, Dangl JL, Mittler R (2009). The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal 2, ra45. |
| [35] |
Mittler R (2006). Abiotic stress, the field environment and stress combination. Trends Plant Sci 11, 15-19.
PMID |
| [36] |
Mittler R, Finka A, Goloubinoff P (2012). How do plants feel the heat? Trends Biochem Sci 37, 118-125.
DOI PMID |
| [37] |
Müller M, Munné-Bosch S (2015). Ethylene response factors: a key regulatory hub in hormone and stress signaling. Plant Physiol 169, 32-41.
DOI PMID |
| [38] |
Nakashima K, Yamaguchi-Shinozaki K (2013). ABA signaling in stress-response and seed development. Plant Cell Rep 32, 959-970.
DOI PMID |
| [39] |
Ohama N, Sato H, Shinozaki K, Yamaguchi-Shinozaki K (2017). Transcriptional regulatory network of plant heat stress response. Trends Plant Sci 22, 53-65.
DOI URL |
| [40] |
Ozaki J, Takemaru KI, Ikegami T, Mishima M, Ueda H, Hirose S, Kabe Y, Handa H, Shirakawa M (1999). Identification of the core domain and the secondary structure of the transcriptional coactivator MBF1. Genes Cells 4, 415-424.
PMID |
| [41] |
Pandey GK, Grant JJ, Cheong YH, Kim BG, Li LG, Luan S (2005). ABR1, an APETALA2-domain transcription factor that functions as a repressor of ABA response in Arabidopsis. Plant Physiol 139, 1185-1193.
DOI URL |
| [42] |
Qin DD, Wang F, Geng XL, Zhang LY, Yao YY, Ni ZF, Peng HR, Sun QX (2015). Overexpression of heat stress-responsive TaMBF1c, a wheat (Triticum aestivum L.) multiprotein bridging factor, confers heat tolerance in both yeast and rice. Plant Mol Biol 87, 31-45.
DOI URL |
| [43] |
Sajid M, Rashid B, Ali Q, Husnain T (2018). Mechanisms of heat sensing and responses in plants. It is not all about Ca2+ ions. Biol Plant 62, 409-420.
DOI URL |
| [44] |
Sakuma Y, Maruyama K, Qin F, Osakabe Y, Shinozaki K, Yamaguchi-Shinozaki K (2006). Dual function of an Arabidopsis transcription factor DREB2A in water-stress- responsive and heat-stress-responsive gene expression. Proc Natl Acad Sci USA 103, 18822-18827.
DOI URL |
| [45] |
Sato H, Mizoi J, Tanaka H, Maruyama K, Qin F, Osakabe Y, Morimoto K, Ohori T, Kusakabe K, Nagata M, Shinozaki K, Yamaguchi-Shinozaki K (2014). Arabidopsis DPB3-1, a DREB2A interactor, specifically enhances heat stress-induced gene expression by forming a heat stress-specific transcriptional complex with NF-Y subunits. Plant Cell 26, 4954-4973.
DOI URL |
| [46] |
Schramm F, Larkindale J, Kiehlmann E, Ganguli A, Englich G, Vierling E, Von Koskull-Döring P (2008). A cascade of transcription factor DREB2A and heat stress transcription factor HsfA3 regulates the heat stress response of Arabidopsis. Plant J 53, 264-274.
DOI URL |
| [47] |
Song C, Ortiz-Urquiza A, Ying SH, Zhang JX, Keyhani NO (2015). Interaction between TATA-binding protein (TBP) and multiprotein bridging factor-1 (MBF1) from the filamentous insect pathogenic fungus Beauveria bassiana. PLoS One 10, e0140538.
DOI URL |
| [48] |
Suzuki N, Bajad S, Shuman J, Shulaev V, Mittler R (2008). The transcriptional co-activator MBF1c is a key regulator of thermotolerance in Arabidopsis thaliana. J Biol Chem 283, 9269-9275.
DOI URL |
| [49] |
Suzuki N, Bassil E, Hamilton JS, Inupakutika MA, Zandalinas SI, Tripathy D, Luo YT, Dion E, Fukui G, Kumazaki A, Nakano R, Rivero RM, Verbeck GF, Azad RK, Blumwald E, Mittler R (2016). ABA is required for plant acclimation to a combination of salt and heat stress. PLoS One 11, e0147625.
DOI URL |
| [50] |
Suzuki N, Rizhsky L, Liang HJ, Shuman J, Shulaev V, Mittler R (2005). Enhanced tolerance to environmental stress in transgenic plants expressing the transcriptional coactivator multiprotein bridging factor 1c. Plant Physiol 139, 1313-1322.
DOI URL |
| [51] |
Suzuki N, Sejima H, Tam R, Schlauch K, Mittler R (2011). Identification of the MBF1 heat-response regulon of Arabidopsis thaliana. Plant J 66, 844-851.
DOI URL |
| [52] |
Takemaru KI, Harashima S, Ueda H, Hirose S (1998). Yeast coactivator MBF1 mediates GCN4-dependent transcriptional activation. Mol Cell Biol 18, 4971-4976.
DOI PMID |
| [53] |
Takemaru KI, Li FQ, Ueda H, Hirose S (1997). Multiprotein bridging factor 1 (MBF1) is an evolutionarily conserved transcriptional coactivator that connects a regulatory factor and TATA element-binding protein. Proc Natl Acad Sci USA 94, 7251-7256.
DOI URL |
| [54] |
Tian X, Qin Z, Zhao Y, Wen J, Lan T, Zhang L, Wang F, Qin D, Yu K, Zhao A, Hu Z, Yao Y, Ni Z, Sun Q, De Smet I, Peng H, Xin M (2021). Stress granule associated TaMBF1c confers thermotolerance through regulating specific mRNA translation in wheat (Triticum aestivum). New Phytol 233, 1719-1731.
DOI URL |
| [55] |
Tsuda K, Tsuji T, Hirose S, Yamazaki KI (2004). Three Arabidopsis MBF1 homologs with distinct expression profiles play roles as transcriptional co-activators. Plant Cell Physiol 45, 225-231.
DOI URL |
| [56] | Tsuda K, Yamazaki KI (2004). Structure and expression analysis of three subtypes of Arabidopsis MBF1 genes. Biochim Biophys Acta 1680, 1-10. |
| [57] |
Wang XL, Du Y, Yu DQ (2019). Trehalose phosphate synthase 5-dependent trehalose metabolism modulates basal defense responses in Arabidopsis thaliana. J Integr Plant Biol 61, 509-527.
DOI URL |
| [58] |
Wang YY, Wei XL, Huang J, Wei JC (2017). Modification and functional adaptation of the MBF1 gene family in the lichenized fungus Endocarpon pusillum under environmental stress. Sci Rep 7, 16333.
DOI URL |
| [59] |
Xu CX, Jiao C, Sun HH, Cai XF, Wang XL, Ge CH, Zheng Y, Liu WL, Sun XP, Xu YM, Deng J, Zhang ZH, Huang SW, Dai SJ, Mou BQ, Wang QX, Fei ZJ, Wang QH (2017). Draft genome of spinach and transcriptome diversity of 120 Spinacia accessions. Nat Commun 8, 15275.
DOI URL |
| [60] |
Yan Q, Hou HM, Singer SD, Yan XX, Guo RR, Wang XP (2014). The grape VvMBF1 gene improves drought stress tolerance in transgenic Arabidopsis thaliana. Plant Cell Tiss Org 118, 571-582.
DOI URL |
| [61] |
Yoshida T, Sakuma Y, Todaka D, Maruyama K, Qin F, Mizoi J, Kidokoro S, Fujita Y, Shinozaki K, Yamaguchi-Shinozaki K (2008). Functional analysis of an Arabidopsis heat-shock transcription factor HsfA3 in the transcriptional cascade downstream of the DREB2A stress-regulatory system. Biochem Biophys Res Commun 368, 515-521.
DOI URL |
| [62] |
Yu RM, Suo YY, Yang R, Chang YN, Tian T, Song YJ, Wang HJ, Wang C, Yang RJ, Liu HL, Gao G (2021). StMBF1c positively regulates disease resistance to Ralstonia solanacearum via its primary and secondary upregulation combining expression of StTPS5 and resistance marker genes in potato. Plant Sci 307, 110877.
DOI URL |
| [63] |
Zandalinas SI, Balfagón D, Arbona V, Gómez-Cadenas A, Inupakutika MA, Mittler R (2016). ABA is required for the accumulation of APX1 and MBF1c during a combination of water deficit and heat stress. J Exp Bot 67, 5381-5390.
PMID |
| [64] |
Zanetti ME, Blanco FA, Daleo GR, Casalongué CA (2003). Phosphorylation of a member of the MBF1 transcriptional co-activator family, StMBF1, is stimulated in potato cell suspensions upon fungal elicitor challenge. J Exp Bot 54, 623-632.
DOI URL |
| [65] |
Zhang X, Xu ZX, Chen LC, Ren ZH (2019). Comprehensive analysis of multiprotein bridging factor 1 family genes and SlMBF1c negatively regulate the resistance to Botrytis cinerea in tomato. BMC Plant Biol 19, 437.
DOI PMID |
| [66] |
Zou LF, Yu BW, Ma XL, Cao BH, Chen GJ, Chen CM, Lei JJ (2019). Cloning and expression analysis of the BocMBF1c gene involved in heat tolerance in Chinese kale. Int J Mol Sci 20, 5637.
DOI URL |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||