

Chinese Bulletin of Botany ›› 2021, Vol. 56 ›› Issue (5): 594-604.DOI: 10.11983/CBB21052 cstr: 32102.14.CBB21052
• TECHNIQUES AND METHODS • Previous Articles Next Articles
Chunyan Zhang1,2,*( ), Xiaojie Pang2
), Xiaojie Pang2
Received:2021-03-23
Accepted:2021-06-18
Online:2021-09-01
Published:2021-08-31
Contact:
Chunyan Zhang
Chunyan Zhang, Xiaojie Pang. The Measurement Principles, Methods and Applications of P515[J]. Chinese Bulletin of Botany, 2021, 56(5): 594-604.
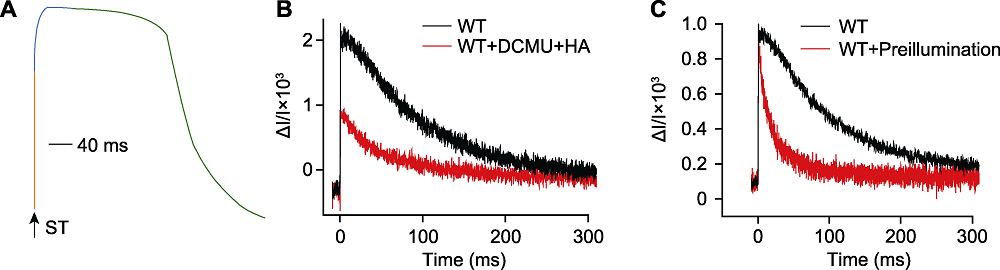
Figure 1 The P515 fast kinetics induced by a single turnover saturating flash (A) The diagram of P515 fast kinetics induced by a single turnover saturating flash, rapid ascent stage (orange), slow ascent stage (blue) and slow descent stage (green), the arrow indicated a single turnover saturating flash (ST); (B) The rapid absorption changes of Chlamydomonas reinhardtii cells (WT) at 520-550 nm induced by a ST (the wavelength of ST was 635 nm, the light intensity was 200000 μmol·m-2·s-1 and the duration was 5 μs), WT (concentration 25 μg·mL-1) was incubated with (red) and without (black) 10 μmol·L-1 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) and 1 mmol·L-1 hydroxylamine (HA) for 20 min in darkness; (C) The affection of preillumination to the P515 fast kinetics, WT algae was incubated in darkness for 20 min (black) and preilluminated for 2 min at 40 μmol·m-2·s-1 followed by 1 min dark (red).
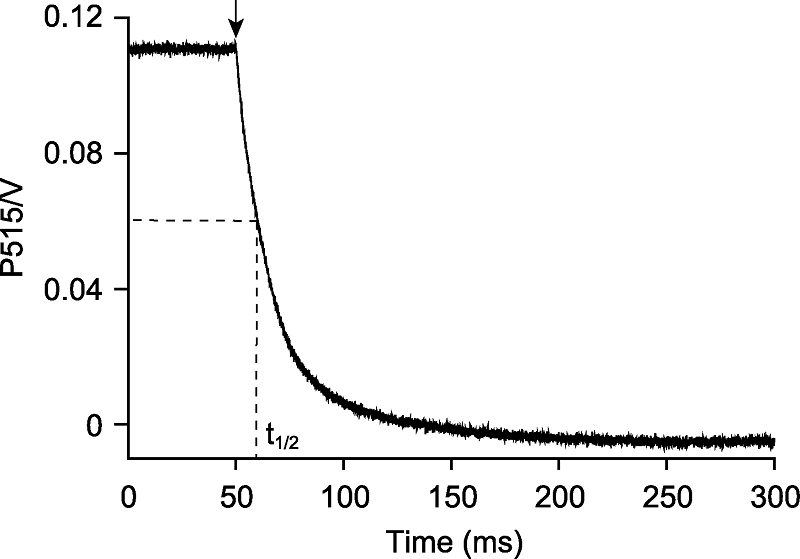
Figure 2 Detection the H+ conductivity of chloroplast ATP synthase in Arabidopsis thaliana The detached leaf of Arabidopsis thaliana was exposed to light (635 nm, 500 μmol·m-2·s-1) for 10 min, and provided stable CO2 (380 μmol·mol-1), humidity (60% atmospheric humidity) and temperature (23°C) during illumination. Detected the P515 decay kinetics curve in darkness that lasted 250 ms, the curve corresponded to 100 independent biological replicates. The H+ conductivity of ATP synthase was 94.3·s-1, which is the reciprocal of the half-time (t1/2) of P515 decay kinetics curve. The arrow indicated light off.
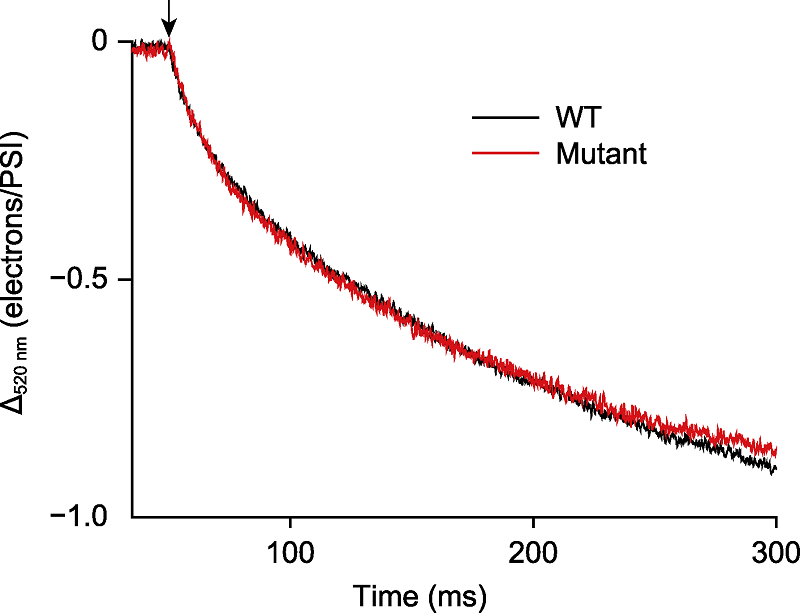
Figure 3 Detection the cyclic electron flow rate around photosystem I in Chlamydomonas reinhardtii The C. reinhardtii cells (WT) and PGR5 mutant (Mutant) were exposed to light (635 nm, 80 μmol·m-2·s-1) for 5 min. The cyclic electron flow rate was detected after the light was turned off and the darkness duration was 250 ms. Before measurement, algal cells (about 25 μg·mL-1) were incubated with 10 μmol·L-1 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU), 1 mmol·L-1 hydroxylamine (HA) and 20% ficoll for 20 min in darkness. The arrow indicated light off.
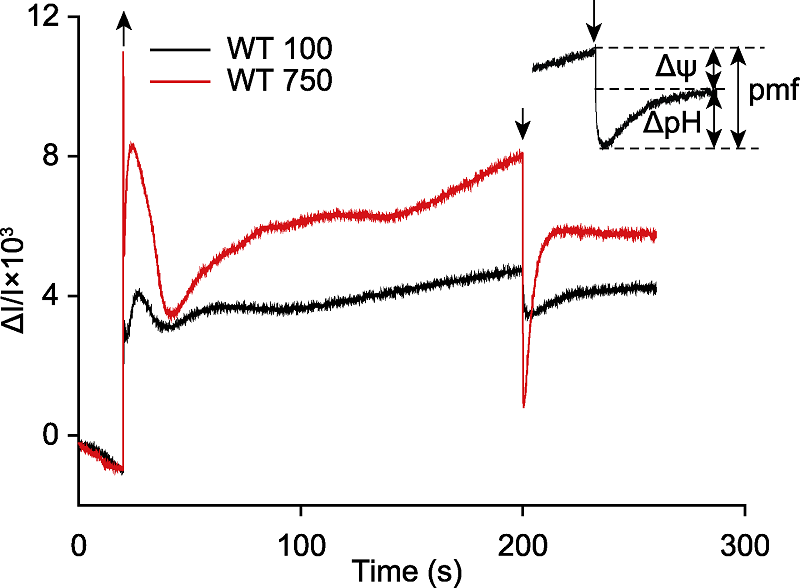
Figure 4 Detection the proton motive force (pmf) and its two components (Δψ and ΔpH) in Arabidopsis thaliana The detached leaf of Arabidopsis thaliana was exposed to light (635 nm, 100 μmol·m-2·s-1, labeled as WT 100, black and 750 μmol·m-2·s-1, labeled as WT 750, red) for 3 min, and provided stable CO2 (380 μmol·mol-1), humidity (60% atmospheric humidity) and temperature (23°C) during illumination. The dark time was 60 s and the curve corresponded to 3 independent biological replicates. The Arabidopsis leaf was darkly adapted for at least 3 hours before measurement. The up arrow indicated light on, and the down arrow indicated light off.
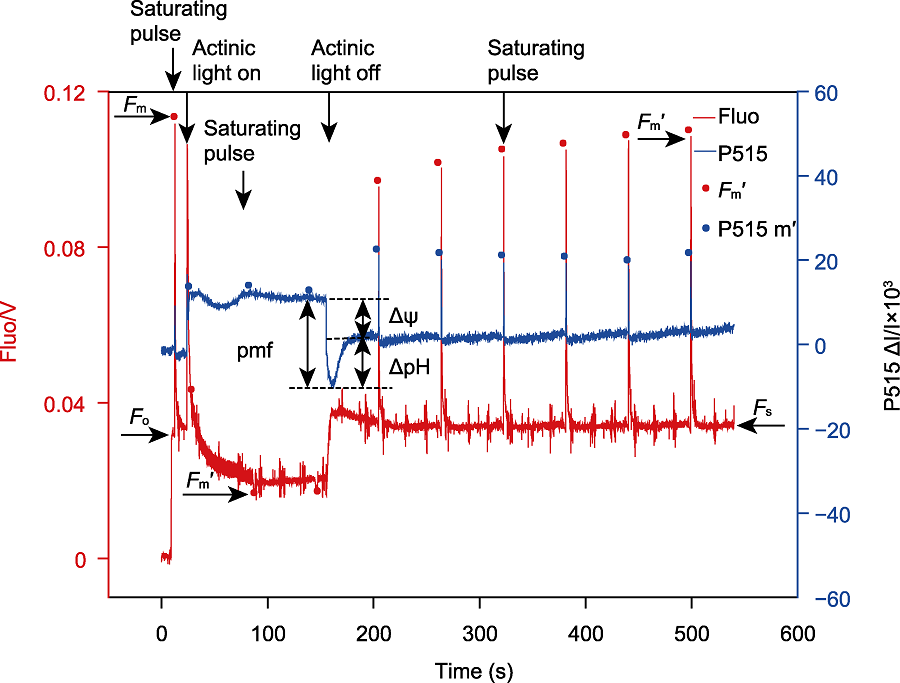
Figure 5 Simultaneous measurement of the P515 signal and fluorescence in Chlamydomonas reinhardtii The C. reinhardtii cells (WT, about 30 μg·mL-1) were placed in the chamber of the chlorophyll fluorometer after dark adaptation for 20 minutes. The measuring lights of the P515/535 module and the chlorophyll fluorescence module were turned on respectively to determine the original fluorescence yield (Fo). Then a saturating pulse (635 nm, 6000 μmol·m-2·s-1, duration 250 ms) was turned on to detect the maximal fluorescence yield (Fm) in darkness. Then an actinic light (635 nm, 1500 μmol·m-2·s-1) was turned on for 140 seconds, during which a saturating flash was applied every 60 seconds to detect the maximum fluorescence yield (Fm') under light. The proton motive force (pmf) and its components (ΔΨ and ΔpH) were measured after the actinic light was turned off, meanwhile a saturating pulse was applied every 60 seconds to check whether the fluorescence yield was restored to the maximum fluorescence yield (Fm) in darkness. Fo: Original fluorescence yield; Fm: Maximal fluorescence yield; Fm′: Maximal fluorescence yield under light; Fs: Steady state fluorescence yield; pmf: Proton motive force; ΔΨ: Transmembrane potential; ΔpH: Transmembrane proton gradient
| [1] | 付振书, 赵世杰, 孟庆伟 (2004). 类囊体腔的酸化与过剩激发能耗散. 植物学通报 21, 486-494. |
| [2] |
Allorent G, Byrdin M, Carraretto L, Morosinotto T, Szabo I, Finazzi G (2018). Global spectroscopic analysis to study the regulation of the photosynthetic proton motive force: a critical reappraisal. Biochim Biophys Acta Bioenerg 1859, 676-683.
DOI URL |
| [3] |
Alric J (2014). Redox and ATP control of photosynthetic cyclic electron flow in Chlamydomonas reinhardtii: (II) involvement of the PGR5-PGRL1 pathway under anaerobic conditions. Biochim Biophys Acta Bioenerg 1837, 825-834.
DOI URL |
| [4] |
Bailleul B, Cardol P, Breyton C, Finazzi G (2010). Electrochromism: a useful probe to study algal photosynthesis. Photosynth Res 106, 179-189.
DOI URL |
| [5] |
Baker NR, Harbinson J, Kramer DM (2007). Determining the limitations and regulation of photosynthetic energy transduction in leaves. Plant Cell Environ 30, 1107-1125.
PMID |
| [6] |
Bennoun P (1994). Chlororespiration revisited: mitochondrial-plastid interactions in Chlamydomonas. Biochim Biophys Acta Bioenerg 1186, 59-66.
DOI URL |
| [7] |
Bonente G, Pippa S, Castellano S, Bassi R, Ballottari M (2012). Acclimation of Chlamydomonas reinhardtii to different growth irradiances. J Biol Chem 287, 5833-5847.
DOI PMID |
| [8] |
Bujaldon S, Kodama N, Rathod MK, Tourasse N, Ozawa SI, Sellés J, Vallon O, Takahashi Y, Wollman FA (2020). The BF4 and p71 antenna mutants from Chlamydomonas reinhardtii. Biochim Biophys Acta Bioenerg 1861, 148085.
DOI URL |
| [9] |
Carraretto L, Formentin E, Teardo E, Checchetto V, Tomizioli M, Morosinotto T, Giacometti GM, Finazzi G, Szabó I (2013). A thylakoid-located two-pore K+ channel controls photosynthetic light utilization in plants. Science 342, 114-118.
DOI PMID |
| [10] |
Carraretto L, Teardo E, Checchetto V, Finazzi G, Uozumi N, Szabo I (2016). Ion channels in plant bioenergetic organelles, chloroplasts and mitochondria: from molecular identification to function. Mol Plant 9, 371-395.
DOI PMID |
| [11] |
Checchetto V, Teardo E, Carraretto L, Formentin E, Bergantino E, Giacometti GM, Szabo I (2013). Regulation of photosynthesis by ion channels in cyanobacteria and higher plants. Biophys Chem 182, 51-57.
DOI PMID |
| [12] |
Checchetto V, Teardo E, Carraretto L, Leanza L, Szabo I (2016). Physiology of intracellular potassium channels: a unifying role as mediators of counterion fluxes? Biochim Biophys Acta Bioenerg 1857, 1258-1266.
DOI URL |
| [13] |
Cruz JA, Sacksteder CA, Kanazawa A, Kramer DM (2001). Contribution of electric field (Δψ) to steady-state transthylakoid proton motive force (pmf) in vitro and in vivo. Control of pmf parsing into Δψ and ΔpH by ionic strength. Biochemistry 40, 1226-1237.
PMID |
| [14] |
Davis GA, Kanazawa A, Schöttler MA, Kohzuma K, Froehlich JE, Rutherford AW, Satoh-Cruz M, Minhas D, Tietz S, Dhingra A, Kramer DM (2016). Limitations to photosynthesis by proton motive force-induced photosystem II photodamage. eLife 5, e16921.
DOI URL |
| [15] |
Davis GA, Rutherford AW, Kramer DM (2017). Hacking the thylakoid proton motive force for improved photosynthesis: modulating ion flux rates that control proton motive force partitioning into Δψ and ΔpH. Philos Trans R Soc Lond B Biol Sci 372, 20160381.
DOI URL |
| [16] |
de Bianchi S, Ballottari M, Dall'Osto L, Bassi R (2010). Regulation of plant light harvesting by thermal dissipation of excess energy. Biochem Soc Trans 38, 651-660.
DOI URL |
| [17] |
Demmig-Adams B, Cohu CM, Muller O, Adams WW III (2012). Modulation of photosynthetic energy conversion efficiency in nature: from seconds to seasons. Photosynth Res 113, 75-88.
DOI URL |
| [18] |
Duan ZK, Kong FN, Zhang L, Li WJ, Zhang J, Peng LW (2016). A bestrophin-like protein modulates the proton motive force across the thylakoid membrane in Arabidopsis. J Integr Plant Biol 58, 848-858.
DOI URL |
| [19] |
Duysens LNM (1954). Reversible changes in the absorption spectrum of Chlorella upon irradiation. Science 120, 353-354.
PMID |
| [20] |
Fork DC, Amesz J (1967). Light-induced shifts in the absorption spectrum of carotenoids in red and brown algae. Photochem Photobiol 6, 913-918.
DOI URL |
| [21] |
Frese RN, Palacios MA, Azzizi A, van Stokkum IHM, Kruip J, Rögner M, Karapetyan NV, Schlodder E, van Grondelle R, Dekker JP (2002). Electric field effects on red chlorophylls, β-carotenes and P700 in cyanobacterial Photosystem I complexes. Biochim Biophys Acta Bioenerg 1554, 180-191.
DOI URL |
| [22] |
Fristedt R, Martins NF, Strenkert D, Clarke CA, Suchoszek M, Thiele W, Schöttler MA, Merchant SS (2015). The thylakoid membrane protein CGL160 supports CF1CFo ATP synthase accumulation in Arabidopsis thaliana. PLoS One 10, e0121658.
DOI URL |
| [23] |
Hind G, Nakatani HY, Izawa S (1974). Light-dependent redistribution of ions in suspensions of chloroplast thylakoid membranes. Proc Natl Acad Sci USA 71, 1484-1488.
DOI URL |
| [24] |
Hirano M, Katoh S (1981). Electrochromic band shifts of carotenoid in a blue-green alga. Photochem Photobiol 34, 637-643.
DOI URL |
| [25] |
Holmes NG, Hunter CN, Niederman RA, Crofts AR (1980). Identification of the pigment pool responsible for the flash-induced carotenoid band shift in Rhodopseudomonas sphaeroides chromatophores. FEBS Lett 115, 43-48.
DOI URL |
| [26] |
Joliot P, Joliot A (2002). Cyclic electron transfer in plant leaf. Proc Natl Acad Sci USA 99, 10209-10214.
DOI URL |
| [27] |
Junge W, Witt HT (1968). On the ion transport system of photosynthesis: investigations on a molecular level. Z Naturforsch B 23, 244-254.
DOI URL |
| [28] |
Klughammer C, Siebke K, Schreiber U (2013). Continuous ECS-indicated recording of the proton-motive charge flux in leaves. Photosynth Res 117, 471-487.
DOI URL |
| [29] |
Kramer DM, Avenson TJ, Edwards GE (2004). Dynamic flexibility in the light reactions of photosynthesis governed by both electron and proton transfer reactions. Trends Plant Sci 9, 349-357.
PMID |
| [30] |
Kramer DM, Cruz JA, Kanazawa A (2003). Balancing the central roles of the thylakoid proton gradient. Trends Plant Sci 8, 27-32.
PMID |
| [31] |
Kramer DM, Evans JR (2011). The importance of energy balance in improving photosynthetic productivity. Plant Physiol 155, 70-78.
DOI PMID |
| [32] |
Kramer H, Mathis P (1980). Quantum yield and rate of formation of the carotenoid triplet state in photosynthetic structures. Biochim Biophys Acta Bioenerg 593, 319-329.
DOI URL |
| [33] |
Liguori N, Roy LM, Opacic M, Durand G, Croce R (2013). Regulation of light harvesting in the green alga Chlamydomonas reinhardtii: the C-terminus of LHCSR is the knob of a dimmer switch. J Am Chem Soc 135, 18339-18342.
DOI URL |
| [34] |
Lucker B, Kramer DM (2013). Regulation of cyclic electron flow in Chlamydomonas reinhardtii under fluctuating carbon availability. Photosynth Res 117, 449-459.
DOI URL |
| [35] |
Nandha B, Finazzi G, Joliot P, Hald S, Johnson GN (2007). The role of PGR5 in the redox poising of photosynthetic electron transport. Biochim Biophys Acta Bioenerg 1767, 1252-1259.
DOI URL |
| [36] |
Nawrocki WJ, Santabarbara S, Mosebach L, Wollman FA, Rappaport F (2016). State transitions redistribute rather than dissipate energy between the two photosystems in Chlamydomonas. Nat Plants 2, 16031.
DOI URL |
| [37] |
Niyogi KK, Grossman AR, Björkman O (1998). Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10, 1121-1134.
PMID |
| [38] |
Pottosin II, Schönknecht G (1996). Ion channel permeable for divalent and monovalent cations in native spinach thylakoid membranes. J Membr Biol 152, 223-233.
DOI URL |
| [39] |
Rott M, Martins NF, Thiele W, Lein W, Bock R, Kramer DM, Schöttler MA (2011). ATP synthase repression in tobacco restricts photosynthetic electron transport, CO2 assimilation, and plant growth by overacidification of the thylakoid lumen. Plant Cell 23, 304-321.
DOI URL |
| [40] |
Ruban AV, Pascal AA, Robert B, Horton P (2002). Activation of zeaxanthin is an obligatory event in the regulation of photosynthetic light harvesting. J Biol Chem 277, 7785-7789.
DOI URL |
| [41] |
Sacksteder CA, Kanazawa A, Jacoby ME, Kramer DM (2000). The proton to electron stoichiometry of steady- state photosynthesis in living plants: a proton-pumping Q cycle is continuously engaged. Proc Natl Acad Sci USA 97, 14283-14288.
DOI URL |
| [42] |
Sacksteder CA, Kramer DM (2000). Dark-interval relaxation kinetics (DIRK) of absorbance changes as a quantitative probe of steady-state electron transfer. Photosynth Res 66, 145-158.
DOI URL |
| [43] | Schmidt S, Reich R, Witt HT (1971). Electrochromism of chlorophylls and carotenoids in multilayers and in chloroplasts. Naturwissenschaften 58, 414. |
| [44] |
Schönknecht G, Hedrich R, Junge W, Raschke K (1988). A voltage-dependent chloride channel in the photosynthetic membrane of a higher plant. Nature 336, 589-592.
DOI URL |
| [45] | Schreiber U, Klughammer C (2008). New accessory for the DUAL-PAM-100: the P515/535 module and examples of its application. PAM Appl Notes 1, 1-10. |
| [46] |
Sonoike K (2011). Photoinhibition of photosystem I. Physiol Plant 142, 56-64.
DOI URL |
| [47] |
Spetea C, Herdean A, Allorent G, Carraretto L, Finazzi G, Szabo I (2017). An update on the regulation of photosynthesis by thylakoid ion channels and transporters in Arabidopsis. Physiol Plant 161, 16-27.
DOI URL |
| [48] | Sukhov V, Surova L, Morozova E, Sherstneva O, Vodeneev V (2016). Changes in H+-ATP synthase activity, proton electrochemical gradient, and pH inPea chloroplast can be connected with variation potential. Front Plant Sci 7, 1092. |
| [49] |
Takahashi H, Clowez S, Wollman FA, Vallon O, Rappaport F (2013). Cyclic electron flow is redox-controlled but independent of state transition. Nat Commun 4, 1954.
DOI PMID |
| [50] |
Takizawa K, Cruz JA, Kanazawa A, Kramer DM (2007). The thylakoid proton motive force in vivo. Quantitative, non-invasive probes, energetics, and regulatory consequences of light-induced pmf. Biochim Biophys Acta Bioenerg 1767, 1233-1244.
DOI URL |
| [51] |
Tolleter D, Ghysels B, Alric J, Petroutsos D, Tolstygina I, Krawietz D, Happe T, Auroy P, Adriano JM, Beyly A, Cuiné S, Plet J, Reiter IM, Genty B, Cournac L, Hippler M, Peltier G (2011). Control of hydrogen photoproduction by the proton gradient generated by cyclic electron flow in Chlamydomonas reinhardtii. Plant Cell 23, 2619-2630.
DOI URL |
| [52] |
Viola S, Bailleul B, Yu JF, Nixon P, Sellés J, Joliot P, Wollman FA (2019). Probing the electric field across thylakoid membranes in cyanobacteria. Proc Natl Acad Sci USA 116, 21900-21906.
DOI URL |
| [53] |
Wang P, Duan W, Takabayashi A, Endo T, Shikanai T, Ye JY, Mi HL (2006). Chloroplastic NAD(P)H dehydrogenase in tobacco leaves functions in alleviation of oxidative damage caused by temperature stress. Plant Physiol 141, 465-474.
DOI URL |
| [54] |
Witt HT (1979). Energy conversion in the functional membrane of photosynthesis. Analysis by light pulse and electric pulse methods: the central role of the electric field. Biochim Biophys Acta 505, 355-427.
PMID |
| [55] |
Yamori W, Takahashi S, Makino A, Price GD, Badger MR, von Caemmerer S (2011). The roles of ATP synthase and the cytochrome b 6/f complexes in limiting chloroplast electron transport and determining photosynthetic capacity. Plant Physiol 155, 956-962.
DOI URL |
| [56] |
Zhang L, Duan ZK, Zhang J, Peng LW (2016). Biogenesis factor required for ATP synthase 3 facilitates assembly of the chloroplast ATP synthase complex. Plant Physiol 171, 1291-1306.
DOI PMID |
| [57] |
Zhang L, Pu H, Duan ZK, Li YH, Liu B, Zhang QQ, Li WJ, Rochaix JD, Liu L, Peng LW (2018). Nucleus-encoded protein BFA1 promotes efficient assembly of the chloroplast ATP synthase coupling factor 1. Plant Cell 30, 1770-1788.
DOI URL |
| [58] |
Zhang R, Cruz JA, Kramer DM, Magallanes-Lundback ME, Dellapenna D, Sharkey TD (2009). Moderate heat stress reduces the pH component of the transthylakoid proton motive force in light-adapted, intact tobacco leaves. Plant Cell Environ 32, 1538-1547.
DOI URL |
| [59] |
Zoschke R, Kroeger T, Belcher S, Schöttler MA, Barkan A, Schmitz-Linneweber C (2012). The pentatricopeptide repeat-SMR protein ATP4 promotes translation of the chloroplast atpB/E mRNA. Plant J 72, 547-558.
DOI URL |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||